Abstract
OBJECTIVE
We sought to prospectively examine whether childbearing is associated with higher incidence of the metabolic syndrome (MetS) after delivery among women of reproductive age.
STUDY DESIGN
In 1451 nulliparas who were aged 18–30 years and free of the MetS at baseline (1985–1986) and reexamined up to 4 times during 20 years, we ascertained incident MetS defined by the National Cholesterol Education Program Adult Treatment Panel III criteria among time-dependent interim birth groups by gestational diabetes mellitus (GDM): (0 [referent], 1 non-GDM, 2 + non-GDM, 1 + GDM births). Complementary log-log models estimated relative hazards of the MetS among birth groups adjusted for race, age, and baseline and follow-up covariates.
RESULTS
We identified 259 incident MetS cases in 25,246 person-years (10.3/1000 person-years). Compared with 0 births, adjusted relative hazards (95% confidence interval [CI]) were 1.33 (95% CI, 0.93–1.90) for 1 non-GDM, 1.62 (95% CI, 1.16–2.26) for 2 + non-GDM (P trend = .02), and 2.43 (95% CI, 1.53–3.86) for 1 + GDM births.
CONCLUSION
Increasing parity is associated with future development of the MetS independent of prior obesity and pregnancy-related weight gain. Risk varies by GDM status.
Keywords: gestational diabetes mellitus, incidence, longitudinal, metabolic syndrome, parity, women’s health
The metabolic syndrome (MetS) is a clustering of metabolic risk factors, including abdominal obesity, atherogenic dyslipidemia (high triglycerides [TG] and low high-density lipoprotein cholesterol [HDL-C]), raised blood pressure, insulin resistance (glucose intolerance), and proinflammatory and prothrombotic states.1–3 Although the MetS is a multiplex risk factor for cardiovascular disease (CVD),1 individuals with this syndrome who have insulin resistance are also at greater risk for type 2 diabetes mellitus.3,4
Physiologic adaptations during healthy pregnancy include marked insulin resistance, atherogenic dyslipidemia, fat accretion, and inflammation.5–7 Although these pregnancy manifestations largely reverse after parturition, lower HDL-C levels, weight gain, and greater abdominal obesity may persist years after delivery.8–11 Pregnancy has lasting adverse physiologic effects and may result in behavioral changes (ie, physical activity), but studies have rarely provided longitudinal evidence directly linking pregnancy-related risk factor changes to disease onset.
Lifetime parity assessed retrospectively has been associated either directly or in a J-shaped pattern with CVD and diabetes12–15 but not in all studies.16–21 In cross-sectional studies, parity showed a graded direct association with prevalence of the MetS later in life22,23 but not independent of obesity.23 In 2 studies, a history of gestational diabetes mellitus (GDM), a strong predictor of type 2 diabetes mellitus,24 was associated with a 2-to 4-fold higher prevalence of the MetS after delivery vs no history of GDM.25,26 Significant limitations of this evidence include the lack of preconception measurements to establish the temporality of pregnancy prior to changes in risk factors that lead to disease and the inability to assess reverse causation by infertility secondary to insulin resistance and obesity- related disorders (eg, polycystic ovarian syndrome). Confounding from both sources would likely bias estimates toward the null. Moreover, previous studies have rarely ascertained history of GDM, which may inflate risk estimates for women in general. One study that prospectively collected reproductive data, including GDM status and controlled for preconception glycemia, the Coronary Artery Risk Development in Young Adults (CARDIA), found a 4-fold higher incidence of type 2 diabetes mellitus for women who developed GDM but no association for women without GDM pregnancy vs nulliparas.21
Given the paucity of evidence, we examined the hypothesis that GDM and non-GDM pregnancies were associated with higher incidence of the MetS during 20 years among premenopausal nulliparas aged 18–30 years at baseline. We controlled for potential confounding by preconception cardiometabolic measures, secular trends, aging, sociodemographics, and behaviors, and examined weight gain and physical activity changes as mediators.
MATERIALS AND METHODS
The CARDIA study is a population-based, multicenter, longitudinal, observational study examining the development of risk for coronary heart disease (CHD) in young black and white men and women.27,28 In 1985–1986, a total of 5115 subjects (2787 women) aged 18–30 years (52% black) were recruited from 4 geographic areas in the United States: Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Retention rates at follow-up examinations 7, 10, 15, and 20 years later (2005–2006) were 81%, 79%, 74%, and 72% of the surviving cohort, respectively.29,30 Institutional review boards at each participating study center approved the study. Written informed consent was obtained from subjects.
Sample selection criteria
Of 2787 women, we excluded 1008 parous at baseline, because our previous analyses showed that HDL-C declines were associated with primiparity,9 as well as women currently pregnant or breastfeeding (n = 11), pregnant within 3 months (n = 3), who had a hysterectomy or removal of both ovaries (n = 4), or type 1 diabetes mellitus (n = 4) at baseline. Finally, we excluded women with the MetS by the National Cholesterol Education Program Adult Treatment Panel III criteria at baseline (n = 92), missing MetS components at baseline and/or all follow-up examinations in the nonpregnant or nonlactating state (n = 208), or missing covariates at baseline (n = 6). The analysis included 1451 women (568 black, 883 white). All 5 MetS component measurements were available for 1451 (100%) at baseline, 593 (41%) at all 4 follow-up examinations, and 444 (31%) at 3 of 4 follow-up examinations. The analytic sample tended to be white (60%), nonsmokers, younger, more educated, had lower body mass index (BMI), and smaller waist girth than women excluded.
