Abstract
Synaptic dysfunction is a key process in the evolution of many neurodegenerative diseases, with synaptic loss preceding that of neuronal cell bodies. In Alzheimer, Huntington, and prion diseases early synaptic changes correlate with cognitive and motor decline, and altered synaptic function may also underlie deficits in a number of psychiatric and neurodevelopmental conditions. The formation, remodelling and elimination of spines and synapses are continual physiological processes, moulding cortical architecture, underpinning the abilities to learn and remember. In disease, however, particularly in protein misfolding neurodegenerative disorders, lost synapses are not replaced and this loss is followed by neuronal death. These two processes are separately regulated, with mechanistic, spatial and temporal segregation of the death ‘routines’ of synapses and cell bodies. Recent insights into the reversibility of synaptic dysfunction in a mouse model of prion disease at neurophysiological, behavioral and morphological levels call for a deeper analysis of the mechanisms underlying neurotoxicity at the synapse, and have important implications for therapy of prion and other neurodegenerative disorders.
Key words: neurodegeneration, prion, synaptic dysfunction, behavior, neurophysiology
Introduction: Synaptopathy in Neurodegeneration
Compromised synaptic function is thought to underlie the earliest symptoms in several neurodegenerative diseases. However, the classic diagnostic neuropathological signatures of these disorders—deposition of amyloid plaques and/or misfolded protein aggregates and neuronal loss—in the context of full blown clinical syndromes represent an end-stage situation, essentially refractory to curative treatments. The critical phase for intervention and for understanding underlying disease mechanisms is earlier in these processes. Loss of spines, dendrites and synapses generally precedes that of neuronal cell bodies—which is irreversible—but the intrinsic potential for synapses to be lost and replaced makes this stage of disease a highly appealing target for treatment. Thus synapses are continuously formed, eliminated and remodeled throughout adulthood. These activity-dependent structural changes in synaptic connectivity likely underlie many forms of experience-dependent plasticity, including learning and memory or cognition, the loss of which is central to many neurodegenerative disorders.
There is growing evidence that synaptic dysfunction is fundamental across the spectrum of these disorders. Cognitive decline is strongly correlated with loss of presynaptic terminals in Alzheimer disease (AD), independent of amyloid plaque load and neuronal loss,1 and in animal models Aβ oligomers are potent inhibitors of synaptic function and memory in the absence of neuronal and even synaptic loss,2 see Haass and Selkoe for review.3 In mouse models of Huntington's disease (HD) there is marked decrease in striatal volume without changes in cell number correlating with motor signs,4,5 consistent with the hypothesis that the loss of neurites and synaptic failure determine phenotype in the earlier stages of disease. Prion diseases are typically diagnosed when dementia and motor symptoms prevail in humans, or by the occurrence of locomotor changes in mice, in each case correlating with extensive neuronal loss and misfolded prion protein, PrPSc, deposition. Here too, however, there are earlier motivational and behavioral symptoms, which likely precede neuronal loss and reflect early synaptic failure. Mice experimentally infected with prions, show changes in species-typical motivational behaviors long before emergence of motor signs6–8 that correlate with early loss of presynaptic terminals in the dorsal hippocampus.9 In one mouse model of an inherited prion disorder, expression of mutant protein is associated with extensive cell death in the cerebellum. Crossing these animals with Bax deficient mice (hence preventing Bax-dependent cell death) rescues neuronal apoptosis but does not rescue synaptic loss, nor symptoms, suggesting it is synaptic dysfunction rather than cell loss that causes the phenotype here.10
Thus defining the pathophysiological mechanisms underlying synaptic dysfunction and its progression in these disorders not only promises to increase our understanding of them, but opens up new avenues for early diagnosis and, critically, for early treatment.
