Abstract
Aims
To determine the relationship between admission systolic blood pressure (SBP) and mortality in older patients hospitalized for heart failure (HF) and among various subgroups.
Methods and results
We evaluated the independent association between initial SBP and 30-day and 1-year mortality, and potential interactions by age, gender, race, previous hypertension, and left ventricular dysfunction using multivariable logistic regression in the National Heart Failure Project, a database of Medicare patients >65 years old recruited from 1998 through 2001. Among 56 942 patients, mean admission SBP was 147.0 ± 92.3 mmHg, 15% presenting with SBP >180 mmHg. Systolic blood pressure showed an inverse relationship with 30-day and 1-year mortality rates in all subgroups analysed. Using admission SBP of 120–149 mmHg as the reference, the adjusted odds ratios (95% confidence intervals) for 1-year mortality were 2.18 (1.77–2.69) for SBP <90 mmHg, 1.57 (1.47–1.69) for SBP 90–119 mmHg, 0.71 (0.67–0.76) for SBP 150–179 mmHg, 0.63 (0.57–0.68) for SBP 180–209 mmHg, and 0.51 (0.44–0.59) for SBP ≥210 mmHg.
Conclusion
Higher SBP on admission is associated with significant lower 30-day and 1-year mortality in patients hospitalized for HF. The relationship is strong, graded, independent of other clinical factors and consistent among subgroups.
Keywords: Systolic blood pressure, Heart failure, Mortality, Prediction
Introduction
Blood pressure is reported to have an inverse relationship with risk of mortality in patients with heart failure (HF). Although elevations in systolic blood pressure (SBP) are associated with an increased risk of developing HF1 and antihypertensive treatment protects against this evolution to HF, more successfully so than against either stroke or myocardial infarction,2,3 among those hospitalized with HF, higher blood pressure at the time of presentation is associated with a lower risk of dying.4–8 Prior studies have described the association between high SBP and lower mortality in these patients, exploring varying cut-offs predominantly ranging from 115 to 160 mmHg,5,9 and some of them with only a short-term follow-up.6 One study examined the continuous relationship between higher SBP levels and long-term mortality,8 although the consistency of this association among important subgroups of patients was not addressed. The different prognostic influence of high blood pressure according to age, gender, or race described by others10–12 emphasizes the importance of the study of the association between SBP and mortality in these different subgroups of patients hospitalized for HF.
On the other hand, even if left ventricular dysfunction is not an important predictor of mortality in HF patients,4,5,8 most patients with normal left ventricular ejection fraction (LVEF) are hypertensive.13 However, the effect of the interaction between LVEF and blood pressure on prognosis has not been previously studied.
A better understanding of the association between blood pressure and mortality in a representative group of HF patients may improve risk stratification and generate questions about the underlying mechanism of this relationship and its therapeutic implications.
To address this issue, we investigated the relationship between the first blood pressure measurement and short- and long-term mortality in a nationally representative cohort of Medicare patients hospitalized for HF from 1998 through 2001 using data from the National Heart Failure (NHF) Project, an initiative funded by the Centres for Medicare & Medicaid Services (CMS) to improve the quality of care of Medicare beneficiaries hospitalized with cardiovascular diseases.14
Methods
The NHF project included the medical chart abstraction of almost 80 000 Medicare beneficiaries hospitalized with a principal discharge diagnosis of HF. Using these data, the project identified fee-for-service Medicare patients who were hospitalized with a principal discharge diagnosis of HF (International Classification of Diseases, Ninth Revision, Clinical Modification codes 402.01, 402.11, 402.91, 404.01, 404.91, or 428) between April 1998 and March 1999 or July 2000 and June 2001. The NHF project sample was restricted to single admissions of patients with valid Social Security numbers who were not receiving long-term haemodialysis and did not leave the hospital (transferred out or against medical advice) during treatment. For each time period, the project used a sampling strategy to randomly select up to 800 discharges in each state, sorted by age, race, gender, and treating hospital; states with <800 medical records were sampled in their entirety. The selected records were reviewed by trained data abstractors at central data abstraction centres. Medical records were abstracted for clinical data including medical history, comorbidity, findings at admission, in-hospital course, status at discharge, medication and procedure use, and laboratory and other diagnostic evaluations.