Data collection
Components of the MetS
Venous blood samples were drawn in the morning after an overnight fast of ≥ 8 hours using a vacutainer tube containing EDTA.31 Procedures for the collection and storage of plasma samples, as well as laboratory quality-control procedures and methodology to assess plasma TG, HDL-C, low-density lipoprotein cholesterol, total cholesterol, and glucose, have been reported.31,32
After an initial 5-minute rest, technicians measured blood pressure 3 times at 1-minute intervals using the Hawksley random-zero sphygmomanometer (Lansing, Sussex, UK) through year 15 and an Omron oscillometer (HEM907XL; Omron Corp., Schaumburg, IL) at year 20; the first and fifth phase Korotkoff sounds were recorded.28 The second and third measurements were averaged. The protocol specified the appropriate cuff size based on the upper arm circumference, which was measured at the midpoint between the acromion and the olecranon.
Anthropometry
Certified technicians measured weight, height, and waist circumference according to a standardized protocol.33 Body-weight was measured to the nearest 0.1 kg using a calibrated balance beam scale in participants wearing light clothing. We measured height (without shoes) to the nearest 0.5 cm using a vertical ruler and waist circumference to the nearest 0.5cmat the minimal abdominal girth.10 BMI was computed as weight in kilo-grams divided by squared height in meters. Weight gain during follow-up was calculated for each time interval by subtraction of the baseline weight.
Definition of incident MetS case
Incident cases of MetS were identified at follow-up examinations in years 7, 10, 15, and 20 using the National Cholesterol Education Program Adult Treatment Panel III criteria for women.34 Diagnosis of MetS was based on the presence of 3 of 5 of the components (waist girth > 88 cm, fasting TG ≥ 150 mg/dL, HDL-C < 50 mg/dL, blood pressure ≥ 130 or ≥ 85 mm Hg and/or treatment with antihypertensive medication, and fasting glucose ≥ 100 mg/dL and/or treatment with diabetes medication).
Main effect time-dependent interim birth groups
At each examination, women reported the number of pregnancies, including abortions, miscarriages, and live births or stillbirths; length of gestation; delivery dates since the last examination; and whether they were currently pregnant or breastfeeding. We classified pregnancies (gravidity) ending in miscarriages, abortions, and/or those < 20 weeks’ gestation as pregnancy losses and ≥ 20 weeks as births. Nulliparity was defined as no live births. At each examination, women also reported ever having diabetes and if they had diabetes only during pregnancy. GDM status was assigned for each interim pregnancy based on self-report and absence of overt diabetes before conception. 21 We classified women into time-dependent interim birth groups (ie, women transitioned into groups as births occurred after baseline) based on the cumulative number of births and GDM status since baseline at CARDIA examinations in years 7, 10, 15, and 20. Woman were categorized into 1 of 4 groups at the end of each time interval: 0 births (referent), 1 non-GDM birth, ≥ 2 (2+) non-GDM births, and ≥ 1 (1+) GDM births. Group assignments continued into subsequent examinations unless new births occurred since the last examination. For example, a woman reporting 1 non-GDM birth between baseline and year 7 and another non-GDM birth between years 7 and 10 would be classified as 1 non-GDM birth at year 7 and 2 + non-GDM births at year 10. If no further births were reported, her group assignment would remain 2 + non-GDM births until the end of follow-up. Once classified as having a GDM pregnancy, a woman would remain in the 1 + GDM birth group until the end of the follow-up regardless of GDM status for subsequent pregnancies. We validated self-report of GDM for a subset of 165 women by abstracting medical record data for 200 births between baseline and year 10.21 Sensitivity for classification by self-report as ever having GDM was 100% (20/20), and specificity was 92% (134/145).
Other covariates
Sociodemographic, medical, and behavioral data (medical history, medications [insulin, oral hypoglycemics, antihypertensives], alcohol intake [mL/day], cigarette smoking, education, marital status, oral contraceptive [OC] use, physical activity) were collected at each examination using self- and interviewer-administered questionnaires. A trained interviewer assessed dietary intake during the previous month using the CARDIA dietary history at baseline. Daily intakes of total fat, protein, carbohydrate, and fiber in grams were calculated as percentages of kilojoules. Baseline categorical variables were smoking (never, former, or current), years of education (≤ 12, 13–15, ≥ 16), marital status (never married, widowed, divorced or separated, or married), and OC use (never/past or current). Positive family history of diabetes was report of ≥ 1 first-degree relative (ie, father, mother, or siblings) with diabetes at examinations in years 0, 5, and 10. This fixed variable covered family history of diabetes no matter when reported. Daily physical activity was assessed at each examination by the interviewer-administered CARDIA physical activity history.35 Physical activity scores (race-specific quartiles) have been correlated positively with symptom-limited graded treadmill exercise test duration.36 Time-dependent covariates included smoking and OC use categories and change in physical activity and weight for each time interval calculated by subtraction of baseline from follow-up measurement.
Statistical methods
Preliminary analyses described baseline characteristics by incident MetS case status and interim birth groups. We used analysis of variance to assess baseline differences in MetS components, BMI, age, and dietary intake and χ2 statistics to examine differences in categorical covariates. Wilcoxon rank-sum and Kruskal-Wallis 1-way tests were used to assess differences in alcohol intake and physical activity scores (median and interquartile range) due to skewedness in the distributions. P values were obtained from 2-sided tests (significance < .05).
The cumulative incidence of MetS (n/N, %) within each fixed time interval (0–7, > 7–10, > 10–15, and > 15–20 years) was calculated by dividing the number of new cases of MetS (n) within the interval by the number of women at risk of MetS (N) at the end of the interval. The cumulative incidence is estimated for each of the 4 intervals to describe the timing of development of new cases during the 20-year period. We also estimated incidence rates (IR) and 95% confidence intervals (CI) by dividing new cases of MetS by the person-time for individuals observed during the 20-year period, including the person-time contributed by noncases. We examined IR of MetS among quartiles of changes in bodyweight and MetS components (fasting plasma HDL-C and glucose, waist girth) to describe the absolute changes in relation to the IR observed for this cohort of relatively young women.