Obstacles to progress in the field include the absence of ‘good’ animal—invariably mouse—models for many of these diseases and a lack of reliable cognitive behavioral assays in mice, in particular. Our recent work looking at early synaptic dysfunction in prion disease addresses both these issues. In contrast to mouse models of AD, HD, and Parkinson disease, where human mutant proteins are expressed in mice with variable ‘phenocopying’ of the human diseases, mice infected with prions are true models of prion disease. These mice replicate infectious mouse prions, develop classic prion pathology—spongiosis, neuronal loss, astrocytosis and PrPSc deposition—and show locomotor and behavioral signs of prion neurodegeneration. Thus studying these animals may give real insights into pathological mechanisms of these disorders. Using mice experimentally infected with prions, we focused on the earliest stages of disease to try and understand the potential for rescue both of function and survival of neurons in these disorders.11
Early Stages of Prion Disease Show Synaptic Dysfunction that can be Reversed
Central to prion pathogenesis is the conversion of a host-encoded prion protein, PrPC, into a partially-protease resistant isoform, PrPSc, which accumulates in the brain and is associated with infectivity. This process is self-propagating, with PrPSc acting as a conformational template recruiting PrPC for further conversion. This conversion reaction is critical to the neurotoxicity of prion diseases as neither loss of PrPC function,12–14 nor deposition of PrPSc is sufficient to cause pathology. We had previously established that prevention of formation of PrPSc by knocking out PrPC in prion infected mice during the course of disease (see below) prevented neuronal loss and progression to clinical disease.15 The animals were effectively clinically cured. A key finding was that early pre-degenerative prion spongiform change reversed—or recovered—after PrPC knockout. This occurred at around eight weeks post infection, four weeks before diagnostic motor symptoms would normally be detected. We therefore asked two questions: did this early pathological change produce detectable functional deficits, and if so, whether its reversal was reflected in functional recovery? Looking for functional deficits allowed us to uncover potential new tests of synaptic dysfunction as a marker of prion disease, rather than focus on signs of neuronal loss and neural network failure such as ataxia and motor changes.
We used prion-infected transgenic mice with and without ‘induced’ PrP depletion in which we had described reversal of early pathology, for our experiments. Thus tg37 mice express PrP from ‘floxed’ PrP sequences (MloxP transgenes) and succumb to Rocky Mountain Laboratories (RML) prion infection ∼13 weeks post inoculation (wpi), showing classic motor signs of prion disease at 12 wpi.15 In these mice the earliest prion pathological changes including spongiosis, gliosis and PrPSc deposition appear by 8 wpi. In double transgenic NFH-Cre/tg37 mice, PrP expression is the same as in tg37 mice until floxed PrP sequences are excised by the DNA recombinase, Cre, at ∼9 weeks of age, when neuronal PrP is depleted.14 NFH-Cre/tg37 mice infected with prions at one week of age develop early hippocampal pathology in parallel with control tg37 mice, but spongiosis reverses soon after Cre-mediated PrP depletion ∼8–9 wpi and the animals survive long term.15
As the focus for the pathological findings and their reversal was the hippocampus, we tested animals in behavioral tasks known to depend on hippocampal integrity in vivo and we measured hippocampal synaptic transmission neurophysiologically in vitro. We used simple behavioral tasks that are independent of mouse strain or genetic background, and measured synaptic transmission and plasticity. Mice were infected with RML prions at 1 week and groups of 8–10 mice of each genotype were tested weekly behaviorally and neurophysiologically from 7 wpi. Tg37 mice were tested up to ∼12 wpi, after which they developed clinical signs of prion disease and were culled. NFH-Cre/tg37 mice were tested up to ∼30 wpi. We found that, in parallel with the onset of spongiosis, mice developed cognitive, behavioral and neurophysiological deficits. Further, in parallel with the reversal of spongiform change in PrP-depleted mice, the functional deficits recovered.
