Abstract
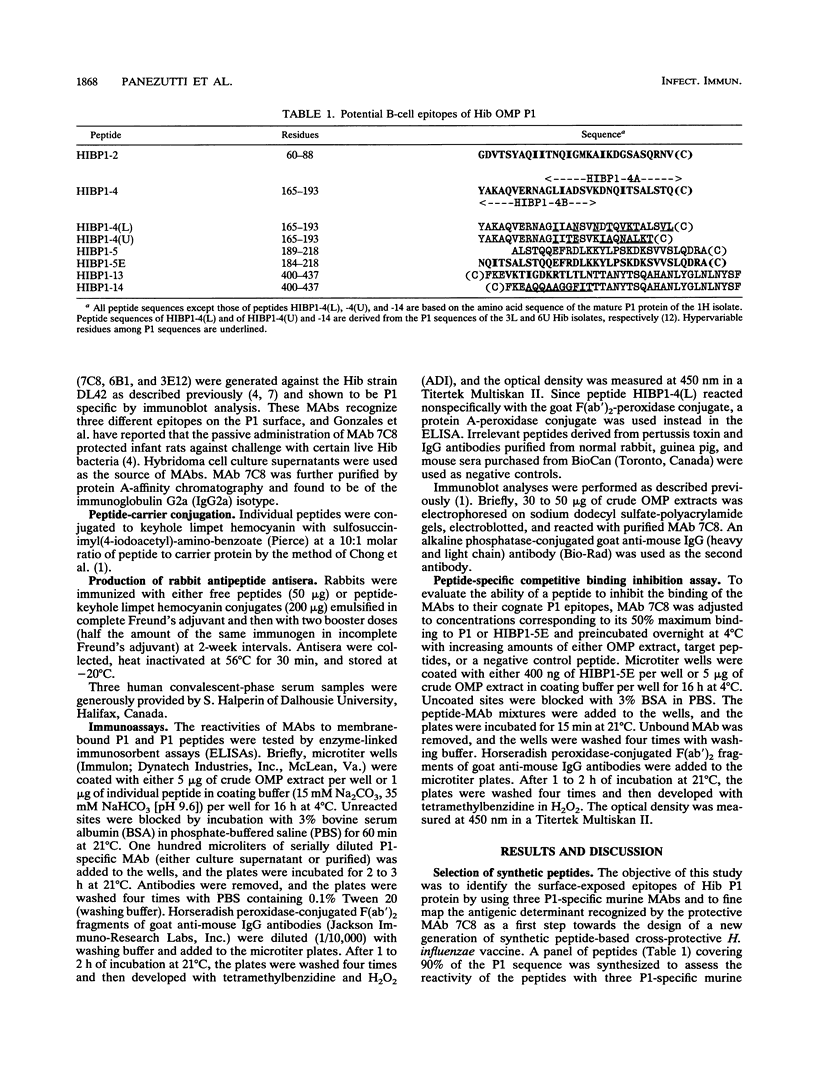
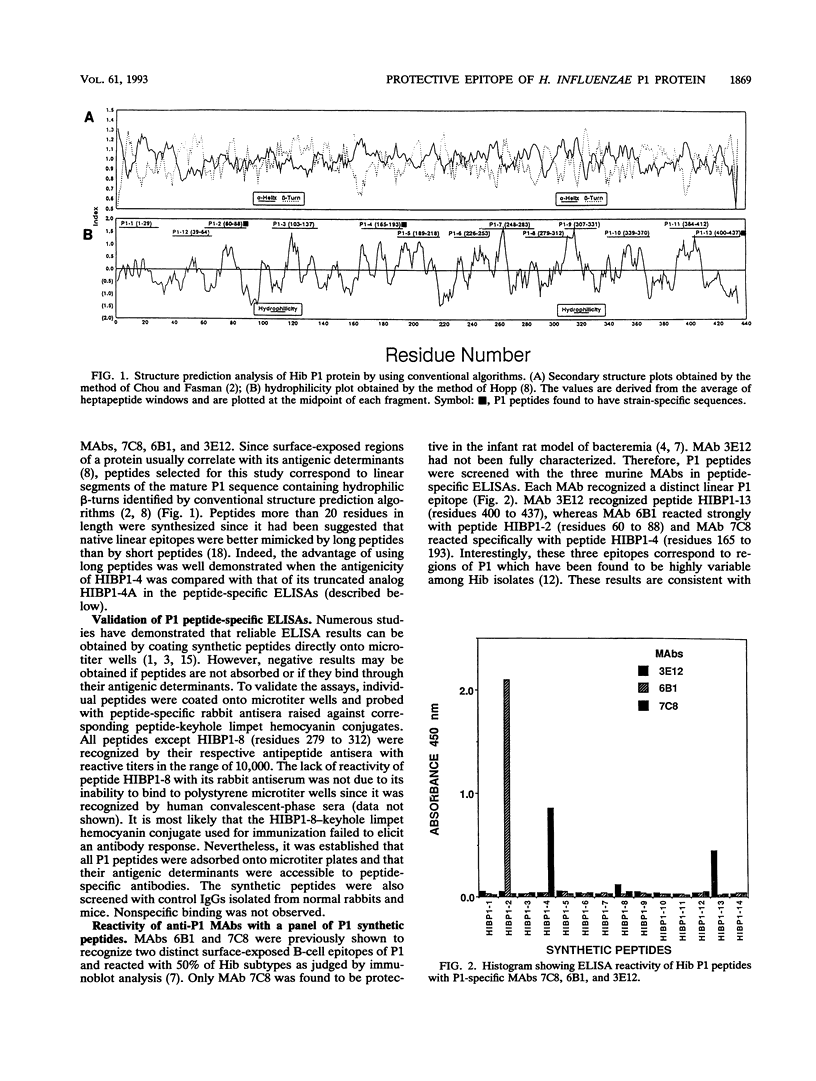
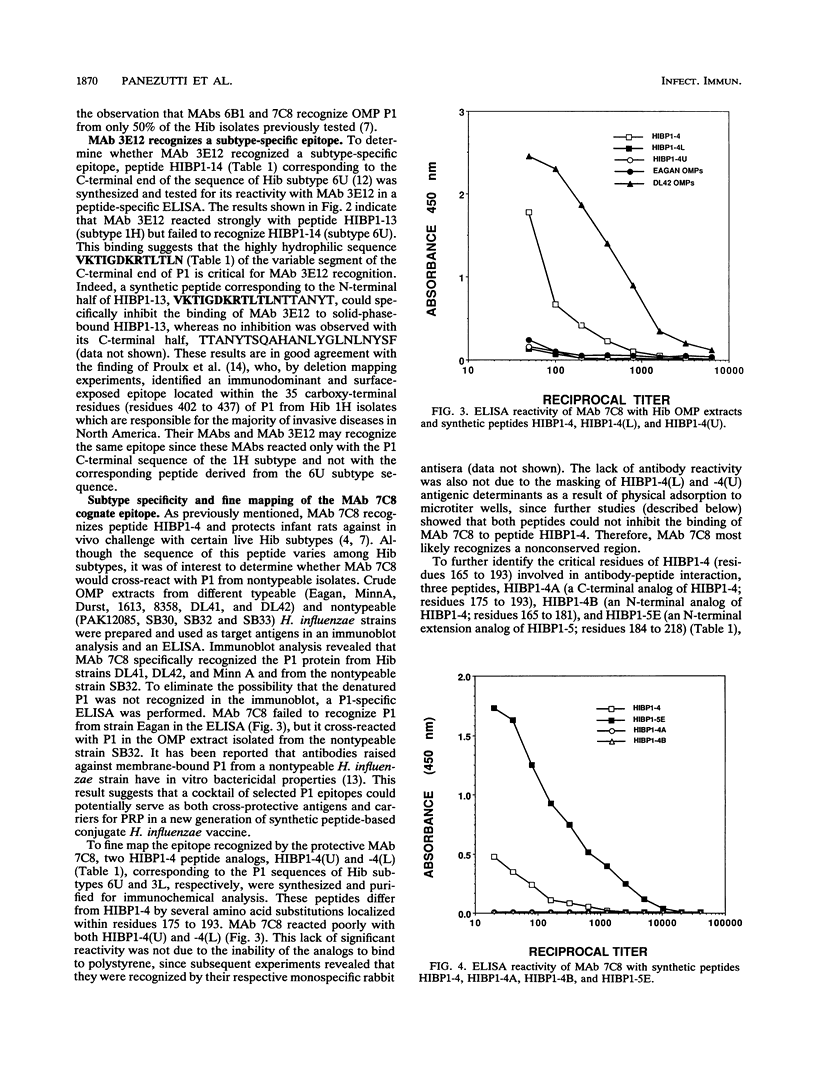
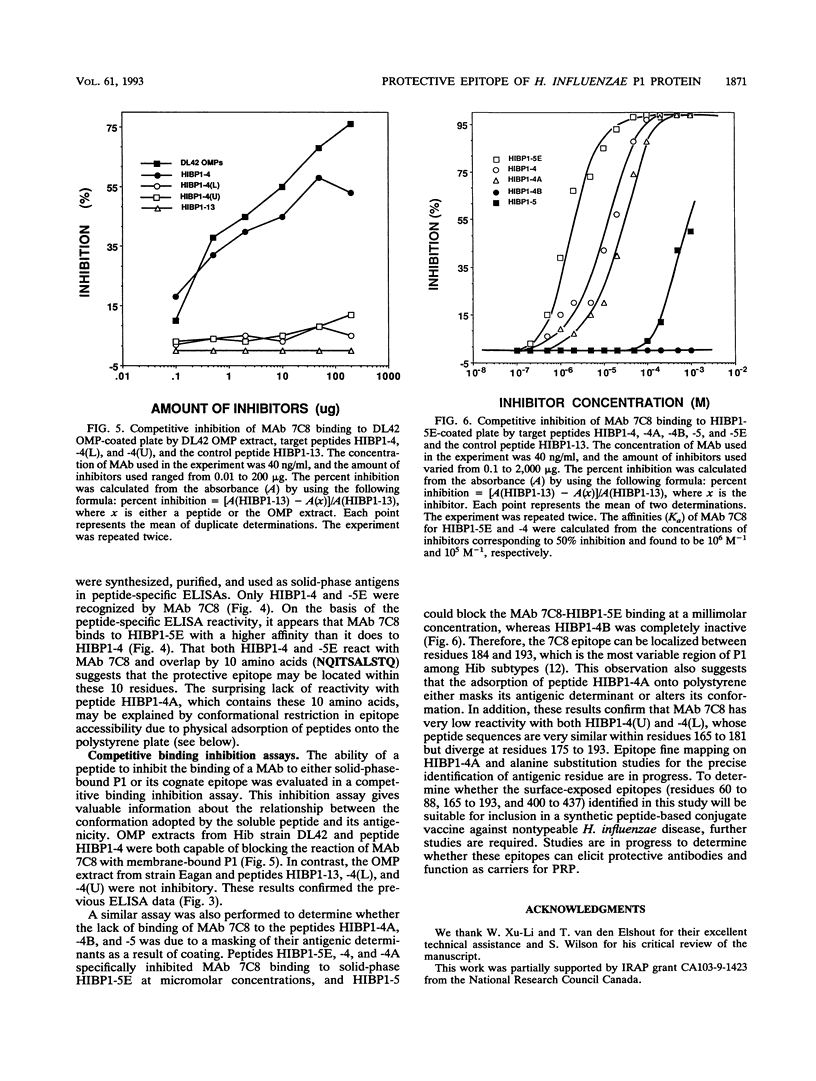
A panel of P1 synthetic peptides was synthesized to map the surface-exposed epitopes of Haemophilus influenzae type b outer membrane protein P1 recognized by three murine monoclonal antibodies (MAbs 7C8, 3E12, and 6B1). By using peptide-specific enzyme-linked immunosorbent assays, MAbs 6B1, 7C8, and 3E12 were shown to recognize distinct epitopes localized within residues 60 to 88, 165 to 193, and 400 to 437 of mature P1, respectively. Since MAb 7C8 was shown previously to be protective against certain H. influenzae type b subtypes in the infant rat model of bacteremia, its cognate epitope was further characterized by using truncated peptide analogs. Fine mapping of the 7C8 epitope by competitive inhibition studies revealed that it was localized within residues 184 and 193.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chong P., Sydor M., Wu E., Zobrist G., Boux H., Klein M. Structural and functional analysis of the S1 subunit of pertussis toxin using synthetic peptides. Mol Immunol. 