Study cohort
The NHF project produced an initial sample of 78 882 hospitalizations. Medical records meeting NHF exclusion criteria (chronic dialysis, no or invalid Social Security number, readmission) were excluded (n = 4281). We included patients aged 65 years and older who were hospitalized for HF, with the aim of evaluating the influence of SBP on 30-day and 1-year mortality. We excluded patients younger than 65 years (n = 6558), those who arrived by inter-hospital transfer (n = 2419) in which accurate admission information could not be identified, those without HF on admission (n = 5003), those with unknown SBP on admission (n = 72), those with severe comorbid diseases (acquired immune deficiency syndrome, chronic liver disease, leukaemia, lymphoma, immunological suppression or cancer) (n = 6216) that could strongly modify 1-year mortality risk, and those with unknown information about death (n = 969). Our final study sample had 56 942 patients.
Systolic blood pressure and other independent variables
The primary independent variable is SBP which was analysed continuously, then categorized into six groups (<90, 90–119, 120–149, 150–179, 180–209, and ≥210 mmHg) for risk estimation. Data on SBP reflect the first measurement obtained in the emergency department or, for patients directly admitted, what was first recorded in the medical record (including nursing notes, progress notes, graphics, and flow sheets). The first diastolic blood pressure (DBP) was also recorded and pulse pressure (PP) calculated as the difference between SBP and DBP.
Other variables included demographic characteristics (age, sex, race, pre-admission setting), non-cardiac comorbidities (mobility, continence, cerebrovascular accident, chronic pulmonary disease, dementia, diabetes, history of hypertension), characteristics on HF presentation (peripheral oedema, pulmonary oedema), other cardiac diseases (coronary artery bypass grafting, myocardial infarction, history of coronary artery disease, severity of left ventricular systolic dysfunction, and atrial fibrillation), laboratory data (serum sodium, albumin, glucose, haematocrit, and white blood cells), and medications (angiotensin-converting enzyme inhibitors, diuretics, angiotensin receptor blockers, digoxin, beta-blockers, and calcium channel blockers) at admission.
Documentation of LVEF was defined as a medical record notation of prior LVEF evaluation when reported, or in-hospital assessment of LVEF. Left ventricular ejection fraction categories were derived through information from current hospital assessment as well as previous LVEF evaluation. The classification of mild, moderate, and severe ventricular dysfunction was made according to the narrative description reported in the medical history. History of hypertension was considered present if there was any documentation by a physician that the patient had a previous hypertension diagnosis.
Outcomes
We determined patient mortality using the Medicare enrollment database, deriving mortality within 30 days and 1 year of admission from the admission date and the death date.
Statistical analysis
We reported data as mean ± standard deviation for continuous variables and number (percentage) for categorical variables. We categorized SBP at admission into groups as previously described and evaluated differences in patient characteristics among the SBP groups using the Pearson χ2 test for categorical variables and analysis of variance for continuous variables.
We compared crude mortality within 30 days and 1 year of admission using the Pearson χ2 test among the SBP groups overall and also stratified by patient age (<75, 75–84, and ≥85 years), gender (male or female), race (white, black, other), history of hypertension (no, yes), and LVEF (unknown, normal, mild, moderate, or severe). We evaluated the independent effect of SBP on mortality using multivariable logistic regression models in the overall cohort and also in the subgroups determined by age, gender, race, history of hypertension, and LVEF, adjusting for all clinical and laboratory variables described above. For SBP analyses, we considered the subgroup for SBP 120–149 mmHg, which includes normal blood pressure values, as the referent.
Owing to the sampling structure, we used survey analysis methods with the consideration of state-level weights and patient clustering within hospitals. We incorporated probability weights based on the inverse sampling fraction for each state in the analyses. We conducted all analyses using SAS 9.1 (SAS Institute Inc., Cary, NC, USA). The Yale University School of Medicine Human Investigation Committee approved the use of the NHF database.
Results
Our study sample had a mean age of 79.7 ± 22.8 years; 59% were women and 85% were white. Baseline characteristics are shown in Table 1. Mean SBP was 147.0 ± 92.3 mmHg and mean DBP was 77.9 ± 55.1 mmHg. More than 50% of the patients had an SBP >140 mmHg, and >15% showed SBP values >180 mmHg. Overall, 30-day mortality was 9.8% and 1-year mortality was 37.5%.
Table 1.