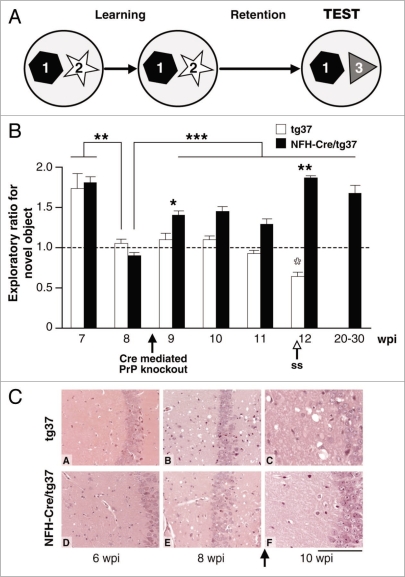
The first task we used was the novel object recognition task (Fig. 1), a non-spatial learning task based on the spontaneous preference of both mice and rats for novelty and their ability to remember previously encountered objects, that is rapidly learnt.16 Novel object recognition is extensively used for testing declarative memory in mice and, critically, performance is independent of mouse strain, or genetic background, unlike most other memory tasks for which performance is highly strain-dependent (Mallucci et al., 2007 for original images).11 At 7 wpi, all prion-infected animals displayed normal novel object discrimination during the test phase preferential exploration of the novel object (mean exploratory ratios greater than 1). However, at 8 wpi, both groups of infected mice showed significant inability to discriminate a novel object during the test phase, with mean exploratory preference ratios of ∼1: i.e., no preference. The loss in novel object discriminatory capacity coincided with the appearance of early spongiform change in all prion-infected mice (see Mallucci et al., 2007 for original images)11 and with reduction of synaptic responses (see Fig. 3 and below). In prion-diseased tg37 mice, this impairment of memory persisted throughout the course of infection and never recovered. In contrast, NFH-Cre/tg37 mice recovered the ability to discriminate novel objects after neuronal PrPC depletion. The exploratory preference for the novel object returned at 9 wpi, very soon after PrP depletion. This functional recovery occurred in parallel with reversal of spongiform pathology seen in NFH-Cre/tg37 mice examined histologically over this period and with recovery in synaptic transmission (Fig. 3). Further, the recovery in memory was sustained up to 20+ wpi.
Figure 1.
The ability to discriminate novel objects is lost in prion-infected mice, but recovers when neuronal PrPC is depleted, in parallel with reversal of pathological change. (A) Mice are allowed to explore two objects in an arena during the learning phase of the test; then, after a retention interval, they are exposed to a novel object (test phase). Time spent actively exploring the novel object compared to the familiar one is a measure of object recognition memory and is expressed as exploratory ratio. (B) Prion-infected tg37 (white bars) and NFH-Cre/tg37 mice (black bars) showed normal object recognition memory at 7 wpi, with preferential exploration of the novel object. This was significantly impaired in all mice by 8 wpi, but recovered in mice with Cre-mediated PrP depletion at 9 wpi. Recovery was sustained up to 30 wpi. *p < 0.05; **p < 0.01; ***p < 0.001 (Student's t test; 2-tails). Open arrow indicates onset of earliest signs of scrapie in tg37 mice (ss). Closed arrow indicates onset of Cre mediated knockout. (C) Haematoxylin and eosin stained sections of hippocampi from prion- infected tg37 and NFH-Cre/tg37 mice have normal appearance at 6 wpi (A and D). Spongiosis develops in all animals (B and E) by 8 wpi when memory is impaired, but by 10 wpi this has reversed in mice with Cre-mediated depletion (panel F), in parallel with recovery of novel object memory. Scale bar represents 160 um.
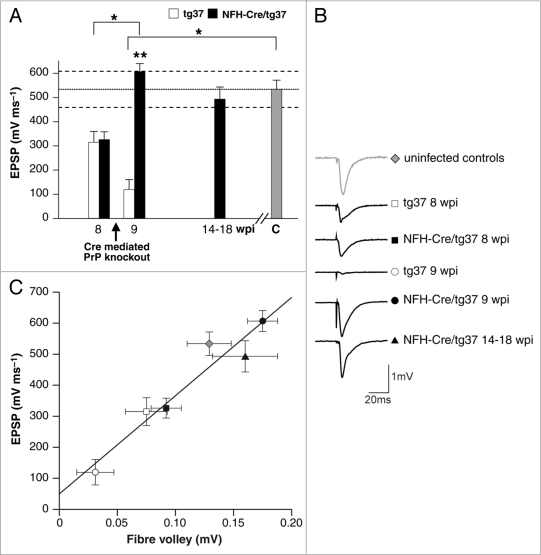
Figure 3.