Because MetS case status was determined at CARDIA examinations, the exact date of diagnosis for a woman who was free of the MetS at a prior examination is unknown. We accounted for interval-censored data using the method of Prentice and Gloeckler37 to provide point and interval estimates of the relative hazard ratios (RH) of MetS after a pregnancy or pregnancies. Individuals were censored from subsequent time intervals once they developed the MetS or reached the end of follow-up. RH estimates were obtained in the context of a generalized linear model for binary outcome with a complementary log-log link function.
RH ratios for the incidence of MetS were estimated for time-dependent interim birth groups (0 births as referent) from multivariable models using examination years 7, 10, 15, and 20 (SAS version 9.1; SAS Institute Inc., Cary, NC). Baseline and time-dependent covariates (sociodemographics, behaviors, medical history, OC use) were introduced into models based on a priori hypotheses to assess confounding. BMI and race were assessed as effect modifiers in the interim birth and incidence of MetS association through introduction of corresponding cross-product terms. We examined potential mediators, time-dependent physical activity, and weight gain from baseline to end of follow-up in separate models.
There was no evidence of effect modification by BMI (interaction P = .14) or race (interaction P = .25) in the association of birth groups and incidence of MetS. Time-dependent race-specific physical activity quartiles and weight gain were added separately to the fully adjusted model as potential mediators of the interim birth and incident MetS association.
RESULTS
Of 1451 nulliparas at baseline, 706 delivered no births and 745 (40% black, 60% white) delivered ≥ 1 births during 20 years of follow-up. Of 745, 88 (12%) women had ≥ 1 birth complicated by GDM. We identified 259 incident cases of the MetS in 25,246 person-years. The IR of the MetS was 10.3 per 1000 person-years, and 127 (49%) women had ≥ 1 birth. Among MetS cases, 24 (9%) reported having GDM during pregnancy, and 103 (40%) had ≥ 1 pregnancy without GDM.
Incident MetS cases (Table 1) were characterized at baseline by black race, lower education, plasma HDL-C and dietary fiber intakes, higher BMI, waist girth, fasting plasma glucose, TG, systolic and diastolic blood pressures, family history of diabetes, and GDM pregnancy. Baseline physical activity scores were similar by case status among black women, but white cases had lower scores. Time from last birth until end of follow-up was similar for MetS cases and noncases: 96.6 (range, 2–226) vs 113.3 (range, 4–231) months.
TABLE 1.
Baseline (1985–1986) characteristics and incident metabolic syndrome (MetS) case status
| Baseline characteristics | Incident MetS case (n = 259) |
Noncase (n = 1192) |
P value | |
|---|---|---|---|---|
| n (%) | ||||
| Race (black) | 134 (51.7) | 434 (36.4) | < .001 | |
| Education (≤ high school) | 86 (33.2) | 298 (25.0) | .002 | |
| Marital status (married) | 33 (12.7) | 165 (13.8) | .21 | |
| Smoker (current) | 67 (25.9) | 269 (22.6) | .48 | |
| OC use (current) | 78 (30.1) | 364 (30.5) | .99 | |
| Gravidity (nulligravida) | 200 (77.2) | 892 (74.8) | .42 | |
| Mean (SD) | ||||
| Age (y) | 24.4 (3.6) | 24.3 (3.7) | .58 | |
| BMI (kg/m2) | 27.8 (6.2) | 22.8 (4.0) | < .001 | |
| Waist (cm) | 81.0 (13.3) | 70.2 (8.0) | < .001 | |
| Fasting plasma glucose (mg/dL) | 81.9 (7.6) | 79.4 (7.1) | < .001 | |
| Fasting HDL-C (mg/dL) | 52.1 (12.9) | 58.6 (12.4) | < .001 | |
| Fasting TG (mg/dL) | 74.4 (35.4) | 61.5 (32.3) | < .001 | |
| Systolic BP (mm Hg) | 109.9 (9.7) | 105.3 (8.9) | < .001 | |
| Diastolic BP (mm Hg) | 69.3 (8.9) | 66.2 (8.6) | < .001 | |
| % KJ as fat | 37.2 (5.9) | 36.9 (6.1) | .44 | |
| % KJ as saturated fat | 13.9 (2.8) | 13.7 (3.0) | .29 | |
| % KJ as CHO | 46.8 (7.4) | 47.1 (7.3) | .54 | |
| Crude fiber (g)/1000 KJ | 0.53 (0.25) | 0.59 (0.29) | < .001 | |
| Median (interquartile range) | ||||
| Alcohol intake (mL/day)a | 2.4 (9.9) | 2.4 (9.7) | .41 | |
| Physical activity score (black)a | 227.5 (286.0) | 254.5 (298.0) | .56 | |
| Physical activity score (white)a | 320.0 (267.0) | 388.0 (363.0) | .004 | |
| n (%) | ||||
| Family history of diabetesb | 113 (43.6) | 286 (24.0) | < .001 | |
| GDM status (ever)b | 24 (9.3) | 64 (5.4) | .02 | |
BMI, body mass index; BP, blood pressure; CHO, carbohydrate; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; OC, oral contraceptives; TG, triglycerides.
Wilcoxon rank-sum test
Data collected during follow-up.
Women who remained nulliparous during follow-up (Table 2) tended to be older, unmarried, or current OC users; have larger waist girth; and have higher diastolic blood pressure and dietary fiber intake at baseline than parous women. Women with ≥ 1 non-GDM birth tended to be of black race, be less educated, and have lower BMI and systolic blood pressure, whereas those with GDM tended to have positive family history of diabetes and higher fasting plasma glucose at baseline.
TABLE 2.
