Abstract
OBJECTIVE:
The development of diastolic dysfunction (DDF) is multifactorial. Possible mechanisms include metabolic disturbances, myocardial fibrosis, chronic inflammation and endothelial dysfunction. Recognizing early stages of DDF may help to identify patients at risk of developing symptomatic DDF. Therefore, biomarkers reflecting pathophysiological changes within the myocardium were investigated in patients with DDF.
METHODS:
Seventy-seven patients submitted for coronary angiography with stable or suspected coronary artery disease (CAD) were consecutively enrolled. Those without known diabetes mellitus (DM) underwent a standardized oral glucose tolerance test. Echocardiography for the diagnosis of DDF was performed according to the European Society of Cardiology. Matrix metalloproteinase 2 (MMP-2) and soluble P-selectin (sP-selectin) serum concentrations were analyzed using the ELISA technique.
RESULTS:
A total of 36% of patients had DM and 74% had CAD. The prevalence of DDF was higher in patients with DM (89% versus 74%) and CAD (84% versus 53%) (P<0.05). DDF in patients with DM was more severe with a significantly lower mitral annulus velocity of 6.5 cm/s versus 7.8 cm/s (P<0.01). Patients with DDF showed significantly higher sP-selectin (140.3 μg/L versus 107.6 μg/L, P<0.05) and MMP-2 (270.5 μg/L versus 224.7 μg/L, P<0.05) levels compared with those without DDF. There was a significant correlation between sP-selectin and MMP-2 (P=0.01), independent of the diagnosis of DM or CAD.
CONCLUSION:
sP-selectin as a marker for platelet hyperactivity, inflammation and endothelial dysfunction, and MMP-2 as a marker for extracellular matrix turnover were significantly elevated in patients with DDF. This elevation was independent of coexisting DM or CAD. This observation may help to identify and monitor patients with DDF.
Keywords: Biomarkers, Coronary artery disease, Diabetes mellitus, Diastolic dysfunction, Glucose metabolism, Heart failure
Diastolic dysfunction (DDF) refers to the presence of abnormalities in relaxation and compliance of the left ventricle. Diastolic and systolic heart failure (HF) is similarly prevalent in the community (1,2), and both are associated with increased morbidity and mortality (2–4). DDF may occur with or without clinical signs of HF. If HF symptoms are present, the condition is called diastolic HF (DHF), although systolic left ventricular (LV) function is preserved. DDF without symptoms of HF is considered to be a precursor to the development of symptomatic DHF.
Known risk factors for the prevalence of DDF are diabetes mellitus (DM) (5), coronary artery disease (CAD), obesity, hypertension, age and female sex. These risk factors are also strongly associated with the development of systolic HF.
Identifying and monitoring systolic HF becomes increasingly popular in routine clinical practice with N-terminal probrain natriuretic peptide (NT-proBNP), a biomarker that reflects pathophysiological changes in systolic HF (6). NT-proBNP is also a marker for symptomatic DHF (7) but, so far, no marker has been established for identifiying and monitoring asymptomatic DDF.
Previous studies support the hypothesis that a change in the extracellular matrix (ECM) of the myocardium, characterized by the formation of excess collagen tissue, is a major cause of worsening DHF (8,9). Furthermore, DHF can be a consequence of repeated episodes of myocardial damage resulting from both structural and functional abnormalities in small vessels or from microvascular functional abnormalities (endothelial dysfunction) (10). Soluble P-selectin (sP-selectin) as a marker for platelet hyperactivity, inflammation and endothelial dysfunction, and matrix metalloproteinase 2 (MMP-2) as a marker for ECM turnover may be suitable target biomarkers because they represent two different mechanisms in the suspected pathogenesis of myocardial remodelling. The aim of the present study was to investigate an association between these parameters and the presence of DDF adjusted for established risk factors.
METHODS
Seventy-seven patients submitted for coronary angiography with stable or suspected CAD were consecutively enrolled in the present study. The protocol was approved by the local ethics committee, and signed informed consent was obtained from all patients. Those without a history of DM were examined with a standardized oral glucose tolerance test and classified according to current World Health Organization criteria. Exclusion criteria were significant valvular heart disease, persistent or chronic atrial fibrillation, obstructive or restrictive cardiomyopathy, pregnancy and pre-existing significant pulmonary disease, creatinine levels greater than 2.5 μmol/L, and active liver disease.
Venous blood samples were taken, centrifuged, divided into aliquots and stored at −80°C until use. MMP-2 and sP-selectin serum concentrations were analyzed using a commercially available ELISA kit (R&D Systems, USA).