Synaptic responses are depressed in prion-infected mice but recover when neuronal PrPC is depleted, while synaptic plasticity is preserved. (A) Field excitatory post synaptic potential (EPSP) slope input-output curves showed marked differences between the experimental groups. Means for the experimental groups in the GLM analysis reveal significantly smaller EPSPs in scrapie-infected tg37 mice (white bars) than in both NFH-Cre/tg37 mice (black bars) after PrP depletion at 9 wpi and uninfected control mice (gray bar). However, after Cre-mediated PrP depletion, synaptic responses return to control levels in NFH-Cre/tg37 mice at 9 wpi and are sustained at this level up to 18 wpi. p < 0.001, pairwise comparisons, using the Bonferroni correction for multiple comparisons; dotted line represents mean EPSP value and dashed lines represent 95% confidence intervals of the control group. (B) Representative sample traces for each group. The amplitude of response in uninfected control mice is shown at the top. This amplitude is reduced at 8 wpi in tg37 and NFH-Cre/tg37 mice and is further diminished in tg37 mice at 9. The response recovers in mice with PrP depletion at 9 wpi, which is sustained in mice up to 18 wpi. (C) Deficits in synaptic responses correlate with reduction in the pre-syanaptic axonal fibre volley: the relationship between EPSP slope and fibre volley (FV) amplitude is unaltered during scrapie infectivity (linear regression, solid line, R2 = 0.97).
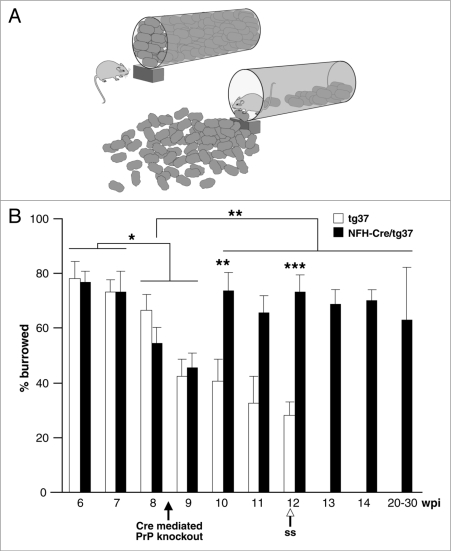
We also tested the spontaneous behaviors of burrowing and nesting, which have a robust association with early prion pathology6–8 and also localise to the dorsal hippocampus. These behaviors have been proposed as powerful tools for elucidating brain function,17 requiring a high degree of organisation and executive function and are thought to reflect motivational aspects of spontaneous behavior in rodents. In the burrowing task (Fig. 2) mice actively burrow in a container that is filled with objects, pushing or carrying them out into the cage until the container is almost emptied (Fig. 2A). We found that all mice burrowed actively, displacing 70–80% of the pellets at 6 and 7 wpi (Fig. 2B). All mice burrowed less from 8 wpi. The timing of onset of the impairment is consistent with observations in other prion-infected mouse strains.6–9 In tg37 mice, burrowing activity continued to decline until the mice reached onset of clinical disease. In contrast, in mice with Cre-mediated PrP knockout there was significant recovery of burrowing behavior by 10 wpi.
Figure 2.
Burrowing behavior is lost in early prion infection in mice but recovers when neuronal PrPC is depleted. (A) Healthy rodents ‘burrow’ food pellets, carrying them out of a container and into their cage. (B) Early in prion infection, all mice burrowed equally. Burrowing then declined significantly in both tg37 and NFH-Cre/tg37 mice respectively at 9 wpi and continued to decline in tg37 mice. In mice with Cre-mediated PrP depletion burrowing behavior resumed by 10 wpi and remained normal up to 30 wpi. Annotations are as for Figure 1.
