Abstract
Members of the spotted fever group (SFG) of rickettsiae spread rapidly from cell to cell by an unknown mechanism(s). Staining of Rickettsia rickettsii-infected Vero cells with rhodamine phalloidin demonstrated unique actin filaments associated with one pole of intracellular rickettsiae. F-actin tails greater than 70 microns in length were seen extending from rickettsiae. Treatment of infected cells with chloramphenicol eliminated rickettsia-associated F-actin tails, suggesting that de novo protein synthesis of one or more rickettsial proteins is required for tail formation. Rickettsiae were coated with F-actin as early as 15 min postinfection, and tail formation was detected by 30 min. A survey of virulent and avirulent species within the SFG rickettsiae demonstrated that all formed actin tails. Typhus group rickettsiae, which do not spread directly from cell to cell, lacked F-actin tails entirely or exhibited only very short tails. Transmission electron microscopy demonstrated fibrillar material in close association with R. rickettsii but not Rickettsia prowazekii. Biochemical evidence that actin polymerization plays a role in movement was provided by showing that transit of R. rickettsii from infected cells into the cell culture medium was inhibited by treatment of host cells with cytochalasin D. These data suggest that the cell-to-cell transmission of SFG rickettsiae may be aided by induction of actin polymerization in a fashion similar to that described for Shigella flexneri and Listeria monocytogenes.
Full text
PDF

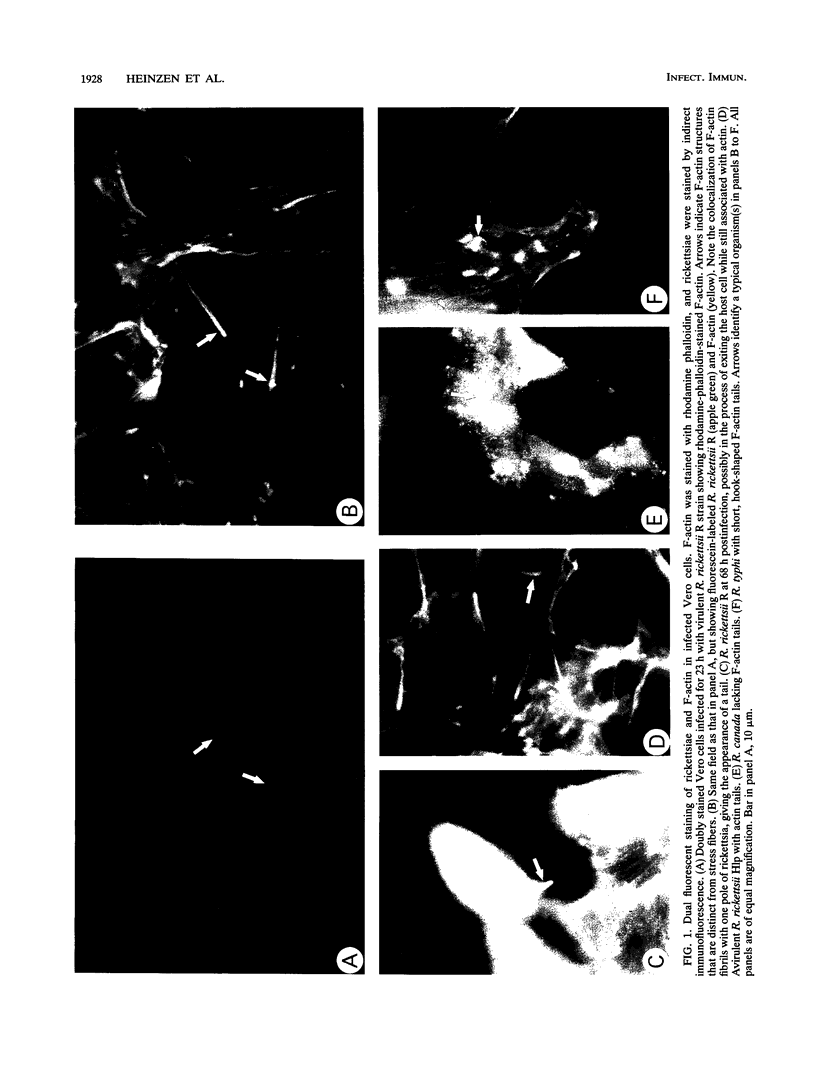


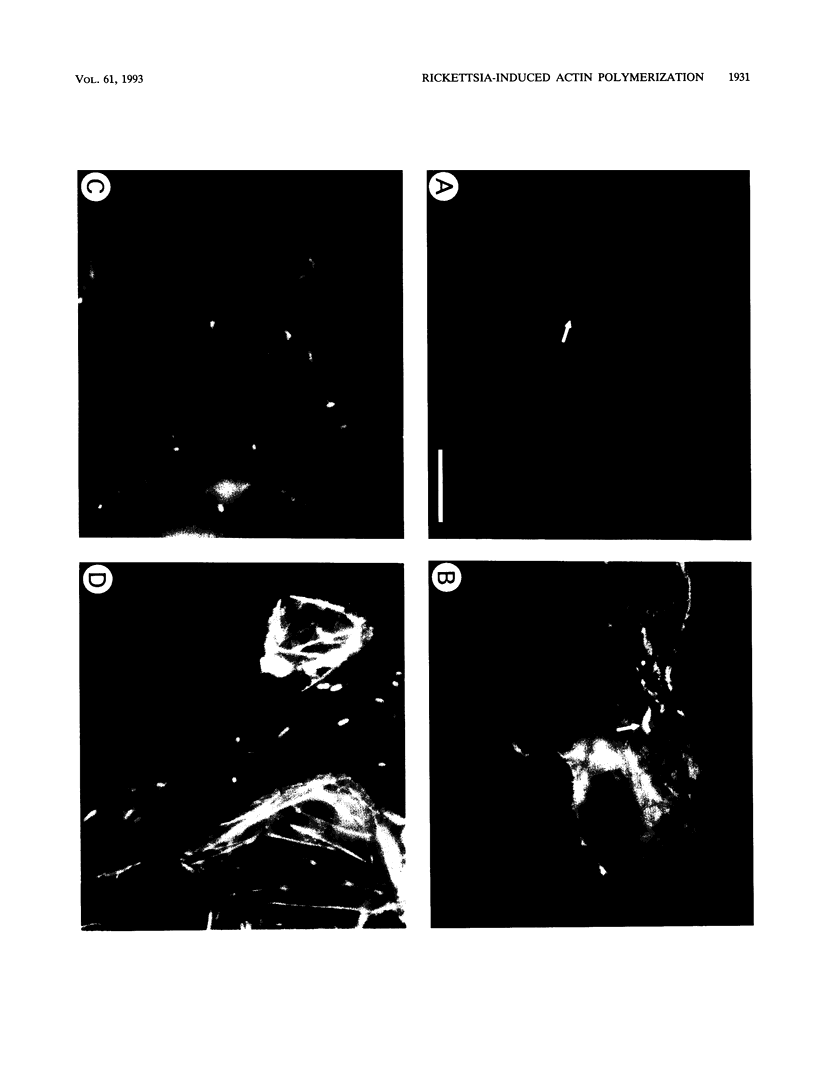

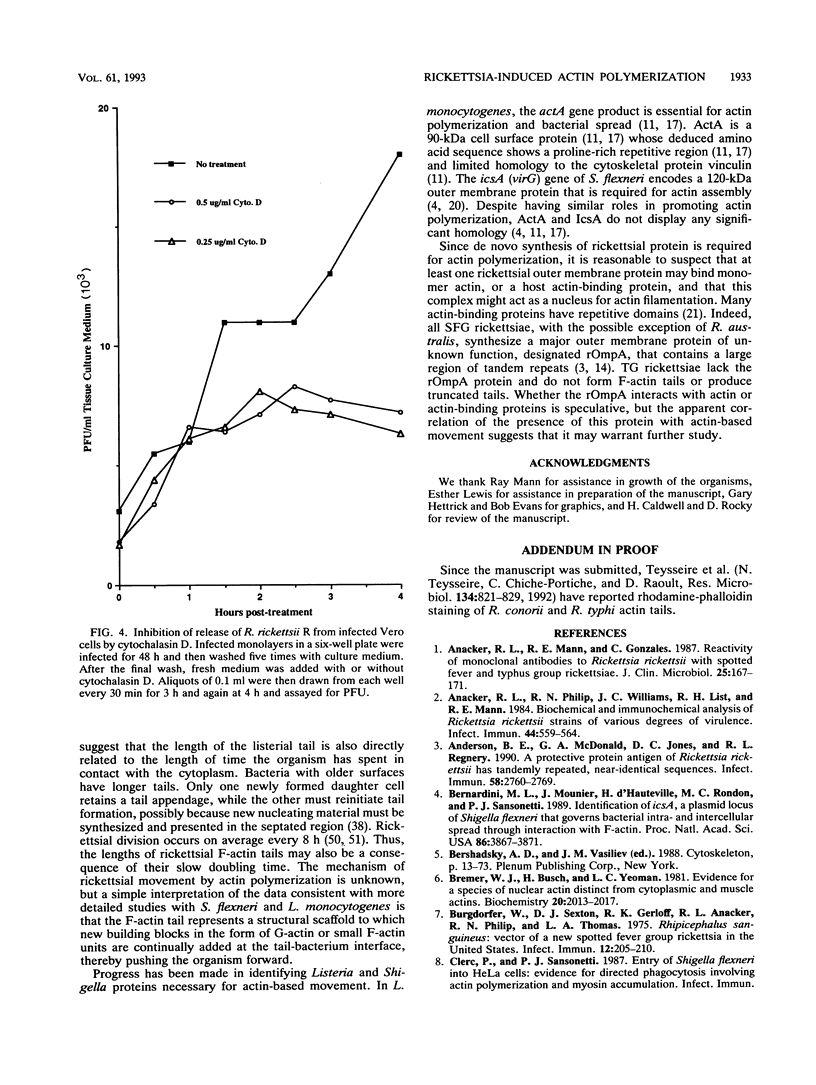


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Mann R. E., Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987 Jan;25(1):167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Williams J. C., List R. H., Mann R. E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. W., Busch H., Yeoman L. C. Evidence for a species of nuclear actin distinct from cytoplasmic and muscles actins. Biochemistry. 1981 Mar 31;20(7):2013–2017. doi: 10.1021/bi00510a042. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Sexton D. J., Gerloff R. K., Anacker R. L., Philip R. N., Thomas L. A. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. doi: 10.1128/iai.12.1.205-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H. R. CULTIVATION OF RICKETTSIAE OF THE ROCKY MOUNTAIN SPOTTED FEVER, TYPHUS AND Q FEVER GROUPS IN THE EMBRYONIC TISSUES OF DEVELOPING CHICKS. Science. 1941 Oct 31;94(2444):399–403. doi: 10.1126/science.94.2444.399. [DOI] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Zobeley S., Rinnerthaler G., Small J. V. Fluorescent phallotoxins as probes for filamentous actin. J Muscle Res Cell Motil. 1988 Oct;9(5):370–383. doi: 10.1007/BF01774064. [DOI] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Hackstadt T. DNA polymorphism in the conserved 190 kDa antigen gene repeat region among spotted fever group Rickettsiae. Biochim Biophys Acta. 1991 Jul 26;1097(1):77–80. doi: 10.1016/0925-4439(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Messer R., Cieplak W., Peacock M. G. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect Immun. 1992 Jan;60(1):159–165. doi: 10.1128/iai.60.1.159-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kokorin I. N. Biological peculiarities of the development of rickettsiae. Acta Virol. 1968 Jan;12(1):31–35. [PubMed] [Google Scholar]
- Kuhn M., Prévost M. C., Mounier J., Sansonetti P. J. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990 Nov;58(11):3477–3486. doi: 10.1128/iai.58.11.3477-3486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett M. C., Sasakawa C., Okada N., Sakai T., Makino S., Yamada M., Komatsu K., Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Wingfield M. E., Formal S. B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985 Apr;48(1):124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Prévost M. C., Lesourd M., Arpin M., Vernel F., Mounier J., Hellio R., Sansonetti P. J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992 Oct;60(10):4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. N., Miyazawa M., Mori S., Caughey B., Evans L. H., Hayes S. F., Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991 Oct;34(3):255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Kaplan J., Hammond M. E., Larson J. K., Buchanan T. M., Schoolnik G. K. Ultrastructural localization of specific gonococcal macromolecules with antibody-gold sphere immunological probes. Infect Immun. 