Abstract
Breast cancer, a leading cause of increased morbidity and mortality among women, overexpresses the human epidermal growth factor receptor 2 in approximately 20% to 30% of cases. Trastuzumab (Trz), a monoclonal antibody against the human epidermal growth factor receptor 2, improves survival in breast cancer patients in both the adjuvant and metastatic settings. Despite the therapeutic benefits of Trz, there is an increased incidence of cardiotoxicity, particularly when administered following anthracycline-based chemotherapy. The pathogenesis underlying Trz-mediated cardiotoxicity remains poorly understood. The present review focuses on the current understanding of Trz-mediated cardiotoxicity from both the basic and clinical science perspectives.
Keywords: Cardiac MRI, Cardiomyopathy, Echocardiography, Renin-angiotensin system, Trastuzumab
BREAST CANCER IN CANADA
Breast cancer is the most prevalent form of malignant disease among women, with an estimated 1.05 million new cases every year worldwide (1). In Canada alone, it is estimated that more than 22,000 Canadian women will be newly diagnosed with breast cancer in 2009, leading to 5400 deaths (2,3).
Recent advances in the management of breast cancer patients have carefully considered predisposing genetic factors underlying its development and progression. With a strong correlation between family history and the onset of breast cancer (4), the BRCA1, BRCA2 and TP53 genes are the most frequently involved (5). Women who carry the BRCA1 and BRCA2 mutations are three to four times more likely to develop breast cancer (5). Among women who develop breast cancer, palpability, neoplasm size, nuclear grade and metastasis to axillary lymph nodes are poor prognostic factors.
The treatment for breast cancer is multifaceted, involving a combination of surgery, radiotherapy and chemotherapy. Mastectomy is often the first-line approach for breast cancer patients. This procedure varies depending on whether any adjacent lymph nodes are affected. Approaches range from radical mastectomy, in which all of the breast tissue is removed, to node-specific lymphadenectomy (6,7). Radiotherapy is an integral component of early-stage breast cancer treatment because it is effective in both surgery and chemotherapy (8). Radiotherapy has been shown to reduce the risk of local recurrence and is particularly effective as an adjuvant treatment. Chemotherapeutic agents generally include cyclophosphamide and anthracyclines, including either doxorubicin or epirubicin. Finally, in patients who express estrogen- or progesterone-positive receptors, adjuvant hormonal therapy is warranted (9).
HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 AND TRASTUZUMAB
It has been well established that overexpression of the human epidermal growth factor receptor 2 (HER2/ErbB2) family of transmembrane tyrosine kinase receptors is present in numerous forms of carcinoma (10). Approximately 20% to 30% of breast cancer patients overexpress the HER2/ErbB2 receptors (11). It is clinically accepted that this population of breast cancer patients experiences more aggressive tumours, with lower overall survival rates (12,13). Also, both cytotoxic agents and radiotherapy are less effective in this subset of breast cancer patients, because cancer cells that overexpress HER2/ErbB2 exhibit resistance (13,14).
Overexpression of HER2 is detected using either cell-based chromogenic in situ hybridization (CISH) or fluorescence in situ hybridization (FISH) (15,16). Based on positive CISH or FISH results from these immunochemical methods of detection, trastuzumab (Trz) is recommended for treatment of patients with strong HER2/ErbB2 overexpression (11). Trz, a monoclonal antibody against the HER2 receptor, improves survival in breast cancer patients in both the adjuvant and metastatic settings (17). Detection of HER2 overexpression in breast cancer patients yields several clinically useful applications including, but not limited to, predicting prognosis and response to drug regimens including endocrine therapy, tamoxifen, chemotherapeutic drugs and anti-HER2 drugs (18).