Synaptic responses and plasticity were tested in the stratum radiatum of the CA1 region of hippocampal slices taken from mice at specific time-points after infection. At 8 wpi, both prion-infected tg37 and NFH-Cre/tg37 mice showed similar significant reductions in evoked excitatory post-synaptic field potentials (EPSPs) at ∼50% of values seen in both uninfected mice and in recovered NFH-Cre/tg37 animals (Fig. 3A). Cre-mediated neuronal PrP depletion in NFH-Cre/tg37 mice resulted in rapid recovery of EPSPs to control levels, a recovery sustained in mice examined up to 18 wpi. In contrast, synaptic responses in the prion-infected tg37 mice continued to decline, and at 9 wpi were 22% of those of uninfected control mice. Action potentials in the presynaptic axons give rise to “fiber volleys”, the amplitude of these was found to be linearly related to the EPSP magnitude at each time point (correlation coefficient 0.97; Fig. 3C), suggesting that the change in EPSP was directly due to a change in the presynaptic action potentials, rather than at a synaptic level. Indeed, when we measured long term potentiation (LTP), a form of synaptic plasticity linked with some forms of learning and memory, we found no reduction in LTP in prion-infected mice with or without PrP depletion throughout the course of the experiment. Thus changes in synaptic transmission in this case are not associated with changes in plasticity, or LTP (see Mallucci et al. 2007 for details).11
Our combined behavioral and neurophysiological analyses provided the first direct evidence for early neuronal dysfunction producing functional cognitive impairment in prion disease. We showed that early hippocampal pathology in prion-infected mice is associated with impaired synaptic responses and with cognitive and behavioral deficits in hippocampal tasks. These deficits precede neuronal and synaptic loss and they are rapidly reversed when PrPC is depleted, demonstrating the potential for recovery of neuronal function at this stage. Further, both their occurrence and recovery are independent of PrPSc accumulation. The development of phenotypic deficits paralleled the development of early spongiform change that we had previously observed, and recovery of these deficits in mice with PrP depletion paralleled its reversal. Interestingly, we found no objective evidence of synapse loss in the early stages of infection associated with early cognitive failure, and there were no changes in levels of synaptophysin detectable semi-quantitatively by immunohistochemistry, by western blotting, or by RNA transcript detection (see Mallucci et al., 2007).11
Anatomy of Synaptic Dysfunction in Early Prion Disease: Mechanistic Implications
The relationship between spongiform change and the functional deficits we observed is not clear. Spongiform degeneration in prion disorders involves the formation of membrane bound vacuoles within neurons and neuropil—predominantly within dendrites; and intradendritic distensions and dendritic spine loss co-localize with vacuolar pathology in the hippocampus.18 The deficits we observe may thus reflect earliest neuronal and dendritic dysfunction, although more detailed neurophysiological analysis implicates impaired function at the level of the presynaptic axon also. Later in prion disease, dendrite loss is known to precede neuronal loss as measured by in vivo imaging of infected mice,19 but this may well be secondary to pre-synaptic changes. Both mRNA and miRNA screens of prion infected mouse brains show that both pre- and post-synaptic proteins are targets of altered gene regulation during prion disease.20 Thus we found that synaptic response depression in our mouse model can be explained here by the proportional loss of the presynaptic fiber volley. Depression of presynaptic fiber volleys has been reported in the progression of another murine prion model,21 where it was tentatively attributed to loss of the presynaptic neurons (CA3 pyramidal cells). The present loss of the fiber volley was rapidly reversible (within a week) and therefore cannot be due to neuronal loss nor to regrowth of axons within this time frame; more likely it was due to axonal dysfunction. Synaptic plasticity appears to be maintained, however: the presence of LTP recordings found in all mice at all stages of disease implies that those synapses still remaining in prion-infected tg37 mice have the intact molecular apparatus both to release transmitter and to sustain LTP. It appears that here memory is impaired due to reduction in effective synaptic integration itself, implicating mechanisms other than CA1 LTP in deficient memory. This is in contrast to the data from AD models, where LTP appears to correlate with behavioral changes due to Aβ oligomers in some cases, see Haass and Selkoe for review.3 Learning and memory have been found associated with impaired synaptic responses and intact LTP in other rodent models.22 However, LTP alterations have been described in prion-infected mice, at a relatively later stage in the disease process, infected with different prion strains and using more intense conditioning stimulation to induce LTP.21 This may reflect later stages of disease—and synaptic pathology—than we have focused on here.