Clinical, routine laboratory and invasive data of the patients were prospectively stored in a database (SPSS Inc, USA). All patients underwent cardiac catheterization. Conventional transmitral flow was measured with pulsed wave Doppler. Early (E) and atrial (A) transmitral peak flow velocities were measured, along with their ratio (E/A). Pulsed wave tissue Doppler imaging was performed at the junction of the LV wall with the mitral annulus. Early diastolic mitral annulus velocity (E′) was recorded. The ratio of E/E′ was calculated. DDF was defined as an inverse E/A ratio of less than 0.5 (when the patient is older than 50 years of age) or less than 1 (when the patient is younger than 50 years; impaired LV relaxation), or an E/A ratio greater than 1.5 and E′ less than 8 cm/s with an E/E′ ratio greater than 15 (pseudonormal pattern).
Statistical analysis was performed with SPSS. The results were expressed as mean ± SD. Unpaired t test and ANOVA analysis were used for continuous variables and the paired t test was used for within-group comparison. Values of P<0.05 were considered statistically significant. Correlation was calculated using the Spearman’s rho correlation coefficient.
RESULTS
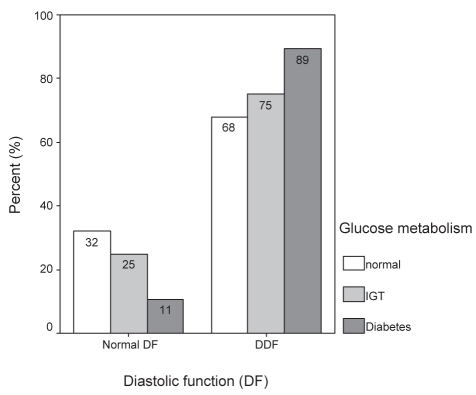
Seventy-seven patients were enrolled in the present study (clinical characteristics are shown in Table 1). A total of 36% had DM and 74% had CAD. A total of 31% had impaired glucose tolerance (IGT) and 33% of enrolled subjects showed normal glucose metabolism. The prevalence of diastolic abnormalities was 68%, 75% and 89% in those with normal glucose metabolism, IGT and DM, respectively (Figure 1).
TABLE 1.
Demographics and clinical characteristics of study patients
| Mean age, years | Female sex | HT | DM | CAD | HbA1c | Mean EF | BMI, kg/m2 | |
|---|---|---|---|---|---|---|---|---|
| DDF | 58* | 23 | 88 | 42 | 80* | 6.4 | 68 | 29.2* |
| Normal DF | 52 | 41 | 82 | 18 | 63 | 6.2 | 68 | 26.3* |
Data presented as percentages unless otherwise indicated.
Significant P<0.05. BMI Body mass index; CAD Coronary artery disease; DDF Diastolic dysfunction; DF Diastolic function; DM Diabetes mellitus; EF Ejection fraction; HbA1c Glycosylated hemoglobin; HT Hypertension
Figure 1).
Prevalence of diastolic dysfunction (DDF) in different stages of glucose metabolism disorders. IGT Impaired glucose tolerance
Prevalence of DDF was significantly higher in patients with DM (89% versus 74%) and CAD (84% versus 53%) (P<0.05) compared with those without DM (including IGT) and with exclusion of CAD, respectively.
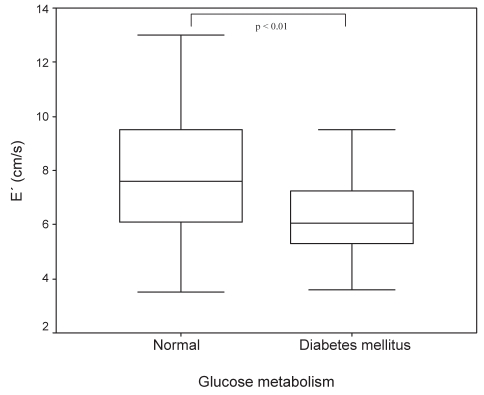
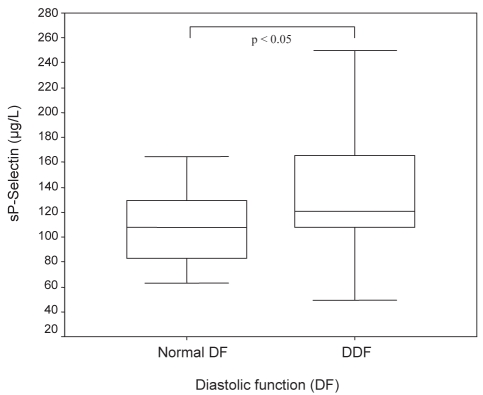
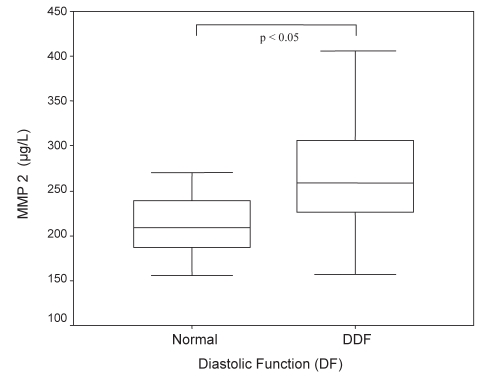
DDF in patients with DM was more severe, with a significantly lower E′ of 6.5 cm/s versus 7.8 cm/s (P<0.01; Figure 2). Patients with DDF showed significantly higher sP-selectin (140.3 μg/L versus 107.6 μg/L, P<0.05; Figure 3) and MMP-2 (270.5 μg/L versus 224.7 μg/L, P<0.05; Figure 4) serum concentrations compared with those without evidence of DDF. There was a significant correlation between sP-selectin and MMP-2 (Spearman’s rho = 0.306, P=0.01). This elevation was independent of the diagnosis of either DM or CAD. Mean LV function and NT-proBNP levels were similar in subjects with and without DDF.