The clinical use of Trz varies between adjuvant therapy and its use in metastatic disease (11,19). In the adjuvant setting of breast cancer, for which anthracycline-based compounds are used, Trz is generally administered following completion of chemotherapy, with a loading dose of 8 mg/kg and a weekly maintenance dose of 6 mg/kg for one year (20). The use of Trz in the metastatic setting is administered with loading and maintenance doses of 4 mg/kg and 2 mg/kg, respectively, generally given every three weeks, as reported in several clinical trials (21). As a first-line treatment, Trz has been added to paclitaxel or anthracyclines, along with cyclophosphamide. The use of Trz has also been evaluated in many chemotherapeutic regimens including capecitabine, cisplatin, gemcitabine and vinorelbine among others. In situations in which patients have significant risk factors for cardiotoxicity, Trz has been administered as monotherapy. Once administered, Trz has a mean half-life of 28.5 days; however, the metabolic pathways for eliminating the drug remain undefined (11,19,22).
Adjuvant setting
In the adjuvant setting of breast cancer, Trz continues to be used because it improves patient survival and reduces rates of reccurrence and progression. Trz use has consistently shown a 50% reduction in the rates of breast cancer reccurrence (23). Many clinical trials have evaluated the efficacy of Trz when combined with chemotherapeutic treatment regimens. Four multicentred, randomized controlled trials have studied the use of Trz in the adjuvant setting, including Herceptin Adjuvant (HERA) trial, National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial, North Central Cancer Treatment Group (NCCTG) trial N9831, and the Breast Cancer International Research Group (BCIRG) 006 trial. Findings from these trials revealed that in those patients who had received at least four cycles of a neoadjuvant chemotherapy followed by one year of Trz therapy, in which the maximum cumulative dose of doxorubicin did not exceed 360 mg/m2 and epirubicin did not exceed 720 mg/m2, there was a 34% to 41% reduction in the reccurence of malignancies along with a 34% to 41% improvement in overall disease-free survival (24,25). Overall, these landmark trials have led to a universally accepted practice that the addition of Trz to adjuvant anthracycline- and taxane-based chemotherapy regimens improves patient survival and lowers the risk of breast cancer reccurrence. As described later in the present review, cardiotoxic side effects limit the use of Trz, and it is for this reason, current adjuvant regimens avoid the concurrent use of Trz with anthracycline treatment (26).
Metastatic setting
Ten per cent of breast cancer patients present with metastatic malignancies (19). Approximately 35% to 45% of breast cancer metastatic tumours have been found to overexpress HER2. The primary therapeutic outcome of metastatic breast cancer chemotherapy is palliative. The use of numerous agents have been evaluated, including anthracyclines, capecitabine, docetaxel, gemcitabine, paclitaxel and vinorelbine, which have all been suggested to improve the survival of metastatic breast cancer patients (27). Most recently, however, significant progress has been made in improving the survival outcomes of these patients with the clinical introduction of Trz. Improvements have occurred among patients who had previously had a prognosis of less than three years (27). The addition of Trz to metastatic breast cancer treatment regimens has shown improvements in the progression of breast cancer (19). In a multicentre study (28) of 114 metastatic HER2-positive patients, there was a 26% overall response rate to Trz as a monotherapy, without the previous use of chemotherapy. The concurrent combination of anthracyclines with Trz has been shown to have a significantly greater responsiveness among HER2-positive patients. Stickeler et al (29) showed that 89% of metastatic breast cancer patients overexpressing HER2 responded to therapy versus 39% of HER2-negative patients.
TRZ-MEDIATED CARDIOTOXICITY: CLINICAL WORLD
Despite the therapeutic benefits of Trz, however, there is an increased incidence of cardiotoxicity, particularly when Trz is administered following anthracycline-based chemotherapy. Standard parameters of cardiac dysfunction have been defined by the Cardiac Review and Evaluation Committee on subanalysis of phase III clinical trials. Four general criteria were established to be sufficient to conclude a diagnosis of Trz-related cardiac dysfunction: congestive heart failure (CHF) symptoms; symptoms associated with CHF including an S3 gallop, tachycardia or a combination; and a greater than 5% decline in left ventricular ejection fraction (LVEF) with associated CHF symptoms, or a greater than 10% decline in LVEF without CHF symptoms. The presence of any one of the aforementioned criteria is used to classify Trz-mediated cardiotoxicity (30).
