Abstract
BACKGROUND:
The test-retest reliability of temporal summation (TS) and diffuse noxious inhibitory control (DNIC) has not been reported to date. Establishing such reliability would support the possibility of future experimental studies examining factors affecting TS and DNIC. Similarly, the use of manual algometry to induce TS, or an occlusion cuff to induce DNIC of TS to mechanical stimuli, has not been reported to date. Such devices may offer a simpler method than current techniques for inducing TS and DNIC, affording assessment at more anatomical locations and in more varied research settings.
METHOD:
The present study assessed the test-retest reliability of TS and DNIC using the above techniques. Sex differences on these measures were also investigated.
RESULTS:
Repeated measures ANOVA indicated successful induction of TS and DNIC, with no significant differences across test-retest occasions. Sex effects were not significant for any measure or interaction. Intraclass correlations indicated high test-retest reliability for all measures; however, there was large interindividual variation between test and retest measurements.
CONCLUSION:
The present results indicate acceptable within-session test-retest reliability of TS and DNIC. The results support the possibility of future experimental studies examining factors affecting TS and DNIC.
Keywords: Diffuse noxious inhibitory control, DNIC, Reliability, Temporal summation
Abstract
HISTORIQUE :
La fiabilité test-retest de la sommation temporelle (ST) et du contrôle inhibiteur diffus nociceptif (CIDN) n’a fait l’objet d’aucun rapport à ce jour. L’établissement de cette fiabilité appuierait la réalisation d’éventuelles études expérimentales sur les facteurs qui affectent la ST et le CIDN. De même, on ne dispose à ce jour d’aucun rapport sur l’utilisation de l’algométrie manuelle pour induire la ST ou d’un garrot pour induire le CIDN de la ST aux stimuli mécaniques. De tels dispositifs offriraient une méthode plus simple que les techniques actuelles pour induire la ST et le CIDN, et faciliteraient en leur évaluation au niveau d’un plus grand nombre de sites anatomiques et dans des contextes de recherche plus variés.
MÉTHODE :
La présente étude a évalué la fiabilité test-retest de la ST et du CIDN à l’aide des techniques décrites plus haut. On y a aussi analysé les différences liées au sexe pour les mesures obtenues.
RÉSULTATS :
L’analyse de variance avec mesures répétées a indiqué une induction réussie de la ST et du CIDN, sans différence significative entre les épisodes test et retest. Les effets liés au sexe n’ont pas été significatifs, peu importe la mesure ou l’interaction. Les corrélations intraclasses ont indiqué une forte fiabilité test-retest pour toutes les mesures. Toutefois, on a noté une importante variation test-retest interinviduelle.
CONCLUSION :
Les résultats actuels indiquent une fiabilité test-retest acceptable pour la ST et le CIDN, à l’intérieur d’une même session. Ces résultats appuient la possibilité de réaliser éventuellement des études expérimentales sur les facteurs qui influent sur la ST et le CIDN.
Temporal summation (TS), defined as the increase in pain rating after repetitive stimulation at a constant stimulus intensity, has been increasingly used to investigate pain processing in healthy and clinical populations (1–3). TS has been demonstrated using repetitive application of thermal, electrical and mechanical stimuli, and is thought to be a psychophysiological correlate of wind-up. Wind-up is the increase in response magnitude of second-order nociceptive neurons and higher structures (4) to repetitive noxious stimulation (5). Increased TS has been found in fibromyalgia patients (1) and healthy women (3,6), while Ashina et al (7) found a trend toward increased TS in chronic tension-type headache (CTH) patients. To date, induction of TS from mechanical pain has used computer-controlled pressure stimulators (1,8). Assessing TS using a hand-held algometer has not been reported to date; however, such devices may offer a simpler method of measurement, affording assessment at more anatomical locations and in more varied research settings.
