Abstract
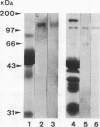
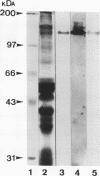
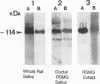
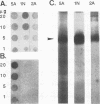
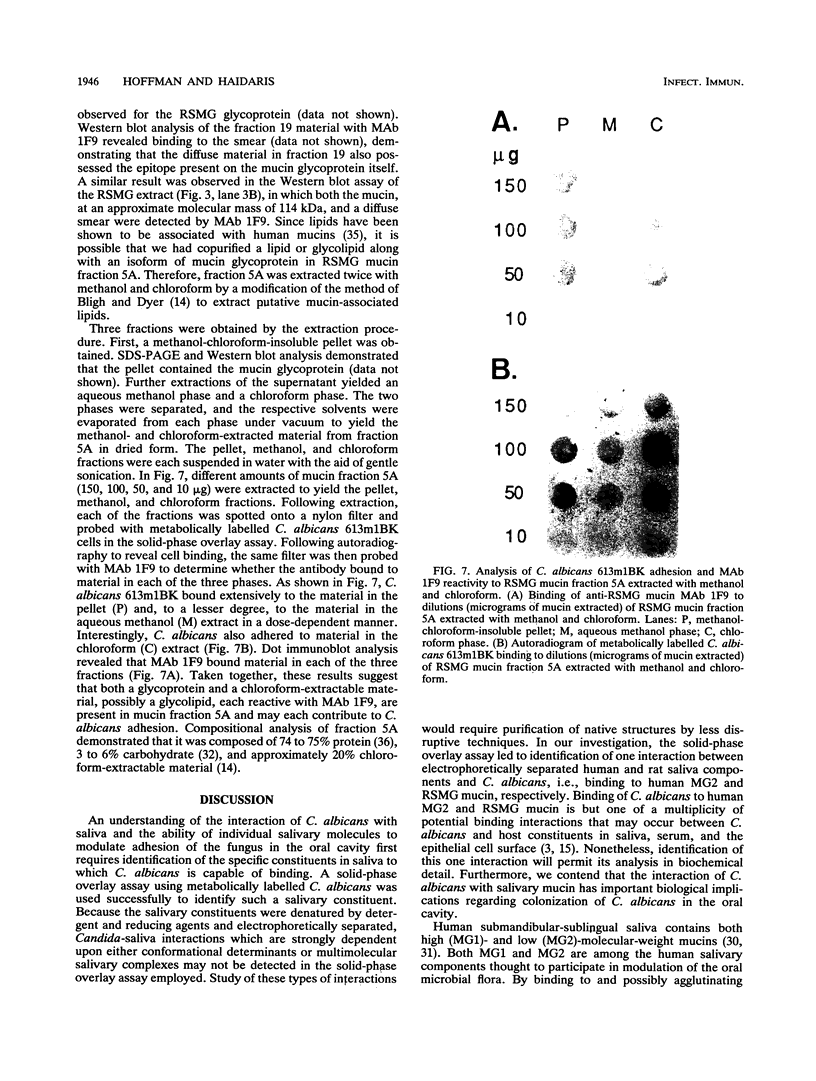
Clearance of Candida albicans from the oral cavity is thought to be mediated via specific receptor-ligand interactions between salivary constituents and the fungus. Since surfaces in the oral cavity are normally coated with a saliva-derived pellicle, specific interactions between salivary constituents and C. albicans may also contribute to adhesion of C. albicans to the oral mucosa and dental prostheses. Therefore, the purpose of this study was to identify salivary constituents to which C. albicans is capable of binding. A solid-phase overlay assay was used in which electrophoretically separated rat and human salivary constituents bound to membrane filters were incubated with radiolabelled C. albicans cells. C. albicans adhered to a single salivary component from each host. Correlation of cell-binding activity with specific monoclonal antibody (MAb)-binding activity indicated that the constituent bound by C. albicans in human saliva was low-molecular-weight mucin (MG2) and that in rat saliva was rat submandibular gland (RSMG) mucin. Further studies showed an identical cell hybridization signal and MAb colocalization by using RSMG ductal saliva and an aqueous RSMG extract in the solid-phase overlay assay. Analysis of cell binding to the aqueous extract of RSMG fractionated by anion-exchange chromatography demonstrated that C. albicans binding was restricted to an acidic subfraction of the RSMG extract, which also bound the RSMG mucin-specific MAb. The Candida-binding fraction contained predominantly RSMG mucin glycoprotein and also a noncovalently associated, chloroform-extractable material. Furthermore, we identified two strains of C. albicans which differed severalfold in the ability to bind RSMG mucin in the overlay assay. These results suggest that C. albicans binds to only a specific subfraction of RSMG mucin and that the two C. albicans strains tested differ in the ability to bind RSMG mucin subfractions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biesbrock A. R., Reddy M. S., Levine M. J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991 Oct;59(10):3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassart D., Woltz A., Golliard M., Neeser J. R. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fuc alpha 1----2Gal beta-bearing complex carbohydrates. Infect Immun. 1991 May;59(5):1605–1613. doi: 10.1128/iai.59.5.1605-1613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. D., Kuhn R. E. Selective binding of Trypanosoma cruzi to host cell membrane polypeptides. Infect Immun. 1990 Jan;58(1):1–6. doi: 10.1128/iai.58.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay I. G. Oral symptoms and candida in the terminally ill. Br Med J (Clin Res Ed) 1986 Mar 1;292(6520):592–593. doi: 10.1136/bmj.292.6520.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Green C. The ABO, Lewis and related blood group antigens; a review of structure and biosynthesis. FEMS Microbiol Immunol. 1989 Jun;1(6-7):321–330. doi: 10.1111/j.1574-6968.1989.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Jay G. D., Culp D. J., Jahnke M. R. Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal Biochem. 1990 Mar;185(2):324–330. doi: 10.1016/0003-2697(90)90302-p. [DOI] [PubMed] [Google Scholar]