Adjuvant setting
Anthracyclines, in particular doxorubicin, have historically been associated with cardiotoxicity. Common risk factors for developing anthracycline-mediated cardiac dysfunction include a cumulative dose that exceeds 400 mg/m2, age older than 70 years, left chest radiotherapy and a history of hypertension (30–32). Recently, however, it has been shown that the incidence of cardiomyopathy is substantially increased when Trz is used sequentially following chemotherapy drugs, limiting the use of both of these drugs. Despite clinical trials demonstrating an overall prevalence of carditoxicity ranging from 10% to 15% following the use of Trz in the adjuvant setting, real-world studies suggest a higher frequency.
Of four major clinical trials using Trz in the adjuvant setting following anthracycline-based chemotherapy, the incidence of symptomatic CHF was reported to range from 0% to 4% (33,34). Asymptomatic cardiac dysfunction was higher in clinical trials, reported as an incidence of 5% to 10% (35). Furthermore, a review of data from the N9831 and NSABP B-31 (20), HERA (36,37), FinHer (38), BCIRG (19) and PACS-04 trials suggests that 8% to 16% of patients required discontinuation of Trz because of cardiac dysfunction (34). In contrast with the clinical trials, we recently reported that 22% of patients developed left ventricular (LV) systolic dysfunction following the administration of Trz in the adjuvant setting in a real-world setting (35). Approximately one in four breast cancer patients undergoing anthracycline-based chemotherapy with subequent Trz use may require discontinuation of the drug as a result of cardiotoxicity (35).
Metastatic setting
Unfortunately, the prevalence of cardiotoxicity is higher in the metastatic setting of breast cancer because Trz is administered concurrently with anthracycline-based chemotherapy. Metastatic breast cancer trials demonstrate that there is a substantially greater incidence of LV cardiac dysfunction, with a heart failure prevalence of approximately 22%. This is due to the aggressive treatment approach because metastatic patients have a poor prognosis. Treatment is often palliative with the use of paclitaxel following Trz therapy, increasing the incidence of cardiac dysfunction to 27% (19,26,39,40). Despite the high incidence of cardiac complications, the side effects are greatly counterbalanced by the benefits that are otherwise limited for treatment options in the metastatic setting (41).
MECHANISMS
HER2/ErbB2
Before discussing the pathogenesis of Trz-induced cardiomyopathy, it is first necessary to briefly review the progress made in the current understanding of the mechanisms underlying the function of HER2/ErbB2. ErbB receptors are a group of tyrosine kinases belonging to the epidermal growth factor receptor family and are responsible for mediating cell growth, differentiation and survival (42,43). In general, signalling of these receptors involves crosstalk and several signalling events arranged in a complex and interdependent cellular network (43). However, the exact mechanisms and signal pathways remain poorly understood, especially for the ErbB2 isoform (43). Four isoforms are expressed: ErbB1, ErbB2, ErbB3 and ErbB4. Each of these isoforms expresses the ability to act as a co-receptor in neuregulin (NRG) signalling. The transmission of cell survival signals and metabolism alteration have been directly implicated as a function of ErbB2 (44,45). Each of the ErbB family receptors exhibit the ability to couple to a unique intracellular signalling pathway whereby ErbB2 does not directly bind to NRG, but instead heterodimerizes with adjacent ErbB receptors for signalling (46,47).