A further mechanistic implication of this work is that synaptic dysfunction is independent of aggregated misfolded protein depostion. Thus both cognitive and neurophysiological impairment and recovery in our model appeared to be independent of PrPSc accumulation. Impaired object recognition memory, burrowing behavior and reduced post synaptic potentials in CA1 occur at 8 wpi, before extensive deposits of PrPSc are apparent. Further, both the loss, and recovery of memory and species-typical behaviors appear to be independent of aggregated PrPSc deposition, which continues to accumulate due to extra-neuronal, predominantly astrocytic, replication. The continued build up of extra neuronal PrPSc in NFH-Cre/tg37 mice after neuronal PrP knockout does not impair memory function or synaptic responses up to 20+ wpi. Thus as for the AD models (see below), the data strongly support a transient, soluble, non-aggregating species—likely generated within neurons—causing synaptic dysfunction in prion disease. Histologically, early synaptic pathology and dendritic dysfunction precede PrPSc accumulation,23 consistent with our observations on behavioral and neurophysiological changes in the absence of extensive PrPSc deposits.
Our data are consistent with findings in other models of neurodegenerative disorders: that neuronal dysfunction and cognitive defects occur before cellular degeneration, and independently of pathological aggregation of disease-associated proteins,5,24,25 and they can result from synaptic dysfunction rather than neuronal loss.2,26–28 In several models of AD there is increasing evidence that this may be due to soluble Aβ oligomers, rather than amyloid (see Haass and Selkoe for review),3 explaining plaque-independent cognitive failure in these animals. Consistent with these studies, our findings support the concept that a transient—as yet unidentified—neurotoxic species is generated within neurons when PrPC is converted to PrPSc, which rapidly impairs neuronal function, and synaptic responses. Depleting neuronal PrPC would halt formation of such an intermediate, whether it be a new molecular species or an oligomeric form of PrPSc, allowing rapid recovery of neuronal function. Interestingly, using lentivirally mediated RNAi of PrP, we recently confirmed that knocking down PrP production at the transcriptional level, prevented the onset of cognitive deficits in tg37 mice.29
Our findings of early reversible neurophysiological and cognitive deficits occurring before neuronal loss have opened new avenues in the prion field. To date, prion infection in mice has conventionally been diagnosed when motor deficits reflect advanced neurodegeneration. Now the identification of earlier dysfunction helps direct the study of mechanisms of neurotoxicity and therapies to earlier stages of disease, when rescue is still possible. The key finding here is that reversal at this synaptic stage of prion disease is that neurons are saved from degeneration. Many questions need addressing still for both prion and other neurodegenerative disorders. The mechanisms by which putative toxic PrP oligomers or intermediates (and indeed the nature or identity of these) cause synaptic dysfunction is still unknown. There is evidence of PrP interaction with NMDA receptors,30 and for PrP mediating toxicity of Aβ oligomers at the level of LTP,31 but clearly there are multiple effects at many levels of the functioning synaptic unit and in the integration of the activity of multiple synapses. Moreover, how synaptic dysfunction ultimately leads to neuronal cell death is not known, nor critically, is the threshold of malfunction up to which sick synapses can be rescued. It may be that individual strategies are needed for individual diseases, or that there are common pathways that will allow a more global intervention, independent of specific pathology. Recently, strengthening synaptic density in the hippocampus by neural stem cell transplantation in a mouse model of AD abrogated memory problems in mice independently of effects on Aβ or tau pathology.32 Could such a system bypass the effects neurotoxic species in many or all of these disorders? Clearly the search for both generic and specific mechanisms and solutions are critically important. A key advance however is the focus on finding new early clinical readouts of neurodegenerative disease, both in mice and ultimately in humans. Early detection is key for any intervention and the possibility of preclinical testing of therapeutic strategies through cognitive endpoints in these disorders is well worth pursuing.