The phenomenon whereby pain from one part of the body inhibits pain elsewhere in the body (diffuse noxious inhibitory control [DNIC]) has also been increasingly used to investigate pain mechanisms in healthy and clinical populations. In DNIC, nociceptive neurons in spinal and trigeminal dorsal horns are inhibited by noxious stimulation remote from the neurons’ excitatory receptive field (9). Impaired DNIC has been found in CTH (9) and fibromyalgia patients (2), and in healthy women (10). DNIC has been demonstrated using cold water (10), electrical stimulation (11) and heat pain (2) as the conditioning stimuli, and phasic electrical (12), thermal (2) and pressure pain ratings (13) as experimental (dependent variable) stimuli. Painful occlusion cuff inflation may represent a simple and reliable method for eliciting DNIC. An occlusion cuff allows continuous control of intensity, and the ischemic pain predominantly involves C-fibre transduction (14). This may be useful because DNIC is more effective on C-fibre- than A-fibre-mediated pain (15). No studies have examined whether occlusion cuff inflation can inhibit pain from pressure algometry, or inhibit TS from any source.
To use TS and DNIC in experimental protocols, the test-retest reliability of these techniques needs confirmation. Such examinations have not been reported to date, although Granot et al (16) found no significant difference between two sessions of heat-induced TS. The present study therefore sought to demonstrate TS and DNIC using manual algometry and occlusion cuff inflation, respectively. We also examined for sex differences on these measures and assessed, for the first time, the test-retest reliability of TS and DNIC. Because TS may be more pronounced for deep tissue than superficial locations (8), we examined these measures at a superficial location (finger) and a deep muscle location (trapezius).
METHODS
Subjects
Subjects were recruited via advertisements in local and University of South Australia (Adelaide, Australia) media requesting volunteers as part of a larger study on headaches. The advertisement requested healthy volunteers to participate as control subjects in a study measuring pain sensitivity. Written consent was obtained. The study was approved by the university’s Human Research Ethics Committee. Inclusion criteria were age between 18 and 65 years; no current or previous psychiatric conditions, chronic pain or major medical conditions; and not currently taking, or having taken in the past three months, any regular medications other than 1000 mg of acetaminophen daily or less, for no longer than 14 consecutive days, and none in the past 14 days. All sessions were conducted in an interview room at the School of Psychology, University of South Australia, between 9:00 and 17:00 from Monday to Friday. The room was maintained at a constant temperature of 23°C.
Self-report questionnaires
Subjects completed an in-house sociodemographic questionnaire detailed elsewhere (17). Subjects also completed the State-Trait Anxiety Inventory (18) and the Centre for Epidemiological Studies Depression Scale (19) due to the known effects of anxiety and depression on pain sensitivity.
TS
TS was induced using an analogue pressure algometer (FPK20, Wagner Instruments, USA) with a circular silicon rubber tip measuring 0.79 cm2. All pain assessments were conducted by the senior author. TS was elicited with 10 applications (‘pulses’) of the algometer at pressure pain detection threshold intensity on the dorsal surface of the right-hand middle finger midway between the first and second distal joints, and at the middle of the right-hand side trapezius belly. To determine pressure pain threshold (PPT), pressure was increased at a rate of approximately 1 kg/s and subjects were asked to say ‘pain’ at the point the sensation first became painful, at which point pressure was released and the readout recorded. Thresholds were taken as the average of two measures taken 30 s apart. The test-retest reliability of the PPT technique was previously confirmed for the author who performed the pain assessment in the present study (20).
At each location, TS elicitation commenced 2 min after the final PPT was taken. This was performed to ensure TS pulses were not contaminated by possible sensitization from pain threshold stimulation. For each pulse, pressure was increased at a rate of approximately 2 kg/s to the previously determined PPT intensity, where it was maintained for 1 s before being released. Pulses were presented with an interstimulus interval (ISI) of 1 s because this has previously been shown to be optimal for inducing TS with pressure pain (8). A timer was used to assure application rate, duration of stimulus and ISI. Before the first pulse, subjects were instructed to verbally rate the pain level of the first, fifth and 10th pulse according to a visual analogue scale (VAS) displayed on a wall in front of them. The VAS was anchored with single digits ranging from 0 to 10, with the ‘0’ end point labelled with ‘no pain’ and the ‘10’ end point labelled as ‘extremely painful’.
DNIC
To establish a baseline for DNIC, subjects sat quietly for 5 min after completion of the previous TS assessment. Replicating a previous method (12), the conditioning stimulus for eliciting DNIC was an occlusion cuff inflated to a painful intensity and maintained at that level while experimental pain measures (TS) were taken. The occlusion cuff was inflated on the opposing (left) arm to the experimental pain stimulation. The cuff was inflated at approximately 20 mmHg/s until subjects reported ‘pain’, at which point inflation was ceased. Subjects adapted to the stimulus for 30 s, then rated the pain on the VAS. Cuff inflation was then increased or decreased until subjects indicated the pain was at level 3 of 10 on the VAS. The left arm was then rested on a table in front of the subject while TS assessment was repeated on the right side as above.