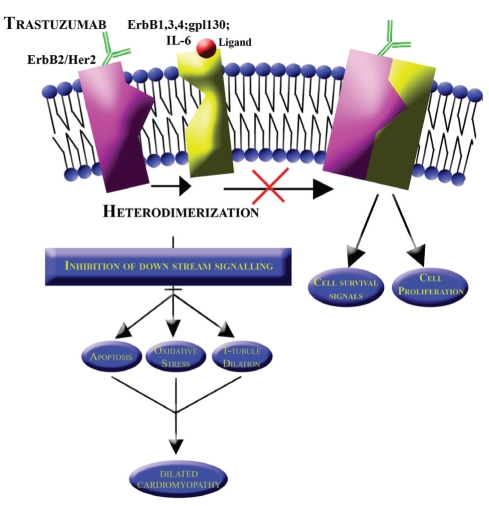
ErbB2 is of specific importance in the setting of breast cancer. This receptor’s extracellular domain is functionally ligand-independent. The receptor is transactivated by signalling G protein-coupled receptors, while it is mediated by intracellular signals (Figure 1). G protein-coupled receptors have a positive effect on ErbB signalling through the activation of matrix metalloproteinases and through the indirect activation of Src (48). On stimulation by a ligand, the receptor heterodimerizes with adjacent ErbB receptors including the cytokine interleukin 6. Because interleukin 6 is a mediator of carcinoma growth, it acts on ErbB2 by inducing tyrosine phosphorylation of ErbB2. In ErbB2 overexpression, this effect results in increased intensity of influence (49). Protein kinase C activating growth factors and hormones counteract this effect by decreasing tyrosine phosphorylation and, hence, the binding affinity of ligands. Intermediate signal mediating sequences of epidermal growth factor receptor are required for ErbB2 activation and transmodulation (50). This mechanism suggests that the overexpression of ErbB2 affects the degree of crosstalk between adjacent receptors and other signalling pathways (48). A study (51) on ErbB2 expression vectors showed that ErbB2 and epidermal growth factor receptors couple with mitogenic signalling pathways. This has direct implications in cellular oxidative stress and apoptotic pathways, which are a key component of Trz-induced cardiomyopathy (Figure 1).
Figure 1).
Normal heterodimerization of the human epidermal growth factor receptor 2 (HER2) with adjacent receptors (ErbB1, ErbB3, ErbB4, gp1130 and IL-6). Trastuzumab binds to HER2, inhibiting downstream signalling with subsequent development of a dilated cardiomyopathy
Trz-mediated cardiomyopathy
Although the pathogenesis of Trz-induced cardiotoxicity remains poorly understood, most investigators agree on the following:
Inhibition of NRG-1/ErbB2 signalling within the heart, as required for G protein-coupled receptor signalling, plays a major role in cardiomyocyte cell death. This is based on the following observations: inhibition or mutation of ErbB2 leads to deterioration of ventricular trabeculations, leading to dilated cardiomyopathy (52,53); blockade of ErbB2 leads to apoptosis via the mitochondrial- and ROS-dependent pathways (47,54); and binding of an anti-ErbB2 antibody reduces ErbB2 activation and downregulates Bcl-xL, while increasing Bcl-xS expression (55).
The addition of Trz to anthracycline treatment regimens significantly increases the cardiotoxic risk. An animal ErbB2 knockout model showed that the sensitivity to anthracyclines was increased in ErbB2-negative cardiac myocytes (47). Although the exact mechanism of this is unknown, several pathways have been implicated. Along with the agreed notion that these effects are synergistic, this combined effect may be attributed to the anthracycline-induced dilation of T-tubules allowing greater access of Trz to ErbB2 within the sarcolemma.
Trz-induced cardiomyopathy is not dose-dependent and is at least partially reversible. The reversibility has been shown in adjuvant clinical trials in which discontinuing the drug with or without concurrent treatment for heart failure has shown recovery of previously diminished LVEF. The mechanism underlying the reversibility itself has scarcely been investigated. It is most conceivable that Trz inhibition of cardiac ErbB2 is only temporary. On discontinuation of its use, the downstream and adjacent pathways resume their normal function. However, as discussed in the present review, the cardiotoxicity likely involves a complex interaction of multiple pathways including apoptotic factors that are together necessary in Trz reversibility (Figure 1).