Figure 2).
Early diastolic mitral annulus velocity (E′) in patients with diabetes mellitus and normal glucose metabolism
Figure 3).
Soluble P-selectin (sP-selectin) levels in patients with normal diastolic function and diastolic dysfunction (DDF)
Figure 4).
Matrix metalloproteinase 2 (MMP-2) levels in patients with normal diastolic function and diastolic dysfunction (DDF)
DISCUSSION
In the present study, we identified a high percentage of patients with DDF apparent on echocardiography. Also, patients with DDF had a high prevalence of DM and CAD. sP-selectin as a marker for platelet hyperactivity, inflammation and endothelial dysfunction, and MMP-2 levels as a marker for ECM turnover were significantly elevated in patients with DDF.
How do these findings fit into the current hypothesis of the development of DDF?
It is generally accepted that a major problem in DHF is a stiff ventricle. It is important to emphasize that, in our study, patients with DDF had no clinical evidence of HF and no differences in NT-proBNP levels compared with those without DDF. Although there is evidence for the contribution of DDF to DHF, the pathogenesis of this transition remains to be understood. Previous studies have shown that myocardial interstitial fibrosis is one of the key pathological features of myocardial remodelling (11–13). Analyzing serum enzymes involved in collagen degradation provides a verified, noninvasive technique to measure the fibrotic process (9,14).
In the present study, we were able to demonstrate that MMP-2 serum levels are significantly elevated in patients with evidence of DDF. This was independent of DM, CAD, hypertension and ejection fraction.
These findings provide further information on collagen metabolism in patients with asymptomatic DDF. Elevated MMP-2 levels may reflect active ECM metabolism. DM or CAD per se were not related to increased MMP-2 concentrations. The elevation of MMP-2 is consistent with previous studies in patients with hypertensive heart disease and obese subjects (15,16). Because of the fact that MMPs are known to be responsible for breakdown of collagen, these findings may seem counterproductive for the development of myocardial fibrosis. Nevertheless, in previous studies, elevated MMP-2 levels have also been associated with profibrotic remodelling (17). MMP-2 activation may lead to alterations in the collagen interface, both in structure and composition, resulting in a loss of normal structural support. To counterbalance this process, ‘overshoot’ and disorganized collagen disposition may occur in later stages, causing the progression from DDF to symptomatic DHF.
Elevated plasma levels of various cellular adhesion molecules, along with other markers of endothelial dysfunction, have been reported in patients with systolic HF (18,19). In the present study, we were also able to show a positive significant correlation between sP-selectin and MMP-2 levels. This may reflect interaction between platelet activation (20), inflammation and ECM turnover. P-selectin mediates platelet-leukocyte interactions with stimulated endothelium, allowing tethering of leukocytes by activated platelets with consecutive secretion of proinflammatory and vasoactive substances (21). These proinflammatory mediators may act as local ‘players’ in the myocardium by activating MMPs and tissue inhibitors of metalloproteinases; thus, they play a role in ECM turnover and amplify the development of DDF. Therefore, the assessment of sP-selectin may be a useful clinical predictor of a pathophysiological cascade leading to diastolic abnormalities.