Test-retest measurements
Following the above assessment of TS and DNIC, subjects remained seated in the interview room for 60 min, during which time they were allowed to watch television and browse supplied newspapers and magazines. Following this 60 min period, the TS and DNIC procedures were repeated, as above. The period of 60 min was chosen as the retest period due to this being a commonly used period for experimental manipulation of pain sensitivity in chronic pain samples. The order of TS and DNIC assessment locations was counterbalanced across subjects and test-retest occasion. That is, for one-half of the subjects, TS and DNIC were conducted in the order of finger then shoulder at time 1 and shoulder then finger at time 2, while one-half received TS and DNIC in the order of shoulder then finger at time 1 and finger then shoulder at time 2. The protocol is presented in Table 1.
TABLE 1.
Temporal summation (TS) and diffuse noxious inhibitory control (DNIC) counterbalancing order at the finger and shoulder
|
Time 1 |
Time 2 |
||
|---|---|---|---|
| TS | DNIC | TS | DNIC |
| F–S | F–S | S–F | S–F |
| S–F | S–F | F–S | F–S |
TS was induced with a pressure algometer. DNIC was TS taken during painful occlusion cuff inflation. F–S TS or DNIC taken in the order of finger (F) then shoulder (S); S–F TS or DNIC taken in the order of S then F; Time 1 and time 2 TS and DNIC assessments taken 60 min apart
Statistical methods
Statistical analyses were conducted using SPSS statistical software (SPSS Inc, USA) (21). Independent samples t tests were used to test for sex differences on age, anxiety and depression measures. For pain measures, a repeated measures ANOVA was conducted to assess for TS, DNIC and sex effects across time 1 and time 2. The model had one between-subjects factor (sex) and three within-subjects factors – occasion (time 1 and 2), DNIC (TS before and during cuff inflation) and TS (pain ratings of the first, fifth and 10th algometer pulse).
For test-retest reliability assessment, intraclass correlations (ICCs) were performed between time 1 and time 2 recordings. The absolute agreement ICC is an ANOVA model including a between- and within-subjects effect, providing a test of both association and systematic bias between raters (or repeated ratings by one rater). This answers the question of whether raters are interchangeable, and not just correlated. In addition to ICCs, we also calculated the coefficient of repeatability (CR) for all pain measures. The CR, defined as two SDs of the mean test-retest difference, provides an estimate of retest ranges expressed in the measurement units. Hence, 95% of repeat measurements for the sample will be in the range of the mean difference ± CR. The ICC and CR therefore provide complementary information on reliability.
To examine reliability of TS, within-condition difference scores were created, calculated as the pain rating of the 10th pulse in the train minus the pain rating of the first pulse in the train. Thus, these scores represented the magnitude of TS at each location (finger and shoulder), under each condition (with and without the cuff) and at each occasion (time 1 and time 2). Additionally, absolute pain ratings (the 10th stimulus rating at the finger and shoulder, before and during cuff inflation, at time 1 and time 2) were also subjected to ICC and CR analyses. This allowed test-retest reliability assessment of not only the magnitude of TS (increase in pain rating from first to 10th pulse, as above), but also assessment of the absolute pain levels induced by TS at each occasion (time 1 and time 2).
To examine reliability of DNIC across conditions, difference scores between maximal pain rating with and without the cuff were created, calculated as the 10th pulse rating without the cuff minus the 10th pulse rating during cuff inflation for each location (finger and shoulder) and at each occasion (time 1 and time 2). Hence, these scores represented the magnitude of pain suppression at each location and occasion.
RESULTS
Sample characteristics
The sample contained nine men and 11 women. The mean (± SD) age was 27±6.4 years for men, and 23±3.6 years for women. Mean state anxiety was 33.2±4.0 for men and 31.1±9.8 for women. Mean trait anxiety was 35.4±6.7 for men and 38.8±11.4 for women, while the mean depression score was 11.3±7.8 for men and 13.4±13.5 for women. Men and women did not significantly differ on age, depression or anxiety measures (all t tests P>0.10).
