Abstract
Background
Chronic abdominal pain is one of the most common gastrointestinal symptoms experienced by patients. Visceral hypersensitivity has been shown to be a biological marker in many patients with chronic visceral pain. We have previously shown that IBS patients with visceral hypersensitivity also have evidence of thermal hyperalgesia of the hand/foot.
Objective
The objective of the current study was to develop an animal model of chronic visceral and somatic hypersensitivity in rats treated with intracolonic trinitrobenzene sulfonic acid.
Design
Male Sprague–Dawley rats (200–250 g) were treated with either 20 mg/rat trinitrobenzene sulfonic acid (TNBS, Sigma Chemical Co.) in 50% ethanol (n = 75), an equivalent volume of 50% ethanol (n = 20) or an equivalent volume of saline (n = 20). The agents were delivered with a 24-gauge catheter inserted into the lumen of the colon. Mechanical and thermal behavioral tests were performed using an automated von Frey and Hargreaves device to evaluate somatic hyperalgesia. Colonic distension was performed using an automated distension device to evaluate visceral pain thresholds. All animals were tested 16 weeks after TNBS treatment following complete resolution of the colitis.
Results
At 16 weeks, 24% of the treated rats (18/75 rats) still exhibited evidence of visceral as well as somatic hypersensitivity compared to saline- and ethanol-treated rats.
Conclusion
Transient colonic inflammation leads to chronic visceral and somatic hypersensitivity in a subset of rats. These findings are similar to the subset of patients who develop chronic gastrointestinal symptoms following enteric infection.
Keywords: Irritable bowel syndrome, Animal model, TNBS, Visceral hypersensitivity, Somatic hypersensitivity
1. Introduction
Chronic abdominal pain is a common gastrointestinal symptom that affects large numbers of patients in the US. Even though the pathophysiology of visceral pain or functional bowel disease is unclear, visceral hypersensitivity is a common biological marker of many functional bowel disorders such as the irritable bowel syndrome (Naliboff et al., 1997; Verne et al., 2001, 2003; Moshiree et al., 2006). Despite the fact that functional abdominal pain is one of the most common gastrointestinal disorders in the United States, the pathophysiological mechanisms of visceral pain are not well understood. It is now well established that most patients with functional abdominal pain demonstrate enhanced perception in response to distension of the gut lumen or visceral hypersensitivity (Naliboff et al., 1997; Verne et al., 2001, 2003). Visceral hypersensitivity is a biological marker for many functional gastrointestinal disorders and may account for symptoms of urgency, bloating, and abdominal pain experienced by many patients with this disorder. The cause of visceral hypersensitivity is unknown but several mechanisms have been postulated and include triggering events such as inflammation, psychological or environmental stress or post-injury sensitization (Mayer and Raybould, 1990; Mayer and Gebhart, 1994; Gebhart, 2000). The effects these triggering events have on primary visceral afferents are now starting to be better understood. In this current study we used colonic inflammation as a trigger for chronic visceral hypersensitivity associated with long-lasting sensitization of the neural pain circuitry after complete resolution of the colitis.
Although the hypersensitivity has been thought to be limited to the gut, many patients with functional abdominal pain frequently complain of pain in body regions somatotopically distinct from the gut, suggesting that central hyperalgesic mechanisms may be involved (Mayer and Gebhart, 1994; Gebhart, 2000). Interestingly, several studies have shown that patients with functional abdominal disorders, such as IBS, also demonstrate hyperalgesia to nociceptive stimuli applied to somatic tissues (Verne et al., 2001, 2003; Bouin et al., 2002; Dunphy et al., 2003). These results suggest that visceral and somatic nociceptive processing overlap (viscerosomatic convergence), particularly in the lumbosacral distribution. Thus, tonic input from the gut may sensitize spinal cord neurons that have viscerosomatic convergence and exhibit somatotopic overlap with the gut.