Baseline characteristics
| Demographics | |
|---|---|
| Age, mean (SD) | 79.7 (22.8) |
| Female (%) | 59.3 |
| White (%) | 85.1 |
| Black (%) | 11.8 |
| Other (%) | 3.1 |
| Nursing facilities (%) | 11.4 |
| Non-cardiac comorbidities (%) | |
| Unable to walk independently | 39.6 |
| Incontinent | 13.7 |
| Chronic pulmonary disease | 33.6 |
| Diabetes | 40.9 |
| Creatinine, mean (SD) | 1.5 (2.17) |
| Cardiac comorbidities (%) | |
| Coronary artery disease | 59.5 |
| Hypertension | 65.6 |
| CABG | 23.8 |
| PCI | 10.0 |
| Revascularization (PCI or CABG) | 29.5 |
| Myocardial infarction | 29.5 |
| Severity of left ventricular systolic dysfunction | |
| Unknown | 34.8 |
| Normal | 24.4 |
| Mild | 9.6 |
| Moderate | 12.0 |
| Severe | 19.2 |
| Left bundle branch block on ECG | 15.4 |
| Atrial fibrillation/flutter on ECG | 30.1 |
| Heart failure severity indicators | |
| Peripheral oedema (%) | 73.1 |
| Pulmonary oedema/heart failure on chest X-ray (%) | 76.5 |
| Sodium, mean (SD) | 138.5 (14.3) |
| History of heart failure (%) | 72.9 |
| Other laboratory, mean (SD) | |
| Albumin (mg/dL) | 3.4 (2.4) |
| Potassium (mEq/L) | 4.3 (1.97) |
| Glucose (mg/dL) | 152.9 (210) |
| White blood cell count, n·103/mm3 | 9.1 (11) |
| Haematocrit (%) | 36.8 (16.8) |
| Vital signs, mean (SD) | |
| Systolic blood pressure (mmHg) | 147.0 (92.3) |
| Diastolic blood pressure (mmHg) | 77.9 (55.1) |
| Pulse pressure (mmHg) | 69.1 (72.5) |
| Heart rate (b.p.m.) | 89.6 (65.1) |
| Respiratory rate | 24.5 (19.5) |
| Medications on admission (%) | |
| Angiotensin-converting enzyme inhibitors | 41.7 |
| Angiotensin receptor blockers | 9.2 |
| Diuretics | 74.3 |
| Digoxin | 39.6 |
| Calcium-channel blockers | 27.3 |
| Beta-blockers | 28.7 |
| Aspirin | 37.1 |
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Baseline patient characteristics differed across SBP groups (see Supplementary material online, Appendix). Patients in higher SBP ranges were more frequently female, younger, and healthier. With the exception of hypertension history, increasing SBP on admission was associated with fewer comorbidities and higher LVEF. Diastolic blood pressure was moderately correlated with SBP (r = 0.63).
Systolic blood pressure and mortality
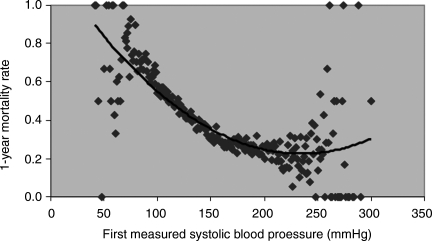
Higher SBP on admission was associated with significantly lower 30-day and 1-year mortality (Table 2). This association persisted even at very high SBP levels (Figures 1 and 2). All levels of SBP above the reference were associated with a lower mortality risk at 30 days and 1 year. This effect was consistent across age, gender, race, history of hypertension, and LVEF subgroups (Table 3). There were no significant interactions between SBP and these groups except for LVEF (P < 0.001). The prognostic importance of SBP was higher for patients with more severe left ventricular dysfunction (Table 3).
Table 2.
Crude outcomes (%) in different systolic blood pressure groups
| Systolic blood pressure (mmHg) |
P-value | ||||||
|---|---|---|---|---|---|---|---|
| <90 | 90–119 | 120–149 | 150–179 | 180–209 | ≥210 | ||
| 30-Day mortality | 34.1 | 18.6 | 10.1 | 5.7 | 4.4 | 2.9 | <0.001 |
| 1-Year mortality | 69.7 | 55.5 | 39.5 | 29.1 | 25.5 | 21.3 | <0.001 |
Figure 1.
Systolic blood pressure and 1-year mortality rates.
Table 3.