Induction of TS and DNIC
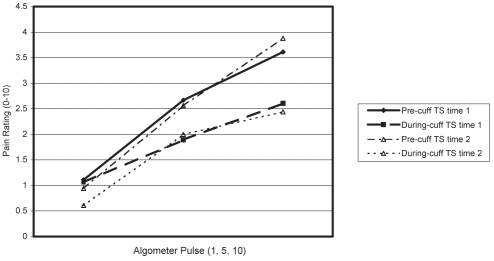
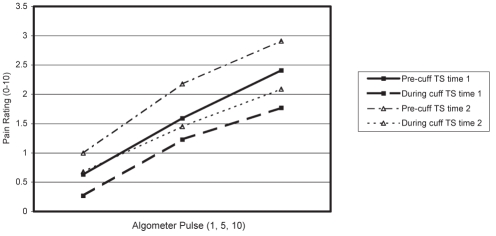
Figures 1 and 2 show pain ratings of algometer pulses 1, 5 and 10 at the shoulder, before and during cuff inflation, at time 1 and time 2, for men (Figure 1) and women (Figure 2).
Figure 1).
Temporal summation (TS) and diffuse noxious inhibitory control of shoulder pain in men
Figure 2).
Temporal summation (TS) and diffuse noxious inhibitory control of shoulder pain in women
The ANOVA results indicated a significant pulse effect (F [2, 38]=40.59, P<0.001), a significant cuff effect (F [1, 18]=13.73, P<0.01) and a significant cuff × pulse interaction (F [2, 36]=6.65, P<0.01). Occasion effects were not significant (F [1, 18]=0.91, P=0.35), nor were sex effects for any measure or interaction (all P>0.10).
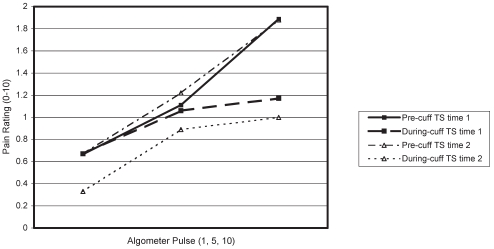
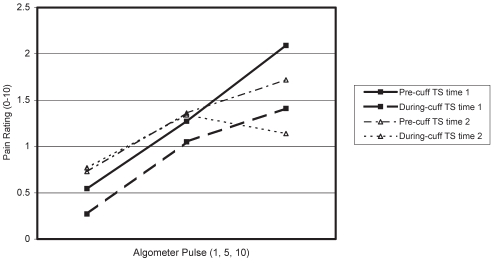
Figures 3 and 4 show pain ratings of algometer pulses 1, 5 and 10 at the finger, before and during cuff inflation, and at time 1 and time 2 for men (Figure 3) and women (Figure 4).
Figure 3).
Temporal summation (TS) and diffuse noxious inhibitory control of finger pain in men
Figure 4).
Temporal summation (TS) and diffuse noxious inhibitory control of finger pain in women
The ANOVA results indicated a significant pulse effect (F [2, 38]=26.19, P<0.01), a significant cuff effect (F [1, 18]=13.71, P<0.01) and a significant cuff × pulse interaction (F [2, 36]=6.14, P<0.01). Occasion effects were not significant (F [1, 18]=0.002, P>0.10), nor were sex effects for any measure or interaction (all P>0.10).
Reliability of TS and DNIC
ICCs and CRs were calculated for combined male and female data due to the lack of significant sex differences (Table 2). ICC coefficients of 0.75 or above represent excellent reliability (22). The ICCs were high to excellent for all measures, except for DNIC at the finger, for which reliability was moderate at ICC=0.57. Table 2 also shows the ICCs between time 1 and time 2 absolute pain ratings, for the finger and shoulder, before and during cuff inflation. The ICCs were high to excellent for all measures. The lowest ICC for absolute pain ratings was ICC=0.69 for finger stimulation during cuff inflation, while the highest was ICC=0.86 for a pain rating of shoulder stimulation during cuff inflation. Table 2 also shows the CR for each measure. The lowest CR was 1.69 for a finger DNIC score, while the highest CR was 3.60 for a precuff TS score at the shoulder.
TABLE 2.