Abbreviations
- AD
Alzheimer disease
- HD
Huntington disease
- Cre
site-specific recombinase from phage P1
- NFH-Cre
mice express Cre under neurofilament heavy gene promoter
- tg37
mice have floxed PrP sequences that can be deleted by Cre
- PrPC
cellular isoform of prion protein
- PrPSc
disease associated isoform of prion protein
- RML
Rocky Mountain Laboratories murine prions (mouse adapted scrapie)
- RNAi
RNA interference
- wpi
weeks post-inoculation
- EPSP
excitatory post synaptic potential
- LTP
long term potentiation
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/9981
References
- 1.Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 2.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 3.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham C, Deacon RM, Chan K, Boche D, Rawlins JN, Perry VH. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol Dis. 2005;18:258–269. doi: 10.1016/j.nbd.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Deacon RM, Raley JM, Perry VH, Rawlins JN. Burrowing into prion disease. Neuroreport. 2001;12:2053–2057. doi: 10.1097/00001756-200107030-00052. [DOI] [PubMed] [Google Scholar]
- 8.Guenther K, Deacon RM, Perry VH, Rawlins JN. Early behavioral changes in scrapie-affected mice and the influence of dapsone. Eur J Neurosci. 2001;14:401–409. doi: 10.1046/j.0953-816x.2001.01645.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz CP, et al. Synaptic changes characterize early behavioral signs in the ME7 model of murine prion disease. Eur J Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiesa R, Piccardo P, Dossena S, Nowoslawski L, Roth KA, Ghetti B, Harris DA. Bax deletion prevents neuronal loss but not neurological symptoms in a transgenic model of inherited prion disease. Proc Natl Acad Sci USA. 2005;102:238–243. doi: 10.1073/pnas.0406173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 13.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behavior of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 14.Mallucci GR, Ratte S, Asante EA, Linehan J, Gowland I, Jefferys JG, Collinge J. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 2002;21:202–210. doi: 10.1093/emboj/21.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 16.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- 18.Jeffrey M, Goodsir CM, Bruce ME, McBride PA, Fraser JR. In vivo toxicity of prion protein in murine scrapie: ultrastructural and immunogold studies. Neuropathol Appl Neurobiol. 1997;23:93–101. [PubMed] [Google Scholar]
- 19.Fuhrmann M, Mitteregger G, Kretzschmar H, Herms J. Dendritic pathology in prion disease starts at the synaptic spine. J Neurosci. 2007;27:6224–6233. doi: 10.1523/JNEUROSCI.5062-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA. A miRNA signature of prion induced neurodegeneration. PLoS ONE. 2008;3:3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiti Z, Knutsen OM, Betmouni S, Greene JR. An integrated, temporal study of the behavioral, electrophysiological and neuropathological consequences of murine prion disease. Neurobiol Dis. 2006;22:363–373. doi: 10.1016/j.nbd.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Brace HM, Jefferys JG, Mellanby J. Long-term changes in hippocampal physiology and learning ability of rats after intrahippocampal tetanus toxin. J Physiol. 1985;368:343–357. doi: 10.1113/jphysiol.1985.sp015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson E, Jeffrey M, Ironside JW, Fraser JR. Apoptosis and dendritic dysfunction precede prion protein accumulation in 87V scrapie. Neuroreport. 2001;12:2147–2153. doi: 10.1097/00001756-200107200-00021. [DOI] [PubMed] [Google Scholar]
- 24.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, et al. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, et al. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 29.White MD, Farmer M, Mirabile I, Brandner S, Collinge J, Mallucci GR. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc Natl Acad Sci USA. 2008;105:10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol. 2008;181:551–565. doi: 10.1083/jcb.200711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]