Baseline (1985–1986) characteristics by interim birth groups and GDM status
| Baseline characteristics | No. of births by GDM status | ||||
|---|---|---|---|---|---|
| 0 births (n = 706) |
1 non-GDM birth (n = 276) |
2 + non-GDM births (n = 381) |
1 + GDM births (n = 88) |
Overall P value |
|
| n(%) | |||||
| Race (black) | 273 (38.7) | 144 (52.2) | 119 (31.2) | 32 (36.4) | < .001 |
| Education (≤ high school) | 179 (25.4) | 93 (33.7) | 93 (24.4) | 19 (21.6) | .007 |
| Marital status (married) | 69 (9.8) | 38 (13.8) | 77 (20.2) | 14 (15.9) | < .001 |
| Smoker (current) | 176 (24.9) | 61 (22.1) | 71 (18.6) | 28 (31.8) | .12 |
| OC use (current) | 245 (34.7) | 77 (27.9) | 99 (26.0) | 21 (23.9) | < .001 |
| Gravidity | 545 (77.2) | 195 (70.7) | 289 (75.9) | 63 (71.6) | .15 |
| Family history of diabetesb | 194 (27.5) | 83 (30.1) | 89 (23.4) | 33 (37.5) | .04 |
| Mean (SD) | |||||
| Age (y) | 24.8 (3.6) | 23.7 (3.6) | 23.9 (3.6) | 23.9 (4.0) | < .001 |
| BMI (kg/m2) | 24.3 (5.4) | 23.4 (4.5) | 22.5 (3.7) | 24.4 (5.9) | < .001 |
| Waist (cm) | 73.7 (11.3) | 71.0 (8.2) | 69.8 (7.3) | 73.5 (12.4) | < .001 |
| Fasting plasma glucose (mg/dL) | 80.3 (7.3) | 79.6 (7.0) | 79.2 (6.9) | 81.0 (8.9) | .04 |
| HDL-C (mg/dL) | 57.5 (13.0) | 57.3 (12.2) | 57.9 (12.5) | 55.1 (12.7) | .31 |
| TG (mg/dL) | 63.8 (30.1) | 63.2 (30.6) | 62.8 (40.4) | 69.7 (30.7) | .36 |
| Systolic BP (mm Hg) | 106.5 (9.5) | 106.4 (9.2) | 104.9 (8.7) | 106.5 (8.8) | .04 |
| Diastolic BP (mm Hg) | 67.7 (8.6) | 66.0 (8.9) | 65.5 (8.8) | 67.0 (8.8) | < .001 |
| % KJ as fat | 36.7 (6.1) | 37.4 (6.2) | 36.7 (6.1) | 37.9 (5.8) | .13 |
| % KJ as saturated fat | 13.7 (2.9) | 13.8 (3.0) | 13.7 (2.9) | 14.2 (3.1) | .39 |
| % KJ as CHO | 7.2 (7.2) | 47.0 (7.6) | 47.1 (7.3) | 46.1 (7.0) | .60 |
| Crude fiber (g)/1000 KJ | 0.60 (0.30) | 0.53 (0.24) | 0.59 (0.31) | 0.53 (0.24) | .002 |
| Alcohol intake (mL/day)a | 2.4 (10.0) | 2.4 (9.5) | 2.4 (9.9) | 2.6 (10.0) | .77 |
| Physical activity (black)a | 257.0 (300.0) | 269.0 (301.0) | 251.0 (316.0) | 223.5 (205.0) | .75 |
| Physical activity (white)a | 374.0 (330.0) | 388.5 (377.0) | 388.0 (351.0) | 332.5 (285.5) | .55 |
BMI, body mass index; BP, blood pressure; CHO, carbohydrate; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; n, number of women within each group at end of follow-up; OC, oral contraceptives; TG, triglycerides.
Median (interquartile range) Kruskal-Wallis test
Data collected during follow-up.
Cumulative incidence of MetS (Table 3) increased during the 20-year period, with largest increases from > 15–20 years compared with earlier intervals. Highest incidence of MetS was among women with GDM during all intervals, except between years 10–15.
TABLE 3.
Cumulative incidence of the metabolic syndrome (MetS) during fixed follow-up intervals
| Year, follow-up | No. of new cases of MetS/no. of individuals at risk through end of interval |
|||
|---|---|---|---|---|
| Years 0–7, n/N (%) |
Years > 7–10, n/N (%) |
Years > 10– 15, n/N (%) |
Years > 15– 20, n/N (%) |
|
| Interim birth groups | ||||
| 0 births | 30/1005 (3.0) | 23/785 (2.9) | 31/585 (5.3) | 48/445 (10.8) |
| Non-GDM | ||||
| 1 birth | 6/260 (2.3) | 5/258 (1.9) | 16/229 (7.0) | 18/181 (9.9) |
| ≥ 2 births | 2/140 (1.4) | 10/251 (4.0) | 19/331 (5.7) | 27/306 (8.8) |
| GDM | ||||
| ≥ 1 births | 6/46 (13.0) | 5/59 (8.5) | 4/70 (5.7) | 9/59 (15.3) |
| Overall total | 44/1451 (3.0) | 43/1353 (3.2) | 70/1215 (5.8) | 102/991 (10.3) |
GDM, gestational diabetes mellitus; MetS, metabolic syndrome.