REVERSIBILITY OF TRZ-MEDIATED CARDIOMYOPATHY
Chemotherapy-related cardiac dysfunction is classified as type I or type II. Type I is characterized by irreversible damage as inflicted by anthracycline and Trz-mediated cardiotoxicity. To some degree, the dysfunction is considered to result from myocyte apoptosis. On the other hand, Trz-related cardiac dysfunction is generally classified as type II, which is a category of reversible cardiomyopathy, following discontinuation of the drug (56). Studies indicate that unlike anthracyclines, Trz-associated cardiotoxicty is not dependant on the cumulative dose administered (57). Following the recovery of cardiac dysfunction via treatment with angiotensin-converting enzyme inhibitors (ACEIs) and beta-blockers, the reintroduction of Trz therapy has been shown to be generally well tolerated (58). However, outside of clinical trials that had stringent screening criteria and short follow-up times, the extent to which Trz-mediated cardiotoxicity is reversible remains undefined (59). Trials (33), such as the HERA trial, suggest that approximately 60% of breast cancer patients with Trz-related diminished LVEF have shown recovery to some extent at six-month follow-up. Real-world studies, on the other hand, indicate that 40% of breast cancer patients show no recovery despite both the withdrawal of Trz and treatment (35). The extent to which this is reversible outside of clinical trials remains ill-defined. One recent study (35), as mentioned above, demonstrated that 22% of adjuvant breast cancer patients recovered from cardiac dysfunction on withdrawal of Trz in a real-world population of 152 patients.
It is clear that a significant predictor of susceptibility to cardiac dysfunction and the potential of reversibility are derived from traditional cardiac risk factors including a family history of coronary artery disease, a history of cardiac disease, smoking and hypertension, and age (35,41). These risk factors predicate prognostic features of increased susceptibility to Trz cardiotoxicity, and are strongly suggested to be included in screening criteria before its use for strategic treatment management (33,35).
PREVENTION
To limit the increasing prevalence of cardiotoxicity associated with Trz, two strategies have been pursued in the clinical setting. The first involves the use of noninvasive cardiac imaging. Current standard preventive strategies use serial multiple-gated acquisition (MUGA) scans for baseline evaluation of LVEF followed by serial monitoring every three months in patients receiving Trz in the adjuvant setting (24). Recently, however, studies (35,60,61) have evaluated the use of other imaging modalities, in particular, echocardiography and cardiac magnetic resonance imaging (MRI) for detecting early myocardial damage due to Trz.
Although the current method of choice for monitoring the cardiac function of patients receiving adjuvant Trz therapy are MUGA scans, more sensitive, early markers of Trz-induced cardiac dysfunction using noninvasive cardiac imaging may allow for a better adaptation of this treatment and a subsequent decrease in cardiac morbidity and mortality. Tissue Doppler imaging (TDI), using echocardiography, is a sensitive, noninvasive echocardiographic technique that allows for measurements of velocity at any point along the ventricular wall during the cardiac cycle. TDI allows for the measurement of maximal systolic endocardial velocity and strain rate. When compared with conventional measures of LVEF, TDI-derived parameters are less influenced by loading conditions, such as the change in intravascular volume that occurs with chemotherapy and Trz therapy. Thus, TDI is a more feasible imaging modality that might provide improved sensitivity in detecting early subclinical LV dysfunction (61).
A recent study (61) evaluated the utility of TDI for the early detection of anthracycline-induced cardiomyopathy, and demonstrated that TDI provides an earlier and more accurate evaluation of cardiac dysfunction during anthracycline therapy in comparison with radionuclide LVEF estimations (MUGA). Similarly, we recently validated the potential application of TDI for the early detection of anthracycline and Trz-mediated cardiac dysfunction in a murine model (61). We demonstrated that TDI was abnormal in mice receiving either doxorubicin or doxorubicin plus Trz as early as 24 h after treatment, and predicted ensuing LV systolic dysfunction with increased mortality.