- Jimenez-Lucho V., Ginsburg V., Krivan H. C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Gal beta 1-4Glc beta 1-1Cer), a possible adhesion receptor for yeasts. Infect Immun. 1990 Jul;58(7):2085–2090. doi: 10.1128/iai.58.7.2085-2090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988;2:73–169. doi: 10.1007/978-1-4612-3730-3_4. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Loomis R. E., Prakobphol A., Levine M. J., Reddy M. S., Jones P. C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987 Nov 1;258(2):452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- MacKay B. J., Pollock J. J., Iacono V. J., Baum B. J. Isolation of milligram quantities of a group of histidine-rich polypeptides from human parotid saliva. Infect Immun. 1984 Jun;44(3):688–694. doi: 10.1128/iai.44.3.688-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D. The functions of saliva. J Dent Res. 1987 Feb;66(Spec No):623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- Meitner S. W., Bowen W. H., Haidaris C. G. Oral and esophageal Candida albicans infection in hyposalivatory rats. Infect Immun. 1990 Jul;58(7):2228–2236. doi: 10.1128/iai.58.7.2228-2236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Moreira J. E., Tabak L. A., Bedi G. S., Culp D. J., Hand A. R. Light and electron microscopic immunolocalization of rat submandibular gland mucin glycoprotein and glutamine/glutamic acid-rich proteins. J Histochem Cytochem. 1989 Apr;37(4):515–528. doi: 10.1177/37.4.2926128. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Prakobphol A., Lee T., Hoover C. I., Fisher S. J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992 Jan;60(1):31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa H., Hamada T. Binding of salivary or serum proteins to Candida albicans in vitro. Arch Oral Biol. 1990;35(7):571–573. doi: 10.1016/0003-9969(90)90090-w. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Yang Y. C., Diamond R. D., Hyslop D., Offner G. D., Troxler R. F. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem. 1986 Jan 25;261(3):1177–1182. [PubMed] [Google Scholar]
- Pollock J. J., Denepitiya L., MacKay B. J., Iacono V. J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984 Jun;44(3):702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Ramasubbu N., Reddy M. S., Bergey E. J., Haraszthy G. G., Soni S. D., Levine M. J. Large-scale purification and characterization of the major phosphoproteins and mucins of human submandibular-sublingual saliva. Biochem J. 1991 Dec 1;280(Pt 2):341–352. doi: 10.1042/bj2800341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Hay D. I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Feb;61(2):397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A., Zielenski J., Mandel I. D. Mucus glycoprotein of human saliva: differences in the associated and covalently bound lipids with caries. Biochim Biophys Acta. 1986 Jun 3;882(1):18–28. doi: 10.1016/0304-4165(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Jain N. K., Bryan A. R., Cohen R. E., Monte L. D., Zawacki S., Nancollas G. H., Slomiany A., Slomiany B. L. Adsorption of human salivary mucins to hydroxyapatite. Arch Oral Biol. 1985;30(5):423–427. doi: 10.1016/0003-9969(85)90070-6. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Mirels L., Monte L. D., Ridall A. L., Levine M. J., Loomis R. E., Lindauer F., Reddy M. S., Baum B. J. Isolation and characterization of a mucin-glycoprotein from rat submandibular glands. Arch Biochem Biophys. 1985 Nov 1;242(2):383–392. doi: 10.1016/0003-9861(85)90222-x. [DOI] [PubMed] [Google Scholar]
- Vasilas A., Molina L., Hoffman M., Haidaris C. G. The influence of morphological variation on Candida albicans adhesion to denture acrylic in vitro. Arch Oral Biol. 1992 Aug;37(8):613–622. doi: 10.1016/0003-9969(92)90123-p. [DOI] [PubMed] [Google Scholar]
- Wilkieson C., Samaranayake L. P., MacFarlane T. W., Lamey P. J., MacKenzie D. Oral candidosis in the elderly in long term hospital care. J Oral Pathol Med. 1991 Jan;20(1):13–16. doi: 10.1111/j.1600-0714.1991.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Schneyer C. A. Composition of saliva in mammalia. Aust J Exp Biol Med Sci. 1981 Feb;59(1):1–53. doi: 10.1038/icb.1981.1. [DOI] [PubMed] [Google Scholar]