Temporal summation (TS) and diffuse noxious inhibitory control (DNIC) ratings for algometer stimulation at the finger and shoulder
| Measure | Time 1 | Time 2 | T1–T2 difference (CR) | ICC |
|---|---|---|---|---|
|
Finger | ||||
| Precuff absolute rating* | 2.95±1.5 | 3.60±1.6 | 0.65 (2.38) | 0.79 |
| During-cuff absolute rating† | 1.30±1.0 | 1.23±.92 | 0.07 (3.12) | 0.69 |
| Precuff TS score‡ | 2.35±1.4 | 2.55±1.4 | 0.20 (2.58) | 0.72 |
| During-cuff TS score§ | 1.42±1.4 | 1.55±1.7 | 0.13 (2.64) | 0.78 |
| DNIC score¶ | 0.63±1.4 | 0.98±1.7 | 0.35 (1.69) | 0.57 |
|
Shoulder | ||||
| Precuff absolute rating | 2.95±1.5 | 3.35±2.0 | 0.40 (2.72) | 0.82 |
| During-cuff absolute rating | 2.15±1.6 | 2.25±2.0 | 0.10 (1.96) | 0.86 |
| Precuff TS score | 2.10±1.3 | 2.38±1.8 | 0.28 (3.60) | 0.67 |
| During-cuff TS score | 1.53±1.5 | 1.60±1.6 | 0.07 (2.60) | 0.80 |
| DNIC score | 0.80±1.2 | 1.10±1.3 | 0.30 (2.38) | 0.69 |
Data presented as mean ± SD unless otherwise indicated.
Pain rating of the 10th algometer pulse before cuff inflation;
Pain rating of 10th algometer pulse during cuff inflation;
10th minus first algometer pulse rating before heterotopic cuff inflation;
10th minus first algometer pulse rating during heterotopic cuff inflation;
10th algometer pulse rating before heterotopic cuff inflation minus 10th algometer pulse rating during heterotopic cuff inflation. CR Coefficient of repeatability within session, equal to 2(SD of the mean time 1 [T1] to time 2 [T2] difference). ICC Intraclass correlation coefficient
DISCUSSION
Induction of TS
The findings from the present study demonstrate TS can be successfully induced at superficial (finger) and deep tissue (trapezius) sites, using a hand-held algometer applied at pressure pain intensity. Compared with present results, previous studies using computer-controlled stimulators have reported less increase in pain ratings to repetitive stimulation, and hence, have demonstrated less TS (1,8). The difference may be due to methodology. We used an ISI of 1 s, whereas Staud et al (1) used ISIs of 3 s and 5 s. Using a computer-controlled algometer, Nie et al (8) showed that a 1 s ISI is better than 3 s, 5 s or 10 s ISIs in inducing TS. Further, the Staud et al (1) and Nie et al (8) studies involved more TS assessments than our study (examining multiple ISIs over multiple locations, and using more pulses within each TS induction). It could be that the greater number of painful stimuli in these studies produced more pain inhibition, and hence less TS compared with our procedure.
Our study affords minimal elucidation of TS mechanisms. However, both peripheral and central mechanisms may be involved. Evidence that TS reflects central mechanisms comes from several lines. In particular, TS occurs even when the site of each stimulus in the train is changed (23); C-polymodal afferents decrease in activity following repeated noxious stimulation (24); and peripheral nociceptors show fatigue from repeated noxious stimulation (25). Furthermore, TS can be induced using intramuscular electrical stimulation, which bypasses the nociceptor and directly activates the nerve fibre (7). However, repeated pressure pain stimulation at the one site, as used in the present study, may induce summation and sensitization in peripheral and central mechanisms, as noted by others (8).
Induction of DNIC
The ANOVA results indicated a significant reduction in TS during painful occlusion cuff inflation, with the magnitude of pain inhibition increasing with the number of pulses. The inhibition was observed at both the finger and shoulder, indicating constant pain from the occlusion cuff inhibited TS from heterotopic pressure pain stimulation at both superficial and deep tissue locations. Pain from an occlusion cuff is reported to increase the pain threshold to electrical stimulation (12). To our knowledge, the present study is the first to demonstrate that pain from an occlusion cuff also increases the pain threshold to pressure stimulation. In previous DNIC studies, pain from a hot water bath has been shown to decrease TS to a heterotopic heat thermode (2), while Serrao et al (10) found TS of the RIII nociceptive reflex was attenuated by a cold pressor test. Our results extend these findings to mechanical conditioning and target stimuli. A benefit of the present method is that an occlusion cuff can assure constant and adjustable conditioning pain, and the ischemic pain predominantly involves C-fibre transduction (14). This may be particularly useful because DNIC is more effective on C-fibre- than A-fibre-mediated pain (15).