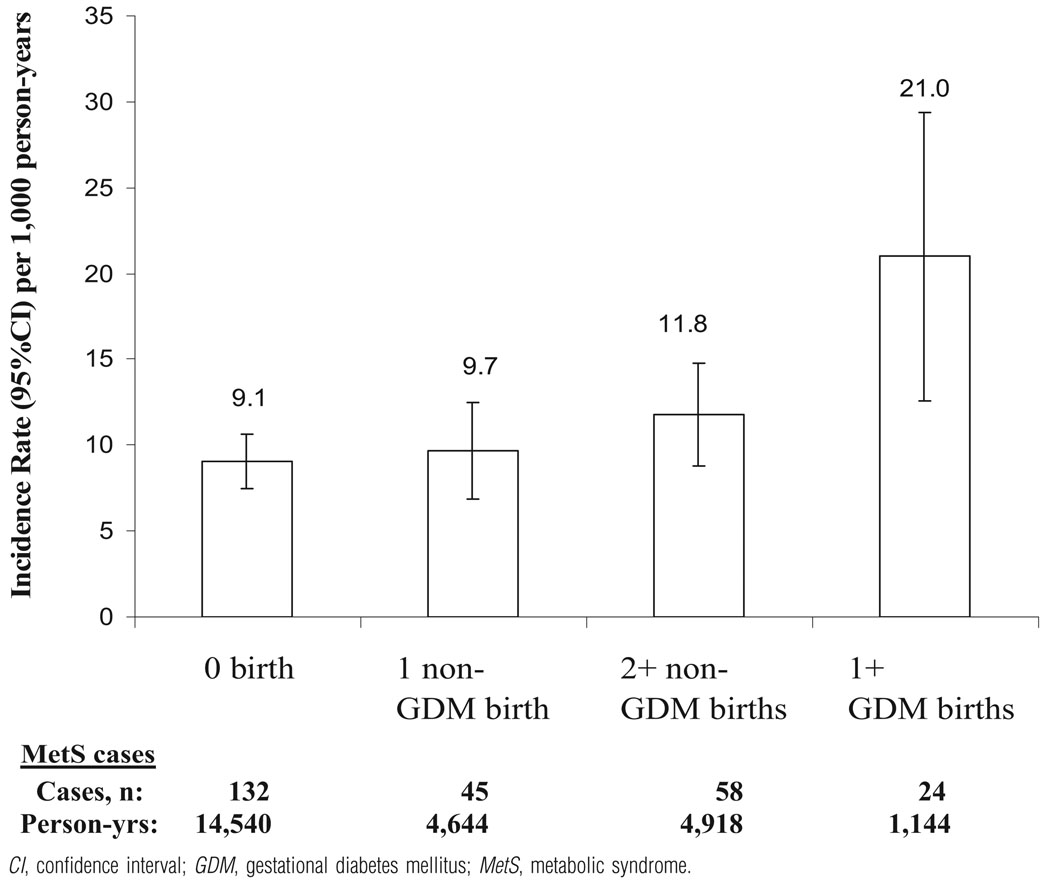
Crude IR (number of cases/1000 person-years [95% CI]) were 2-fold higher (Figure) for 1 + GDM births (21.0) vs none (9.1) and modestly higher among non-GDM groups (9.7 and 11.8) for the entire follow-up period.
FIGURE.
Crude incidence rates (95% CI) for the MetS by interim births
Table 4 describes the absolute changes in bodyweight and specific MetS components in relation to the IR of the MetS. Crude IR were 12–24 times greater among women who experienced the largest decrements (Table 4) in plasma HDL-C and largest increments in waist girth and weight gain (all P trend < .001); IR were 3-fold higher for the highest vs 3 lowest quartiles of fasting plasma glucose increments (P trend < .001). The majority of MetS cases (n = 195, 75%) were identified by the presence of only 3 risk factors. The most frequent combinations for all groups, except 1 + GDM births, were high waist girth and low HDL-C plus 1 of the following: elevated blood pressure, fasting TG, or fasting glucose. Among MetS cases with 1 + GDM births, 71% had high fasting glucose or diabetes as 1 of the components compared with 43–53% for other birth groups.
TABLE 4.
Crude incidence rates of the metabolic syndrome (MetS) by changes in components of the MetS and bodyweight
| Changes in bodyweight and MetS components during follow-up |
Incident cases of MetS/1000 person-y IR and 95% CI limits |
||||
|---|---|---|---|---|---|
| n | Person-y | IR | Lower, upper limits |
P value for trend |
|
| Fasting plasma glucosea (mg/dL) | < .001 | ||||
| First quartile (−41 to 2) | 39 | 5545 | 7.0 | 4.8, 9.2 | |
| Second quartile (3–9) | 47 | 6326 | 7.4 | 5.3, 9.5 | |
| Third quartile (10–16) | 51 | 7333 | 7.0 | 5.1, 8.9 | |
| Fourth quartile (17–217) | 122 | 6042 | 20.2 | 16.6, 23.8 | |
| Plasma HDL-Ca (mg/dL) | < .001 | ||||
| First quartile (−50 to −8) | 127 | 5681 | 22.4 | 18.5, 26.3 | |
| Second quartile (−7 to 0) | 91 | 6294 | 14.5 | 11.5, 17.5 | |
| Third quartile (1–9) | 32 | 6536 | 4.9 | 3.2, 6.6 | |
| Fourth quartile (10–70) | 9 | 6735 | 1.3 | 0.4, 2.2 | |
| Waist girtha (cm) | < .001 | ||||
| First quartile (−37.5 to 5.0) | 12 | 6084 | 2.0 | 0.9, 3.1 | |
| Second quartile (5.1–11.7) | 23 | 6399 | 3.6 | 2.1, 5.1 | |
| Third quartile (11.8–19.3) | 71 | 6361 | 11.2 | 8.6,13.8 | |
| Fourth quartile (19.3–65.0) | 153 | 6402 | 23.9 | 20.1, 27.7 | |
| Bodyweight (kg)a,b | < .001 | ||||
| First quartile (−45.4 to 4.4) | 6 | 6277 | 1.0 | 0.2, 1.7 | |
| Second quartile (4.5–11.3) | 27 | 6391 | 4.2 | 2.6, 5.8 | |
| Third quartile (11.4–21.5) | 74 | 6270 | 11.8 | 9.1, 14.5 | |
| Fourth quartile (21.6–80.7) | 152 | 6288 | 24.2 | 20.3, 28.0 | |
CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; IR, incidence rates; MetS, metabolic syndrome; n, number of new cases of MetS.