Improvements in spatial and temporal resolution have made cardiac MRI a powerful tool that has become the gold standard for the noninvasive assessment of LV systolic function. Cardiac MRIs are frequently used in the assessment of dilated cardiomyopathies secondary to ischemia and myocarditis. Of interest is the utility of cardiac MRI in the assessment of chemotherapy-induced cardiomyopathy. Recently, it was demonstrated that delayed enhancement imaging using cardiac MRI was positive in assessing for scar and fibrosis of the lateral portion of the LV wall in Trz-induced cardiomyopathy (60). Use of delayed enhancement imaging as an early predictor of LV dysfunction remains unexplored in this patient population and needs further study (35).
Currently, dexrazoxane is used as a cardioprotective agent against anthracycline cardiotoxicity. Dexrazoxane is prescribed in the metastatic setting as a free-radical scavenger in patients who have exceeded a cumulative doxorubicin dose of 300 mg/m2 (62). Although dexrazoxane has been shown to be highly effective in preventing cardiac dysfunction in the metastatic setting, there is concern that it may reduce the efficacy of chemotherapy (63). There have been conflicting results with phase III clinical trials, in which one study concluded that the antitumour efficacy of doxorubicin is reduced when dexrazoxane is used, whereas other trials suggest otherwise (63).
ACEIs, angiotensin receptor blockers (ARBs) and betareceptor antagonists (beta-blockers) incur a standard use in the setting of heart failure treatment and specifically in asymptomatic LV dysfunction (64,65). The use of ACEI has been shown to prolong survival in heart failure patients (66).
Similarly, in the setting of chemotherapy-related CHF, ACEIs, ARBs and beta-blockers are widely used in many combinations for the treatment of asymptomatic and symptomatic CHF. Clinically, in the treatment of CHF, including anthracycline-induced cardiomyopathy, ACEIs and ARBs are proven class I medications as per the American College of Cardiology/American Heart Association and Canadian Cardiovascular Society guidelines (42,43). Furthermore, ACE inhibition has been shown to attenuate symptomatic CHF as a result of anthracycline-mediated cardiotoxicity (67,68). ACEIs have been implicated in the prevention of LVEF drops associated with high-dose chemotherapy (69). Recent small trials (44) have proposed the prophylactic use of ACEIs for the prevention of doxorubicin-mediated cardiomyopathy in breast cancer patients. The prophylactic administration of ACEIs, including zofenopril and lisinopril, has demonstrated cardioprotective effects in this clinical setting, likely from their free radical scavenging and antioxidant properties. The nonselective beta-blocker carvedilol has proven efficacy in preventing doxorubicin-associated cardiomyopathy (70,71). The potential use of ACEIs and beta-blockers for their cardioprotective effect in the setting of Trz-mediated cardiac dysfunction remains ill-defined.
CONCLUSION
The mechanisms underlying Tratuzumab-associated cardiotoxicity in both the adjuvant and metastatic settings remain ill-defined and necessitate future investigation. Cardioprotective drugs, such as antioxidants, ACEIs and beta-blockers that mitigate reactive oxidative stress pathways and reduce cardiac stress, may prevent the onset of cardiotoxicity and optimize treatment outcomes for breast cancer patients receiving Trz.