One-year mortality stratified by systolic blood pressure group
| Characteristics | Overall | Systolic blood pressure (mmHg) |
P-value | |||||
|---|---|---|---|---|---|---|---|---|
| <90 | 90–119 | 120–149 | 150–179 | 180–210 | >210 | |||
| Crude rate (%) | ||||||||
| Age (years) | ||||||||
| <75 | 27.7 | 60.0 | 46.0 | 29.6 | 19.2 | 16.1 | 14.0 | <0.001 |
| 75 to <85 | 36.0 | 68.7 | 55.2 | 37.5 | 27.4 | 24.3 | 19.2 | <0.001 |
| ≥85 | 49.2 | 81.1 | 65.6 | 51.6 | 41.4 | 36.3 | 34.7 | <0.001 |
| Race | ||||||||
| White | 38.7 | 70.8 | 56.6 | 40.4 | 30.0 | 26.3 | 22.5 | <0.001 |
| Black | 30.0 | 53.7 | 46.9 | 33.3 | 24.7 | 20.8 | 19.5 | <0.001 |
| Other | 32.0 | 89.4 | 48.9 | 35.7 | 23.5 | 25.1 | 8.6 | <0.001 |
| Gender | ||||||||
| Male | 40.3 | 72.0 | 58.2 | 40.9 | 29.1 | 25.8 | 17.5 | <0.001 |
| Female | 35.5 | 67.1 | 52.7 | 38.4 | 29.1 | 25.3 | 22.9 | <0.001 |
| Hypertension | ||||||||
| No | 42.6 | 77.5 | 56.9 | 42.1 | 31.4 | 27.9 | 21.2 | <0.001 |
| Yes | 34.8 | 62.9 | 54.2 | 37.8 | 28.2 | 24.8 | 21.3 | <0.001 |
| Severity of LVSD | ||||||||
| Unknown | 42.9 | 76.5 | 60.5 | 45.1 | 33.5 | 30.5 | 27.2 | <0.001 |
| Normal | 28.4 | 44.6 | 45.0 | 30.6 | 24.4 | 21.0 | 20.6 | <0.001 |
| Mild | 31.8 | 72.5 | 45.0 | 35.9 | 26.4 | 24.6 | 18.8 | <0.001 |
| Moderate | 35.1 | 69.1 | 50.3 | 37.9 | 29.1 | 24.2 | 11.4 | <0.001 |
| Severe | 43.5 | 73.4 | 59.7 | 41.4 | 29.9 | 25.7 | 16.4 | <0.001 |
| Adjusted OR (95% CI) | ||||||||
| Age (years) | ||||||||
| <75 | 1.99 (1.38–2.88) | 1.60 (1.40–1.84) | 1.00 | 0.64 (0.56–0.73) | 0.57 (0.48–0.69) | 0.49 (0.37–0.66) | ||
| 75 to <85 | 2.38 (1.73–3.27) | 1.67 (1.50–1.87) | 1.00 | 0.72 (0.65–0.79) | 0.63 (0.55–0.72) | 0.46 (0.36–0.59) | ||
| ≥85 | 2.36 (1.50–3.72) | 1.49 (1.31–1.69) | 1.00 | 0.74 (0.66–0.83) | 0.64 (0.55–0.75) | 0.60 (0.46–0.78) | ||
| Race | ||||||||
| White | 2.19 (1.74–2.74) | 1.58 (1.47–1.70) | 1.00 | 0.72 (0.67–0.77) | 0.63 (0.57–0.69) | 0.51 (0.43–0.61) | ||
| Black | 1.47 (0.85–2.52) | 1.47 (1.16–1.86) | 1.00 | 0.73 (0.60–0.89) | 0.68 (0.53–0.86) | 0.62 (0.44–0.88) | ||
| Other | 17.51 (4.07–75.29) | 1.42 (0.89–2.26) | 1.00 | 0.58 (0.38–0.89) | 0.71 (0.40–1.24) | 0.25 (0.08–0.72) | ||
| Gender | ||||||||
| Male | 2.18 (1.61–2.96) | 1.63 (1.47–1.80) | 1.00 | 0.69 (0.63–0.77) | 0.64 (0.55–0.74) | 0.41 (0.30–0.56) | ||
| Female | 2.08 (1.56–2.79) | 1.48 (1.35–1.64) | 1.00 | 0.74 (0.68–0.80) | 0.64 (0.58–0.72) | 0.58 (0.49–0.69) | ||
| Hypertension | ||||||||
| No | 2.81 (2.04–3.86) | 1.52 (1.37–1.68) | 1.00 | 0.71 (0.63–0.79) | 0.65 (0.54–0.77) | 0.48 (0.32–0.70) | ||
| Yes | 1.79 (1.36–2.36) | 1.59 (1.44–1.74) | 1.00 | 0.73 (0.67–0.79) | 0.64 (0.58–0.72) | 0.54 (0.46–0.64) | ||
| Severity of LVSD | ||||||||
| Unknown | 2.62 (1.87–3.67) | 1.62 (1.43–1.83) | 1.00 | 0.69 (0.62–0.77) | 0.65 (0.57–0.76) | 0.55 (0.43–0.70) | ||