Change
1 woman missing weight change (noncase).
For the multivariable models (Table 5) the total number of women classified within each birth group is shown as of the end of follow-up. In unadjusted models, incidence of the MetS did not increase among those with non-GDM births (Table 5) but was 2-fold higher for 1 + GDM births vs 0 births. In multivariable models, age, race, and baseline covariates (education, smoking) had minimal impact. Addition of baseline BMI and waist girth strengthened associations to 1.30 (0.91, 1.85) and 1.58 (1.13, 2.21) for 1 non-GDM and 2 + non-GDM births and 2.53 (1.60, 4.00) for 1 + GDM births. Full adjustment for other MetS components and physical activity score measured at baseline increased RH for non-GDM groups to 1.33 (0.93, 1.90) and 1.62 (1.16, 2.26); P trend = .02, with little effect on RH for 1 + GDM.
TABLE 5.
Relative hazards (95% CI) of incident metabolic syndrome among interim birth groups
| Models | No. of births by GDM status | |||
|---|---|---|---|---|
| 0 births (referent) (n = 706) |
1 non-GDM birth (n = 276) |
2 + non-GDM births (n = 381) |
1 + GDM births (n = 88) |
|
| Unadjusted | 1.0 | 0.96 (0.68–1.35) | 0.94 (0.69–1.29) | 1.88 (1.21–2.91) |
| Model 1 = adjusted for race and baseline age | 1.0 | 0.93 (0.66–1.31) | 1.04 (0.75–1.43) | 1.94 (1.25–3.01) |
| Model 2 = model 1 + baseline education and smoking | 1.0 | 0.92 (0.65–1.30) | 1.11 (0.80–1.53) | 2.15 (1.38–3.34) |
| Model 3 = model 2 + baseline BMI | 1.0 | 1.12 (0.79–1.59) | 1.44 (1.04–2.01) | 2.27 (1.45–3.57) |
| Model 4 = model 3 + baseline waist girth | 1.0 | 1.30 (0.91–1.85) | 1.58 (1.13–2.21) | 2.53 (1.60–4.00) |
| Model 5 = model 4 + all other baseline MetS components | 1.0 | 1.33 (0.93–1.90) | 1.62 (1.16–2.26) | 2.42 (1.52–3.83) |
| Model 6 (fully adjusted) = model 5 + baseline physical activity | 1.0 | 1.33 (0.93–1.91) | 1.62 (1.16–2.26) | 2.43 (1.53–3.86) |
| Model 6 + time-dependent physical activity (mediator) | 1.0 | 1.25 (0.87–1.79) | 1.41 (1.01–1.97) | 2.05 (1.29–3.27) |
| Model 6 + time-dependent weight gain (mediator) | 1.0 | 1.09 (0.76–1.58) | 1.35 (0.95–1.90) | 1.77 (1.10–2.84) |
BMI, body mass index; CI, confidence interval; GDM, gestational diabetes mellitus; MetS, metabolic syndrome; n, number of women within each group at end of follow-up.
Both time-dependent physical activity and weight gain acted as mediators and attenuated associations between interim birth groups and incident MetS, although the association for 1 + GDM births remained significant.
Only 4–5% of MetS cases arose from the lowest quartile of baseline BMI or waist girth. In sensitivity analyses where we omitted thinner women (data not shown), findings were similar, except for attenuation of RH (95% CI) for 1 non-GDM birth from 1.33 (95% CI, 0.93–1.90) to 1.14 (95% CI, 0.78–1.67), with exclusion of the lowest quartile of baseline BMI, and 1.16 (95%CI, 0.79–1.72) with parallel exclusion for waist girth.
COMMENT
Childbearing was directly associated with incidence of the MetS among women with GDM, but not among women without GDM, when models were adjusted only for race and socio-demographics. After controlling for preconception (baseline) measurements of BMI, all MetS components, and physical activity, we found that both primiparity and multiparity among women without GDM were independently associated with higher incidence of the MetS compared with nulliparity: 33% and 62% higher, respectively. Among women with GDM, adjustment for baseline (preconception) measurements strengthened the association from 2-fold to 2.4-fold higher incidence of the MetS. These findings indicate that baseline risk factor differences among parity groups resulted in negative confounding, which biased the association toward the null in models that did not control for measurements before pregnancy. Finally, weight gain and physical activity during follow-up acted as mediators in that each modestly attenuated the associations.
The study strengths include preconception measurements of all MetS components (including diagnoses of hypertension and diabetes before pregnancies) in the population-based sample of premenopausal nulliparas, repeated measurements at 3- to 7- year intervals, and high cohort retention (72% after 20 years). The cohort consisted of healthy women of reproductive age who infrequently used medications to treat hyperlipidemia (~ 1–6%), hypertension (~ 9%), and diabetes (~ 4%) during the 20-year follow-up. Moreover, measurements of potential confounders or mediators—including sociodemographic, medical, and behavioral attributes; weight change; and physical activity—were collected prospectively. The study’s limitations include self-report of GDM status; variable time intervals to conception and from delivery until CARDIA examinations; and inability to examine CVD outcomes, because average age was 44 years in 2006. We validated GDM by self-report. Given nondifferential misclassification, our findings would be biased toward the null. When we excluded other pregnancy complications (ie, hypertensive disorders [n = 166], multiple gestations [n = 27]), risk estimates were only slightly attenuated.
Our longitudinal study found a 30% higher incidence per non-GDM birth compared with 13–16% higher prevalence per birth in cross-sectional studies where GDM status was unknown.22,23 These differences are consistent with our findings of negative confounding by preconception risk factor levels. Similarly, pregnancy cohort studies reported a 2-to 4-fold higher prevalence of the MetS for previous GDM vs non-GDM pregnancies. 25,26 Our 2.4-fold higher incidence of the MetS more closely reflects the true impact of GDM pregnancy.