1991 Mar;28(3):239–245. doi: 10.1016/0161-5890(91)90068-u. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Geerligs H. J., Weijer W. J., Bloemhoff W., Welling G. W., Welling-Wester S. The influence of pH and ionic strength on the coating of peptides of herpes simplex virus type 1 in an enzyme-linked immunosorbent assay. J Immunol Methods. 1988 Feb 10;106(2):239–244. doi: 10.1016/0022-1759(88)90203-7. [DOI] [PubMed] [Google Scholar]
- Gonzales F. R., Leachman S., Norgard M. V., Radolf J. D., McCracken G. H., Jr, Evans C., Hansen E. J. Cloning and expression in Escherichia coli of the gene encoding the heat-modifiable major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2993–3000. doi: 10.1128/iai.55.12.2993-3000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Peltola H., Eskola J., Rönnberg P. R., Kela E., Karanko V., Mäkelä P. H. Immunogenicity of Haemophilus influenzae oligosaccharide-protein and polysaccharide-protein conjugate vaccination of children at 4, 6, and 14 months of age. Pediatrics. 1989 Dec;84(6):995–999. [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Grass S., Einhorn M., Bailey C., Newell C. Comparative analysis of the structures of the outer membrane protein P1 genes from major clones of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3300–3305. doi: 10.1128/iai.57.11.3300-3305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx C., Munson R. S., Jr, Grass S., Hamel J., Martin D., Brodeur B. R. Identification of a surface-exposed immunodominant epitope on outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1991 Mar;59(3):963–970. doi: 10.1128/iai.59.3.963-970.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Houghten R., Taulane J. P., Carson D., Vaughan J. The immune response to Epstein-Barr nuclear antigen: conformational and structural features of antibody binding to synthetic peptides. Mol Immunol. 1984 Nov;21(11):1047–1054. doi: 10.1016/0161-5890(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Tarr P. I., Peter G. Demographic factors in the epidemiology of hemophilus influenzae meningitis in young children. J Pediatr. 1978 Jun;92(6):884–888. doi: 10.1016/s0022-3476(78)80353-9. [DOI] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]