| Normal | 0.99 (0.60–1.62) | 1.55 (1.31–1.83) | 1.00 | 0.86 (0.75–0.98) | 0.75 (0.62–0.90) | 0.74 (0.56–0.97) | ||
| Mild | 3.73 (1.61–8.65) | 1.18 (0.91–1.53) | 1.00 | 0.66 (0.53–0.82) | 0.63 (0.47–0.83) | 0.49 (0.31–0.77) | ||
| Moderate | 2.27 (1.19–4.31) | 1.29 (1.04–1.59) | 1.00 | 0.70 (0.58–0.85) | 0.57 (0.43–0.74) | 0.28 (0.15–0.50) | ||
| Severe | 2.45 (1.65–3.65) | 1.69 (1.47–1.94) | 1.00 | 0.70 (0.60–0.82) | 0.58 (0.45–0.75) | 0.32 (0.18–0.56) | ||
CI, confidence interval; LVSD, left ventricular systolic dysfunction; OR, odds ratio.
Figure 2.
Kaplan–Meier crude survival curves for each subgroup of systolic blood pressure.
In the multivariable analysis, higher SBP persisted as a powerful independent predictor of lower short-term and 1-year mortality in the entire spectrum of SBP after adjustment for confounders (Figure 3A). Patients with SBP ≥210 mmHg had an adjusted odds ratio for 1-year mortality of 0.51 (95% confidence interval 0.44–0.59) with respect to the 120–149 mmHg SBP range. The independent and inverse association between SBP and mortality was observed in all age, sex, and race subgroups as well as in patients with all degrees of LVEF (Table 3).
Figure 3.
Adjusted odds ratios and 95% confidence intervals for 1-year mortality rate for systolic blood pressure (A), diastolic blood pressure (B), and pulse pressure (C) groups. Reference value = 120–149 mmHg for SBP and 60–89 mmHg for diastolic and pulse pressures.
Diastolic blood pressure, pulse pressure, and mortality
Median DBP was 77 mmHg (interquartile range: 65–90 mmHg). Similar to SBP, higher DBP levels were associated with lower 30-day and 1-year mortality rates in unadjusted analysis that persisted after adjustment for baseline characteristics and SBP values (Figure 3B).
Median PP was 66 mmHg (interquartile range: 50–84 mmHg). Higher PP values were mostly driven by higher SBP levels than lower DBP, as both were correlated. Higher PP levels were associated with lower short- and long-term crude mortality rates. Contrary to the increasing benefit observed with higher levels of SBP and DBP, we did not find additional benefits in adjusted mortality over 120 mmHg (Figure 3C).
Discussion
This study demonstrates the independent and continuous relationship between higher first SBP measurement and lower short- and long-term mortality in older patients hospitalized for HF, with no upper threshold. This association is independent of patient clinical characteristics, is present across the entire spectrum of SBP even at the highest levels, and is consistent among groups of patients. Systolic blood pressure on admission is a strong prognostic factor, with implications for mortality risk, in a paradox that persists to very high levels of blood pressure.