1984 Nov;46(2):361–366. doi: 10.1128/iai.46.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., BOZEMAN F. M., SMADEL J. E. Study on the growth of Rickettsiae. II. Morphologic observations of living Rickettsiae in tissue culture cells. Virology. 1957 Feb;3(1):160–172. doi: 10.1016/0042-6822(57)90030-2. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W., Southwick F. S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992 Sep;60(9):3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr Top Microbiol Immunol. 1992;180:1–19. doi: 10.1007/978-3-642-77238-2_1. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. doi: 10.1128/iai.26.2.714-727.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Portnoy D. A. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990 Dec;111(6 Pt 2):2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992 Jul;118(1):71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Weber A., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. II. Nucleation, actin filament polarity, filament assembly, and evidence for a pointed end capper. J Cell Biol. 1992 Jul;118(1):83–93. doi: 10.1083/jcb.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Burgdorfer W., Wray G. P. Detection of fibrils associated with Rickettsia rickettsii. Infect Immun. 1983 Sep;41(3):1252–1260. doi: 10.1128/iai.41.3.1252-1260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Hellio R., Sansonetti P. J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Prevost M. C., Hellio R., Sansonetti P. J. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. doi: 10.1128/iai.59.5.1723-1732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsia species (as organisms). Annu Rev Microbiol. 1990;44:131–153. doi: 10.1146/annurev.mi.44.100190.001023. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Edlinger E. A., Waddell A. D., Jones M. R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]