Although our methods allow limited conclusions on the underlying mechanisms, the fact that the occlusion cuff stimulation was heterotopic to the phasic pain suggests that peripheral or segmental mechanisms are not solely responsible for the inhibiting effect of the cuff. Rather, our results support the notion of a DNIC effect (11). The mechanisms of DNIC are not completely elucidated, but are thought to involve spinalbulbo-spinal loops and supraspinal mechanisms inhibiting second-order spinal and trigeminal dorsal horn neurons. Hence, although our TS findings may reflect peripheral and central mechanisms, it is likely that the pain inhibition from the occlusion cuff was due to central mechanisms. However, a spinally mediated effect cannot be ruled out because previous research demonstrates contralateral effects of pain stimulation at mirror image points (11,15).
Reliability of TS and DNIC
The results of the ICC analyses on maximal pain ratings indicated no significant change in rating of the 10th pulse at time 1 and time 2, at both the finger and shoulder, either before or during cuff inflation. In fact, the ICCs indicate a high to excellent level of absolute agreement between these measures at time 1 and time 2. This suggests that assessment of TS and DNIC does not affect absolute pain levels in subsequent with-in-session TS and DNIC assessment, and suggests that these techniques have high with-in-session test-retest reliability when maximal pain ratings are measured.
In addition to maximal pain ratings, we also calculated change scores for assessing the within-session test-retest reliability of TS magnitude (increase in pain from pulse 1 to pulse 10) at time 1 and time 2. Using change scores also controlled for potential changes in pressure pain detection thresholds between time 1 and time 2. This was considered important because these were used to determine initial pulse intensity for all TS inductions. The results of the ICC analyses on these data showed the magnitude of TS was similar at time 1 and time 2 for both finger and shoulder locations, and under both cuff and no-cuff conditions. Indeed, the ICC results showed a high to excellent level of absolute agreement between time 1 and time 2 magnitudes of TS, at both the finger and shoulder, and during both cuff and no-cuff conditions. This suggests that pain from TS and DNIC induction does not affect subsequent TS magnitudes, and that TS and DNIC have high within-session test-retest reliability when TS magnitude scores are used. Although the ICCs were acceptable, the CR results for TS indicate a considerable interindividual variability in this measure. Thus, while the mean difference in absolute pain rating at the shoulder between time 1 and time 2 was 0.40, 95% of repeat observations will, in fact, be within a difference in pain rating of ±2.72.
Reliability of DNIC was assessed by conducting ICCs on difference scores, calculated as the 10th pulse rating without cuff minus the 10th pulse rating during cuff inflation for each location (finger and shoulder) and at each occasion (time 1 and time 2). Hence, these scores represent the magnitude of DNIC at each location and occasion. The results of the ICC analyses indicated an excellent level of absolute agreement between these measures. This suggests that assessment of TS and DNIC does not affect the magnitude of subsequent DNIC, and suggests that DNIC has excellent within-session test-retest reliability. To our knowledge, such reliability has not been examined previously. As with TS, the CR results for DNIC indicate considerable interindividual variability in repeat assessment of this measure. Thus, while the mean difference in pain inhibition at the finger between time 1 and time 2 was 0.35, 95% of repeat observations will, in fact, be within a pain rating difference of ±1.69.
Sex differences
We found no significant sex differences in TS or DNIC. This is in contrast to some previous studies (3,10), but is consistent with others (8,26). A speculative explanation for our results concerns psychological effects of the procedures. Robinson et al (6) found that increased TS in women could be accounted for by sex role expectations. Previous studies that found sex differences were conducted in laboratories with specialist equipment, such as computer-controlled pressure stimulators and electrical stimulation equipment. In contrast, our procedures were conducted in an interview room using simpler and less specialized equipment. Our subjects were also predominantly university students. It may be that our procedure and population invoked fewer sex role expectations. This speculation requires further investigation.
Limitations of the present study
A number of limitations of the present study need to be mentioned. In inducing TS, we used only one ISI (1 s) and one application rate, and all assessments were conducted by one rater. Hence, the generalizability of our findings to other ISI, etc, are limited, and previous research has indicated these factors affect TS (8). Similarly, we used predominantly university student volunteers, which limits the generalizability of our results to wider populations. Finally, despite a considerable age range in our sample, we did not examine for possible age effects. Future research would be useful to examine these issues.