There are some limitations of the present study that need to be considered. First, patients with DDF were older than those with normal diastolic function. This may have an impact on myocardial remodelling processes. We did not correct for lifestyle factors such as smoking, since smoking is known to influence endothelial function. The main limitation of the study may be the lack of myocardial biopsies to directly link circulating and tissue concentrations of MMPs and sP-selectin, but obtaining ventricular muscle biopsies in asymptomatic patients with CAD is ethically difficult.
CONCLUSION
To our best knowledge, the present study is the first to describe an association between sP-selectin and MMP-2 levels in patients with DDF. This may be interesting in different ways; even mild impairment of diastolic function was associated with an eightfold increased risk of all-cause mortality compared with normal diastolic function (2) and despite growing awareness of the burden of DDF, there have been few randomized clinical trials of drug therapies for these patients. Thus, biomarkers that identify DDF at a very early stage could be very helpful to stratify patients into a high-risk group for symptomatic HF. On the other hand, biomarkers of asymptomatic DDF could be very helpful to monitor the course and influence of potential drug therapies. Furthermore, the association of these two biomarkers may lead to a better understanding of the pathophysiology of DDF and will consequently provide a rationale for the development of plausible therapy strategies for the disorder. Our observations may be one important step to identify and monitor patients with DDF.
Footnotes
CONFLICT OF INTEREST: Reiner Füth, none declared; Wilfried Dinh, none declared; Werner Nickl, none declared; Lars Bansemir, none declared; Michael Coll Barroso, none declared; Alexander Bufe, none declared; Armin Sause, none declared; Thomas Scheffold, none declared; Thomas Krahn, none declared; Peter Ellinghaus, none declared; Mark Lankisch, none declared.
REFERENCES
- 1.Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–8. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Piccini JP, Klein L, Gheorghiade M, Bonow RO. New insights into diastolic heart failure: Role of diabetes mellitus. Am J Med. 2004;116(Suppl 5A):64S–75S. doi: 10.1016/j.amjmed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Olsson LG, Swedberg K, Cleland JG, et al. Prognostic importance of plasma NT-pro BNP in chronic heart failure in patients treated with a beta-blocker: Results from the Carvedilol Or Metoprolol European Trial (COMET) trial. Eur J Heart Fail. 2007;9:795–801. doi: 10.1016/j.ejheart.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 8.Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–95. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: Relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 10.Yngen M, Ostenson CG, Hu H, Li N, Hjemdahl P, Wallen NH. Enhanced P-selectin expression and increased soluble CD40 Ligand in patients with Type 1 diabetes mellitus and microangiopathy: Evidence for platelet hyperactivity and chronic inflammation. Diabetologia. 2004;47:537–40. doi: 10.1007/s00125-004-1352-4. [DOI] [PubMed] [Google Scholar]
- 11.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–41. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 12.Ciulla M, Paliotti R, Hess DB, et al. Echocardiographic patterns of myocardial fibrosis in hypertensive patients: Endomyocardial biopsy versus ultrasonic tissue characterization. J Am Soc Echocardiogr. 1997;10:657–64. doi: 10.1016/s0894-7317(97)70028-2. [DOI] [PubMed] [Google Scholar]
- 13.Dinh W, Bansemir L, Füth R, et al. Increased levels of laminin and collagen type VI may reflect early remodelling in patients with acute myocardial infarction. Acta Cardiol. 2009;64:329–34. doi: 10.2143/AC.64.3.2038017. [DOI] [PubMed] [Google Scholar]
- 14.Alla F, Kearney-Schwartz A, Radauceanu A, Das Dores S, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur J Heart Fail. 2006;8:147–53. doi: 10.1016/j.ejheart.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Derosa G, D’Angelo A, Ciccarelli L, et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;3:227–31. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 16.Derosa G, Ferrari I, D’Angelo A, et al. Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium. 2008;15:219–24. doi: 10.1080/10623320802228815. [DOI] [PubMed] [Google Scholar]
- 17.Matsusaka H, Ide T, Matsushima S, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47:711–7. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 18.Devaux B, Scholz D, Hirche A, Klovekorn WP, Schaper J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J. 1997;18:470–9. doi: 10.1093/oxfordjournals.eurheartj.a015268. [DOI] [PubMed] [Google Scholar]
- 19.Yin WH, Chen JW, Jen HL, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003;5:507–16. doi: 10.1016/s1388-9842(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 20.Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci U S A. 2000;97:13835–40. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bienvenu JG, Tanguay JF, Chauvet P, Merhi Y. Relationship between platelets and neutrophil adhesion and neointimal growth after repeated arterial wall injury induced by angioplasty in pigs. J Vasc Res. 2001;38:153–62. doi: 10.1159/000051042. [DOI] [PubMed] [Google Scholar]