Acknowledgments
Mr Jonathan R Walker was supported by the St Boniface General Hospital and Research Foundation, and by the Manitoba Health Research Council. Dr Pawan K Singal is the holder of the Naranjan S Dhalla Chair in Cardiovascular Research, and is supported by the St Boniface Hospital and Research Foundation. Dr Davinder S Jassal holds the FW DuVal Clinical Research Professorship and the Heart and Stroke Foundation of Canada New Investigator Award.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J. A new anti-ErbB2 strategy in the treatment of cancer: Prevention of ligand-dependent ErbB2 receptor heterodimerization. Cancer Cell. 2002;2:93–5. doi: 10.1016/s1535-6108(02)00098-3. [DOI] [PubMed] [Google Scholar]
- 3.Marrett LD, De P, Airia P, Dryer D, Steering Committee of Canadian Cancer Statistics 2008 Cancer in Canada in 2008. CMAJ. 2008;179:1163–70. doi: 10.1503/cmaj.080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasche B. Recent advances in breast cancer genetics. In: Gradishar WJ, Wood WC, editors. Advances in Breast Cancer Management. Second Edition. New York: Springer; 2008. pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalloo F, Varley J, Ellis D, et al. Early Onset Breast Cancer Study Group Prediction of pathogenic mutations in patients with early-onset breast cancer by family history. Lancet. 2003;361:1101–2. doi: 10.1016/S0140-6736(03)12856-5. [DOI] [PubMed] [Google Scholar]
- 6.Hazard HW, Gorla SR, Scholtens D, Kiel K, Gradishar WJ, Khan SA. Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer. 2008;113:2011–9. doi: 10.1002/cncr.23870. [DOI] [PubMed] [Google Scholar]
- 7.Hazard HW, Hansen NM. Sentinel lymphadenectomy in breast cancer. Cancer Treat Res. 2008;141:11–36. doi: 10.1007/978-0-387-73161-2_2. [DOI] [PubMed] [Google Scholar]
- 8.Taghian AG, Powell SN. The role of radiation therapy for primary breast cancer. Surg Clin North Am. 1999;79:1091–115. doi: 10.1016/s0039-6109(05)70063-3. [DOI] [PubMed] [Google Scholar]
- 9.Olver IN. Trastuzumab as the lead monoclonal antibody in advanced breast cancer: Choosing which patient and when. Future Oncol. 2008;4:125–31. doi: 10.2217/14796694.4.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Pegram M, Slamon D. Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. Semin Oncol. 2000;27:13–9. [PubMed] [Google Scholar]
- 11.Plosker GL, Keam SJ. Trastuzumab: A review of its use in the management of HER2-positive metastatic and early-stage breast cancer. Drugs. 2006;66:449–75. doi: 10.2165/00003495-200666040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60:3384–8. [PubMed] [Google Scholar]
- 13.Halyard MY, Pisansky TM, Dueck AC, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: Tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009;27:2638–44. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–21. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 15.Xing WR, Gilchrist KW, Harris CP, Samson W, Meisner LF. FISH detection of HER-2/neu oncogene amplification in early onset breast cancer. Breast Cancer Res Treat. 1996;39:203–12. doi: 10.1007/BF01806187. [DOI] [PubMed] [Google Scholar]
- 16.Press MF, Slamon DJ, Flom KJ, Park J, Zhou JY, Bernstein L. Evaluation of HER-2/neu gene amplification and overexpression: Comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol. 2002;20:3095–105. doi: 10.1200/JCO.2002.09.094. [DOI] [PubMed] [Google Scholar]
- 17.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14:437–44. doi: 10.1016/j.cardfail.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Harris LA, Yu Y, Donato B. Prescription patterns for metastatic breast cancer patients previously treated with anthracycline and taxane: An assessment in a United States managed care database. Am J Clin Oncol (Meeting Abstracts) 2007;25:17056. [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 21.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. New Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 22.McKeage K, Perry CM. Trastuzumab: A review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs. 2002;62:209–43. doi: 10.2165/00003495-200262010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–33. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 24.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 25.Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer. 2008;8:324–33. doi: 10.3816/CBC.2008.n.037. [DOI] [PubMed] [Google Scholar]
- 26.Rayson D, Richel D, Chia S, Jackisch C, van der Vegt S, Suter T. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: Current experience and future strategies. Ann Oncol. 2008;19:1530–9. doi: 10.1093/annonc/mdn292. [DOI] [PubMed] [Google Scholar]
- 27.Olin JJ, Muss HB. New strategies for managing metastatic breast cancer. Oncology (Williston Park) 2000;14:629–41. [PubMed] [Google Scholar]
- 28.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 29.Stickeler E, Klar M, Watermann D, et al. Pegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: A multicenter phase II trial. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-008-0306-9. [DOI] [PubMed] [Google Scholar]