CONCLUSIONS
The present results are the first to demonstrate the following: TS can be elicited using a hand-held mechanical algometer at both superficial (finger) and deep tissue (trapezius muscle belly) locations; DNIC from tonic mechanical pain (occlusion cuff) inhibits TS elicited from a manual algometer; and TS and DNIC have acceptable test-retest reliability, although there is considerable interindividual variation across assessments. This should be considered if future studies wish to examine factors affecting TS and DNIC. This noted, our results support the possibility of such research, particularly in chronic pain conditions such as fibromyalgia and CTH, in which increased TS and impaired DNIC have been observed (1,2,9). For example, because stress is related to headache, and known to affect pain processing throughout the central nervous system, it is possible that stress could be related to headache through its effects on TS and DNIC (6). Further research is needed to examine these possibilities.
REFERENCES
- 1.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 2.Staud R, Robinson ME, Vierck CJ, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–74. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 3.Ge HY, Madeleine P, Arendt-Nielson L. Gender differences in pain modulation evoked by repeated injections of glutamate into the human trapezius muscle. Pain. 2005;113:134–40. doi: 10.1016/j.pain.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Staud R, Craggs JG, Robinson ME, Perlsein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–23. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Ashina S, Bendtsen L, Ashina M, Magerl W, Jensen R. Generalized hyperalgesia in patients with chronic tension-type headache. Cephalalgia. 2000;26:940–8. doi: 10.1111/j.1468-2982.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- 8.Nie H, Arendt-Nielsen L, Anderson H, Graven-Nielsen T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J Pain. 2005;6:348–55. doi: 10.1016/j.jpain.2005.01.352. [DOI] [PubMed] [Google Scholar]
- 9.Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–23. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Serrao M, Rossi P, Sandrini G, et al. Effects of noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:353–60. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Le Bars D, Dickenson AH, Besson J. Diffuse noxious inhibitory controls (DNIC). 1. Effects on dorsal horn convergent neurons in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 12.Fujii K, Motohashi K, Umino M. Heterotopic ischemic pain attenuates somatosensory evoked potentials induced by electrical tooth pulp stimulation: Diffuse noxious inhibitory controls in the trigeminal territory. Eur J Pain. 2006;10:495–504. doi: 10.1016/j.ejpain.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Ge HY, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: An evaluation using repeated bilateral experimental induction of muscle pain. Pain. 2004;110:72–8. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Crews JC, Cahall M, Behbehani MM. The neurophysiologic mechanisms of tourniquet pain. Anesthesiology. 1994;81:730–6. doi: 10.1097/00000542-199409000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Kakigi R. Diffuse noxious inhibitory control; reappraisal by pain related somatosensory evoked potentials following CO2 laser stimulation. J Neurol Sci. 1994;125:198–205. doi: 10.1016/0022-510x(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: Tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Cathcart S, Pritchard D. Daily stress and pain sensitivity in chronic tension-type headache sufferers. Stress Health. 2007;24:123–7. doi: 10.1111/j.1468-1331.2008.02124.x. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CD, Gorsuch RL, Lushene RL. Manual for the State Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–481. [Google Scholar]
- 20.Cathcart S, Pritchard D. Reliability of pain threshold measurement in young adults. J Headache Pain. 2006;7:21–6. doi: 10.1007/s10194-006-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SPSS Inc Statistical Package for the Social Sciences, Release 15.0. Vesta Services Inc; 2008 [Google Scholar]
- 22.Shrout PE, Fleiss JL. Intra-class correlation: Uses in assessing rater reliability. Psychol Bull. 1976;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 23.Sarlani E, Greenspan JD. Gender differences in temporal summation of mechanically evoked pain. Pain. 2002;97:163–9. doi: 10.1016/s0304-3959(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 24.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 25.Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fibre nociceptors in the monkey to controlled-force stimuli. J Neurophysiol. 2000;83:2179–91. doi: 10.1152/jn.2000.83.4.2179. [DOI] [PubMed] [Google Scholar]
- 26.Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26:782–9. doi: 10.1111/j.1468-2982.2006.01130.x. [DOI] [PubMed] [Google Scholar]