The mechanisms of this association are not clear. Patients with higher SBP on admission are younger and healthier; however, baseline characteristics do not explain the better prognosis, as the effect on mortality after adjustment for these differences remains very strong. A second explanation may be related to differences in treatment patterns or responses. Physicians may be more aggressive using vasodilator therapies, e.g. angiotensin-converting enzyme inhibitors or beta-blockers, in patients with higher blood pressure levels. In addition, patients with higher SBP may better tolerate higher doses of these treatments than patients with lower levels of blood pressure. However, it is unlikely that a greater use or the use of higher doses explained the differences in short-term mortality, as the survival benefit of these drugs take months or years to be seen in randomized clinical trials. It is possible that the treatments used for patients with HF and low SBP increased mortality, as has been suggested for many inotropic agents.15–17 However, that would only partially explain the well-known higher mortality of patients with HF admitted with low SBP.5,8,18 The effect of treatments, such as beta-adrenergic receptor blockers and angiotensin-converting enzyme inhibitors, could differ across the SBP spectrum. This hypothesis cannot be ruled out because, although a number of trials have demonstrated a similar effect of the active drug independently of the level of SBP, most of these studies either used only two categories for comparison19,20 or excluded patients with very high SBP.20,21 It is possible that the better prognosis of the patients with highest SBP is partially due to their hospitalization having been caused by a hypertensive pulmonary oedema, rather than having been within the context of chronic HF with the full syndrome of other organ- and neurohormonal-associated changes.22 The existence of a distinct pathophysiology in patients with HF and higher blood pressure levels on admission seems also likely. The ability to raise blood pressure in the acute phase of HF regardless of LVEF may reflect a greater ‘cardiovascular reserve’ that can be mediated by a greater ability to achieve a vasoconstrictive response and/or a greater myocardial contractile reserve, translating into greater cardiac output. Thus, the capability of raising blood pressure during the acute phase of HF may be inversely associated with the severity of the disease, which is not well predicted by LVEF.
Previous studies have demonstrated the prognostic importance of SBP in HF patients.3–6,8 Some have mainly focused on lower levels of SBP as a predictor of higher mortality.5,18 Fonarow et al.5 reported that blood pressure above 115 mmHg was the best cutpoint to indicate lower risk. The association of high SBP and better prognosis has been described in patients with chronic stable HF23,24 and in patients requiring hospital admission for decompensation.4,6,25,26
However, there are a number of differences between the current study and previous studies. Many previous studies included a small number of selected patients,26 did not adequately examine higher SBP levels,26 or were focused on short-term mortality.6,27 Our study of a representative population confirms previous findings and demonstrates the association of higher SBP levels and lower mortality across the full spectrum of SBP values, in short- and long-term follow-up, and includes information about the consistency of the association across different patient subgroups. Even if the number of cases in each subgroup is not very high, our sample contained 2176 patients with SBP of 210 mmHg or above, which provides a reasonable number of cases in each subgroup with which to make comparisons.
Diastolic blood pressure, pulse pressure, and mortality
Few studies have evaluated the relationship between DBP and mortality in HF patients. Lee et al.24 demonstrated that DBP < 60 mmHg increased mortality in chronic HF patients with left ventricular dysfunction. When they analysed the relationship, considering DBP as a continuous variable, the association between higher DBP and lower mortality was evident, but the upper DBP levels in their study population were lower than those in our population. Similarly, the OPTIMIZE-HF registry found a decrease in hospital mortality per each 10 mmHg of DBP increase up to 100 mmHg.27 We have shown that even higher levels of DBP are associated with a better prognosis.
Pulse pressure at admission is also a strong independent predictor of better survival in patients hospitalized with HF in our study. Elevated PP relates to arterial stiffness and is associated with greater cardiovascular events and mortality risk. In older patients, PP seems to be a better predictor of HF development than SBP and DBP.28 However, in patients with HF, it has been speculated that the better prognosis observed in patients with higher PP values may be due to a greater inotropic reserve.25
The results of this study should be evaluated in the context of its limitations. The analysis was restricted to fee-for-service Medicare patients—the predominate form of government coverage for the elderly in the USA in which doctors are paid for each service they provide—aged 65 years or older and hospitalized for HF, and the findings may not be applicable to other populations. Nevertheless, the majority of HF patients are aged 65 years or older, and the proportion of patients included in the Medicare managed care system is very low.
The retrospective analysis of the results of a database is also associated with other limitations, which theoretically might have had an influence on the findings of this study, such as the risk of HF ‘upcoding’,29 the use of only first blood pressure measurement, or the lack of standardized blood pressure measurement, including time of the day, person who measured it or relationship between the taking of medications and timing of BP recordings. However, the large number of cases, and the remarkable consistency of the findings across subgroups as well as the exclusion of patients who did not have a diagnosis of HF at the time of admission makes it unlikely that these limitations played an important role in the results. Finally, the hypothesis that patients with higher SBP at admission received different treatments, agents or doses of vasodilators during follow-up, cannot be discounted as noted.