Other evidence also supports a biological effect of pregnancy or stress of child-rearing. Previously, we reported waist girth increases with each birth,11 while bodyweight increased only after the first birth,10,11 suggesting that parity has a greater impact on central than overall adiposity. We also found −3 to −4 mg/dL greater decrements in HDL-C for primiparas vs nulliparas independent of weight gain, waist girth increases, aging, secular trends, sociodemographics, and lifestyle behaviors.9 Cross-sectional studies reported an inverse association of parity and HDL-C levels in women38,39 independent of obesity but no association with number of biological children in men.40,41 Multiparity has been directly associated with CHD in women,13,14,41–43 with a J-shaped relationship showing higher risk for nulliparas.19,41 However, others found null associations with increasing number of children in both women17,19 and men.44,45 Conflicting findings may stem from varying definitions of CHD, age effects, or socioeconomic status, but negative confounding from preconception risk status may explain the discrepant findings, as well as the fact that BMI and other potential confounders were measured many years after pregnancy.
One mechanism for pregnancy’s persistent metabolic effects may be changes in fat distribution. Specifically, central adiposity is of greater importance than overall obesity, because intraabdominal (visceral) fat may be associated with development of obesity-related insulin resistance46 and production of adipocytokines that regulate insulin sensitivity.47 Intraabdominal adipose tissue was positively associated with parity in a cross-sectional study.48 A 5-year longitudinal study in CARDIA found a 40% increase in visceral fat from preconception to postpartum vs 14% among nonparous women adjusted for total and subcutaneous fat changes.49 Pregnancy may affect long-term health by increasing abdominal obesity relative to weight gain, unmasking future glucose intolerance, and worsening risk profiles (ie, lower plasma HDL-C postpartum) regardless of gains in overall and central adiposity.
Our findings suggest that childbearing contributes to development of the MetS and that the association is partially mediated through weight gain and lack of physical activity. Although women with GDM had the highest relative risk, those with non-GDM pregnancies had a greater absolute risk, contributing 4 times as many cases as GDM pregnancies (40% vs 9% MetS cases). The MetS predicts CHD and diabetes during midlife and early mortality in women.50–52 In the San Antonio Heart Study, women who had both diabetes and the MetS experienced an 8-fold higher CVD mortality.53 Recent studies suggest a stronger link to diabetes than CHD.54,55 Women of reproductive age often do not receive early risk factor assessment, and few data are available to determine whether postpartum screening of cardiometabolic risk factors is warranted. Of women, 10–15% experience substantial postpartum weight retention of ≥ 5 kg, with 7–20% becoming overweight.56,57 Because prevalence of the MetS increases from 18–37% between the ages of 20–39 and 40–59 years in women,58 the childbearing years may be a critical period for its development. Future studies may determine whether reductions in weight retention, central obesity, and dyslipidemia after pregnancy may prevent disease later in life. Postpartum screening of car-diometabolic risk factors may offer an important opportunity for primary prevention (eg, structured lifestyle interventions) among women of reproductive age who might otherwise not receive early risk factor assessment.
Acknowledgments
This study was supported by the National Institutes of Health (contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095 from the National Heart, Lung, and Blood Institute and the Career Development Award, Grant K01 DK059944, from the National Institute of Diabetes, Digestive and Kidney Diseases) and a Research Award from the American Diabetes Association.
Footnotes
Presented orally at the 46th Annual Conference on Cardiovascular Disease Epidemiology and Prevention of the American Heart Association, Phoenix, AZ, March 2–5, 2006.
Reprints not available from the authors.
REFERENCES
- 1.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Grundy SM, Becker D, et al. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Grundy SM, Becker D, et al. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the ”metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 5.Desoye G, Schweditsch MO, Pfeiffer KP, Zechner R, Kostner GM. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab. 1987;64:704–712. doi: 10.1210/jcem-64-4-704. [DOI] [PubMed] [Google Scholar]
- 6.Ledoux F, Genest J, Nowaczynski W, Kuchel O, Lebel M. Plasma progesterone and aldosterone in pregnancy. Can Med Assoc J. 1975;112:943–947. [PMC free article] [PubMed] [Google Scholar]
- 7.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 8.van Stiphout WA, Hofman A, de Bruijn AM. Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol. 1987;126:922–928. doi: 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith WD, Sidney S. Long-term plasma lipid changes associated with a first birth: the coronary artery risk development in young adults study. Am J Epidemiol. 2004;159:1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy: the CARDIA study; coronary artery risk development in young adults study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 11.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: the coronary artery risk development in young adults study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Sullivan JB, Gordon T. Childbearing and diabetes mellitus. United States-1960–1962. Vital Health Stat 11. 1966;21:1–19. [PubMed] [Google Scholar]
- 13.Rosenberg L, Palmer JR, Rao RS, Adams-Campbell LL. Risk factors for coronary heart disease in African American women. Am J Epidemiol. 1999;150:904–909. doi: 10.1093/oxfordjournals.aje.a010098. [DOI] [PubMed] [Google Scholar]
- 14.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328:1528–1533. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 15.Humphries KH, Westendorp IC, Bots ML, et al. Parity and carotid artery atherosclerosis in elderly women: the Rotterdam study. Stroke. 2001;32:2259–2264. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Rimm EB, Colditz GA, et al. Parity and incidence of non-insulin-dependent diabetes mellitus. Am J Med. 1992;93:13–18. doi: 10.1016/0002-9343(92)90674-z. [DOI] [PubMed] [Google Scholar]
- 17.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7:641–643. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology. 1999;10:255–259. [PubMed] [Google Scholar]
- 19.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. 1987;126:861–870. doi: 10.1093/oxfordjournals.aje.a114723. [DOI] [PubMed] [Google Scholar]