We found that a subset of rats (5 of 16 rats) retained visceral and somatic hypersensitivity after resolution of colitis induced by colonic trinitrobenzene sulfonic acid (TNBS) (Zhou et al., 2006b). Surprisingly, this subset of rats was hypersensitive on both thermal and mechanical tests. These preliminary results suggest the possibility of a prospective animal model that has some characteristic features of functional abdominal pain and that could address the development of this condition. Thus, our current study evaluated visceral and somatic hypersensitivity in rats following intracolonic instillation of either TNBS, ethanol (vehicle) or saline (control). In this study, we examined behavioral changes at 16 weeks as measured by nociceptive visceral, mechanical, and thermal behavioral tests following complete resolution of colitis. Thus, our specific hypothesis was that approximately 25% of TNBS-treated rats would develop visceral and somatic hypersensitivity following resolution of TNBS colitis, similar to the percent of humans that develop chronic gastrointestinal symptoms such as IBS after infectious diarrhea (Gwee et al., 1996). We anticipated somatic hypersensitivity in somatic areas associated with convergence of colonic and somatic afferents onto common spinal neurons (e.g., paw and/or tail receptive fields).
2. Methods
2.1. Animals and experimental design
A total of 115 male adult Sprague–Dawley rats (Harlan, Indianapolis, Indiana) weighing 200–250 g were used in this experiment. Female rats were not used in this experiment to eliminate changes in sensitivity due to the estrus cycle. Sprague –Dawley rats were used since TNBS colitis has been best characterized in this species (Morris et al., 1989). The rats were housed in pairs under constant temperature and humidity with 12-h light–dark cycles, and were given free access to food and water. Administration of a single dose of TNBS with 50% ethanol was used to produce colonic inflammation as previously described (Morris et al., 1989). Prior to administration of TNBS in the colon, animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50–90 mg/kg). Using 5–6 cm of 24 gauge catheter, 20 mg of TNBS (Sigma–Aldrich, St. Louis, Missouri) in 50% ethanol (total volume, 0.4 ml) was instilled into the lumen of the colon 3–4 cm proximal to the anus (n = 75). An equivalent volume of saline or ethanol was administered into control rats (n = 20) or vehicle rats (n = 20), respectively. Rats were kept in a vertical position for 10 min to avoid leakage of the instilled intracolonic solutions. Rats were monitored daily for changes in body weight, body condition, physical appearance, and behavior during the 16 weeks period following treatment.
The animals were observed daily for changes in body weight, body condition, physical appearance, and behavior. Any animal that loses >10% of its body weight will be examined twice a day. Animals that exhibit signs of weight loss >15% from expected weight, loss of body condition, ascites (severely swollen abdomen), internal bleeding (presence of pale paws), gross rectal bleeding or general distress (hair standing on end) will be euthanized immediately. All procedures were approved by the North Florida/South Georgia Veterans Health System Institutional Animal Care & Use Committee.
Somatic and visceral pain testing were performed 16 weeks following administration of TNBS, ethanol or saline under blind conditions and the order of testing was counterbalanced across groups. Behavioral testing was done following a 12-h fast. The rats were euthanized after all behavior tests were completed and the colon was removed for histopathological study.
2.2. Visceral pain testing
2.2.1. Colonic distension
A polyethylene balloon (3 cm long, 1.5 cm max. diameter) was secured to tubing attached to an automated distension device (G & J Electronic Inc., Toronto, Canada) used to perform colonic distension. The balloon was lubricated and placed into the rat’s distal colon so that the tip of the balloon was 2 cm proximal to the anus. The rats were allowed 10 min to acclimatize before behavior testing began. Using the automated distension device, rats were restrained in a plastic containment device and allowed to acclimate for 15 min before testing. The rats received phasic distension (0–80 mmHg in 5-mmHg ascending increments) of the colon until the first contraction of the testicles, tail or abdominal musculature occurred. This visualization was performed by three independent observers. The visceral pain threshold indicative was defined as the first nociceptive response as previously described (Ness and Gebhart, 1998; Wesselmann et al., 1998). Compliance of the colon was not measured given the rapid distension protocol used in this study. The colonic distensions were repeated four times with 10-min interstimulus intervals and the mean pressures at the nociceptive threshold were recorded for each rat.
2.3. Somatic pain testing
2.3.1. Mechanical stimulation
Mechanical hypersensitivity was measured using an electronic von Frey device (Dynamic Plantar Aesthesiometer; Electronic Unit/Filaments and Calibration Weights, from Ugo Basile S.R.L. Biological Research Apparatus, Italy). Rats were placed on a wire mesh floor in a plastic enclosure. A computer driven filament was then extended up through the mesh floor and exerted an increasing amount of pressure (maximum 50 g) onto the rat’s hind-paw. The force in grams required until the rat withdrew its hind-paw was defined as the mechanical pain threshold. Both hind-paws and both forepaws were tested in each rat. The stimulus was repeated four times following a 5-min interstimulus interval and the mean was calculated for each rat’s hind-paw; the mean was also calculated for each rat’s forepaw.