In conclusion, our study enriches the profiling of admission SBP as a risk factor, raises the issue of an underlying pathophysiological mechanism contributing to better prognosis of high blood pressure in HF patients and, more importantly, the issue of potential therapeutic implications. Whether the response to the recommended therapies (i.e. angiotensin-converting enzyme inhibitors or beta-blockers) differs in patients with very high blood pressure levels, and if the doses and titration should vary accordingly, merit further research.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
M.T.V. and H.B. were supported in part by grants of the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III, Spain (BA08/90012 and BA08/90010, respectively) during their stay at the Centre for Outcomes Research and Evaluation, Yale-New Haven Hospital, where they participated in the development of this study. J.S.R. is supported by the National Institute on Aging (K08 AG032886) and by the American Federation of Aging Research through the Paul B. Beeson Career Development Award Program.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. J Am Med Assoc. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 2.Moser M, Hebert PR. Prevention od disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 3.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ HYVET Study Group. Treatment of hypertension in patients 80 of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. J Am Med Assoc. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Gattis Stough W, Yancy CW, Young JB, Fonarow GC for the OPTIMIZE-HF Investigators Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. J Am Med Assoc. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 7.Huynh BC, Rovner A, Rich MW. Long-term survival in elderly patients hospitalized for heart failure: 14-year follow-up from a prospective randomized trial. Arch Intern Med. 2006;166:1892–1898. doi: 10.1001/archinte.166.17.1892. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. J Am Med Assoc. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 9.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinand KC. Recommendations for the management of special populations: racial and ethnic populations. Am J Hypertens. 2003;16:50S–54S. doi: 10.1016/j.amjhyper.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Glynn RJ, L'Italien GJ, Sesso HD, Jackson EA, Buring JE. Development of predictive models for long-term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002;39:105–110. doi: 10.1161/hy1201.097199. [DOI] [PubMed] [Google Scholar]
- 12.Oates DJ, Berlowitz DR, Glickman ME, Silliman RA, Borzecki AM. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55:383–388. doi: 10.1111/j.1532-5415.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Masoudi FA, Ordin DL, Delaney RJ, Krumholz HM, Havranek EP. The National Heart Failure Project: a Health Care Financing Administration initiative to improve the care of Medicare beneficiaries with heart failure. Congest Heart Fail. 2000;6:337–339. doi: 10.1111/j.1527-5299.2000.80175.x. [DOI] [PubMed] [Google Scholar]
- 15.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. J Am Med Assoc. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, O'Connor CM. Inotropic therapy for heart failure: an evidence-based approach. Am Heart J. 2001;142:393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 17.Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096.e1–e1098. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh BC, Rovner A, Rich MW. Identification of older patients with heart failure who may be candidates for hospice care: development of a simple four-item risk score. J Am Geriatr Soc. 2008;56:1111–1115. doi: 10.1111/j.1532-5415.2008.01756.x. [DOI] [PubMed] [Google Scholar]
- 19.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 20.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH for the US Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 21.CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS) Circulation. 1994;90:1765–1773. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 22.Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 23.Grigorian-Shamagian L, Gonzalez-Juanatey JR, Vazquez R, Cinca J, Bayes-Genis A, Pascual D, Fernández-Palomeque C, Bardají A, Almendral J, Nieto V, Macaya C, Pavón-Jimenez R, Bayés de Luna A. Association of blood pressure and its evolving changes with the survival of patients with heart failure. J Card Fail. 2008;14:561–568. doi: 10.1016/j.cardfail.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee T, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med. 2004;116:466–473. doi: 10.1016/j.amjmed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, Mebazaa A, Juilliere Y, Cohen-Solal A, Guize L, Alla F, Rouge P, Blin P, Barlet MH, Paolozzi L, Vincent C, Desnos M, Samii K. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, ÓConnor CM, Sun JL, Yancy CW, Young JB on behalf of the OPTIMIZE-HF Investigators Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. J Am Med Assoc. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 29.Psaty BN, Boineau R, Kuller LH, Luepker RV. The potential costs of upcoding for heart failure in the United States. Am J Cardiol. 1999;84:108–109. doi: 10.1016/s0002-9149(99)00205-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.