- 20.Hanley AJ, McKeown-Eyssen G, Harris SB, et al. Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care. 2002;25:690–695. doi: 10.2337/diacare.25.4.690. [DOI] [PubMed] [Google Scholar]
- 21.Gunderson EP, Lewis CE, Tsai AL, et al. A 20-year prospective study of childbearing and incidence of diabetes mellitus in young women controlling for glycemia before conception: the coronary artery risk development in young adults study. Diabetes. 2007;56:2990–2996. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lao XQ, Thomas GN, Jiang CQ, et al. Parity and the metabolic syndrome in older Chinese women: the Guangzhou Biobank cohort study. Clin Endocrinol (Oxf) 2006;65:460–469. doi: 10.1111/j.1365-2265.2006.02615.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen A, Pieper CF, Brown AJ, Bastian LA. Number of children and risk of metabolic syndrome in women. J Womens Health (Larchmt) 2006;15:763–773. doi: 10.1089/jwh.2006.15.763. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 25.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 26.Bo S, Monge L, Macchetta C, et al. Prior gestational hyperglycemia: a long-term predictor of the metabolic syndrome. J Endocrinol Invest. 2004;27:629–635. doi: 10.1007/BF03347494. [DOI] [PubMed] [Google Scholar]
- 27.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults: the CARDIA baseline monograph. Control Clin Trials. 1991;12 suppl:1–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 28.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 29.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study; coronary artery risk development in young adults study. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 30.Steffen LM, Kroenke CH, Yu X, et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the coronary artery risk development in young adults (CARDIA) study. Am J Clin Nutr. 2005;82:1169–1177. doi: 10.1093/ajcn/82.6.1169. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA study. Ann Epidemiol. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CE, Funkhouser E, Raczynski JM, Sidney S, Bild DE, Howard BV. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women: the CARDIA study; coronary artery risk development in young adults study. Am J Epidemiol. 1996;144:247–254. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 33.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR., Jr Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 35.Anderssen N, Jacobs DR, Jr, Sidney S, et al. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the coronary artery risk development in young adults study (CARDIA) Am J Epidemiol. 1996;143:351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 36.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24:177–183. [PubMed] [Google Scholar]
- 37.Prentice R, Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 38.Haertel U, Heiss G, Filipiak B, Doering A. Cross-sectional and longitudinal associations between high density lipoprotein cholesterol and women’s employment. Am J Epidemiol. 1992;135:68–78. doi: 10.1093/oxfordjournals.aje.a116203. [DOI] [PubMed] [Google Scholar]
- 39.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The relationship between multiparity and lipoprotein levels in older women. J Clin Epidemiol. 1992;45:761–767. doi: 10.1016/0895-4356(92)90053-p. [DOI] [PubMed] [Google Scholar]
- 40.Kritz-Silverstein D, Barrett-Connor E, Friedlander NJ. Parenthood and lipid and lipoprotein levels in older men. Ann Epidemiol. 1997;7:275–279. doi: 10.1016/s1047-2797(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 41.Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British women’s heart and health study and the British regional heart study. Circulation. 2003;107:1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 42.Ness RB, Schotland HM, Flegal KM, Shofer FS. Reproductive history and coronary heart disease risk in women. Epidemiol Rev. 1994;16:298–314. doi: 10.1093/oxfordjournals.epirev.a036155. [DOI] [PubMed] [Google Scholar]
- 43.Dekker JM, Schouten EG. Number of pregnancies and risk of cardiovascular disease. N Engl J Med. 1993;329:1893–1894. [PubMed] [Google Scholar]
- 44.Ness RB, Cobb J, Harris T, D’Agostino RB. Does number of children increase the rate of coronary heart disease in men? Epidemiology. 1995;6:442–445. doi: 10.1097/00001648-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 45.Hardy R, Lawlor DA, Black S, Wadsworth ME, Kuh D. Number of children and coronary heart disease risk factors in men and women from a British birth cohort. BJOG. 2007;114:721–730. doi: 10.1111/j.1471-0528.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- 46.Weidner MD, Gavigan KE, Tyndall GL, Hickey MS, McCammon MR, Houmard JA. Which anthropometric indices of regional adiposity are related to the insulin resistance of aging? Int J Obes Relat Metab Disord. 1995;19:325–330. [PubMed] [Google Scholar]
- 47.Altomonte J, Harbaran S, Richter A, Dong H. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism. 2003;52:958–963. doi: 10.1016/s0026-0495(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 48.Blaudeau TE, Hunter GR, Sirikul B. Intra-abdominal adipose tissue deposition and parity. Int J Obes (Lond) 2006;30:1119–1124. doi: 10.1038/sj.ijo.0803252. [DOI] [PubMed] [Google Scholar]
- 49.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–1084. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women: risk within the ’normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 51.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 52.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 53.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National cholesterol education program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio heart study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 54.Sattar N. Why metabolic syndrome criteria have not made prime time: a view from the clinic. Int J Obes (Lond) 2008;32 suppl:S30–S34. doi: 10.1038/ijo.2008.33. [DOI] [PubMed] [Google Scholar]
- 55.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31:1898–1904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 1999;21:261–275. doi: 10.1093/oxfordjournals.epirev.a018001. [DOI] [PubMed] [Google Scholar]
- 57.Gunderson EP, Quesenberry CP, Jr, Lewis CE, et al. Development of overweight associated with childbearing depends on smoking habit: the coronary artery risk development in young adults (CARDIA) study. Obes Res. 2004;12:2041–2053. doi: 10.1038/oby.2004.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung BM, Ong KL, Man YB, Wong LY, Lau CP, Lam KS. Prevalence of the metabolic syndrome in the United States national health and nutrition examination survey 1999–2002 according to different defining criteria. J Clin Hypertens (Greenwich) 2006;8:562–570. doi: 10.1111/j.1524-6175.2006.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]