- 30.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 31.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: Course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 32.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. New Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 33.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31:459–67. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 34.Guarneri V, Frassoldati A, Bruzzi P, et al. Multicentric, randomized phase III trial of two different adjuvant chemotherapy regimens plus three versus twelve months of trastuzumab in patients with HER2-positive breast cancer (Short-HER Trial; NCT00629278) Clin Breast Cancer. 2008;8:453–6. doi: 10.3816/CBC.2008.n.056. [DOI] [PubMed] [Google Scholar]
- 35.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: A retrospective study. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 36.Smith I, Procter M, Gelber RD, et al. HERA study team 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 37.Yaal-Hahoshen N, Safra T. Herceptin (trastuzumab): Adjuvant and neoadjuvant trials. Isr Med Assoc J. 2006;8:416–21. [PubMed] [Google Scholar]
- 38.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. FinHer Study Investigators Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 39.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–65. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 40.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–83. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–15. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 42.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: Critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–9. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 43.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(Suppl 12):60–70. [PubMed] [Google Scholar]
- 44.Chien KR. Myocyte survival pathways and cardiomyopathy: Implications for trastuzumab cardiotoxicity. Semin Oncol. 2000;27:9–14. [PubMed] [Google Scholar]
- 45.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14:437–44. doi: 10.1016/j.cardfail.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–55. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 50.Worthylake R, Wiley HS. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997;272:8594–601. doi: 10.1074/jbc.272.13.8594. [DOI] [PubMed] [Google Scholar]
- 51.Di Fiore PP, Segatto O, Taylor WG, Aaronson SA, Pierce JH. EGF receptor and erbB-2 tyrosine kinase domains confer cell specificity for mitogenic signaling. Science. 1990;248:79–83. doi: 10.1126/science.2181668. [DOI] [PubMed] [Google Scholar]
- 52.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 53.Ozcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–5. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon LI, Burke MA, Singh ATK, et al. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. 2009;284:2080–7. doi: 10.1074/jbc.M804570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grazette LP, Boecker W, Matsui T, et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: Implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231–8. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 56.Jones AL, Barlow M, Barrett-Lee PJ, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: Updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684–92. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 58.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 59.de Azambuja E, Bedard P, Suter T, Piccart-Gebhart M. Cardiac toxicity with anti-HER-2 therapies: What have we learned so far? Targeted Oncol. 2009;4:77–88. doi: 10.1007/s11523-009-0112-2. [DOI] [PubMed] [Google Scholar]
- 60.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jassal DS, Han S-Y, Hans C, et al. Utility of tissue doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009;22:418–24. doi: 10.1016/j.echo.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15:1333–40. doi: 10.1200/JCO.1997.15.4.1333. [DOI] [PubMed] [Google Scholar]
- 63.Cvetković RS, Scott LJ. Dexrazoxane : A review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65:1005–24. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- 64.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. New Engl J Med. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 65.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 66.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. New Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 67.Jensen BV, Nielsen SL, Jensen TS. Angiotensin-converting enzyme inhibitor in the treatment of epirubicin-induced dilated cardiomyopathy. Ugeskr Laeger. 1997;159:1945–9. [PubMed] [Google Scholar]
- 68.Jensen BV, Nielsen SL, Skovsgaard T. Treatment with angiotensin-converting-enzyme inhibitor for epirubicin-induced dilated cardiomyopathy. Lancet. 1996;347:297–9. doi: 10.1016/s0140-6736(96)90469-9. [DOI] [PubMed] [Google Scholar]
- 69.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 70.Machado V, Cabral A, Monteiro P, Goncalves L, Providencia LA. Carvedilol as a protector against the cardiotoxicity induced by anthracyclines (doxorubicin) Rev Port Cardiol. 2008;27:1277–96. [PubMed] [Google Scholar]
- 71.Armstrong SC. Anti-oxidants and apoptosis: Attenuation of doxorubicin induced cardiomyopathy by carvedilol. J Mol Cell Cardiol. 2004;37:817–21. doi: 10.1016/j.yjmcc.2004.07.001. [DOI] [PubMed] [Google Scholar]