2.3.2. Thermal stimulation
A thermal stimulus was delivered using the Hargreaves technique (7371 Plantar Test. from Ugo Basile S.R.L. Biological Research Apparatus, Italy) (Hargreaves et al., 1998). Rats were placed in a plastic enclosure on a plexiglass surface and the heat stimulus was applied underneath the plastic chamber. The time in seconds (latency) until the rat withdrew its hind-paw was recorded for each rat. Both hind-paws and both fore-paws were tested in each rat. The stimulus was repeated four times following a 5-min interstimulus interval and the mean was calculated for each rat’s hind-paw; the mean was also calculated for each rat’s forepaw.
2.3.3. Tail flick reflex
The tail reflex was performed by immersing 6–7 cm of the rat’s tail in 50 °C water. The length of time in seconds (latency) until the rat withdrew its tail was measured. The stimulus was repeated four times following a 5-min interstimulus interval and the mean was calculated for each rat.
2.4. Histopathological evaluation of colonic tissues
Immediately following the somatic and visceral pain testing, all rats were euthanized using sodium pentobarbital (120 mg/kg, IP). Following euthanasia, 3 cm of the descending colon was removed and processed for histopathology. Cross-sections of the colons were fixed in formalin, dehydrated in xylene and alcohol, and embedded in paraffin. All of the colonic sections were cut into 50-μm sections and evaluated using standard techniques for H & E staining following administration of saline, ethanol or TNBS. Microscopic evaluation of tissue was done in a blinded fashion by a single pathologist.
2.5. Statistical analysis
Power calculations were performed prior to the study to determine that the number of rats in each group was sufficient. All statistics were run using Prism version 6 (Prism, San Diego, CA). Frequency distribution was used to classify the groups of rats. The rats were separated into two distinct groups from TNBS-treated rats (recovered rats and hypersensitive rats) based on the distribution of the behavioral pain testing. One-way ANOVA followed by Tukey comparison was used to analyze all behavioral test data. Values are expressed as means ± standard deviation (SD) (Table 1).
Table 1.
Summary of visceral and somatic pain testing
| Tests | Saline control rats (n = 20) | Ethanol control rats (n = 20) | Recovered rats (n = 57 of 75) | Hypersensitive rats (n = 18 of 75) | One-way ANOVA Followed by Tukey comparison |
|---|---|---|---|---|---|
| Colonic distention (mmHg) | 53.85 ± 10.27 | 52.42 ± 9.17 | 51.05 ± 11 31 | 17.92 ± 5.03 | p < 0.001 |
| Mechanical stimuli (force/g) | 33.52 ± 6.73 | 32.79 ± 6.08 | 30.82 ± 7.23 | 9.99 ± 4.22 | p < 0.001 |
| Thermal stimuli (latency/s) | 16.63 ± 3.00 | 16.73 ± 3.23 | 16.36 ± 3.75 | 4.99 ± 1.45 | p < 0.001 |
| Tail flick reflex (latency/s) | 5.65 ± 1.10 | 5.94 ± 1.02 | 5.75 ± 1.17 | 2.39 ± 0.72 | p < 0.001 |
All values represent means ± standard deviation (SD).
3. Results
No observable differences were seen in any of the conditions monitored (i.e. weight loss, bleeding, etc.) in any of the treatment groups.
3.1. Histological examination of colons
Regardless of experimental treatment, rats had no evidence of colitis at 16 weeks following administration of saline, ethanol, and TNBS, respectively. There was no evidence of neutrophils in the lamina propria or interstitial edema.
3.2. Behavior testing
TNBS-treated rats demonstrated abnormal posturing and behavior that included repeated licking of the lower abdomen, testicles, and hind-paw and a hunched posture. This behavior started several hours after TNBS administration and lasted for several days. This abnormal behavior has been previously described in other experimental models of visceral inflammation (Ness and Gebhart, 1998; Wesselmann et al., 1998). The remaining results apply to observations made 16 weeks after treatment with TNBS, ethanol, and saline and after histological resolution of colitis.
3.2.1. Colonic distension
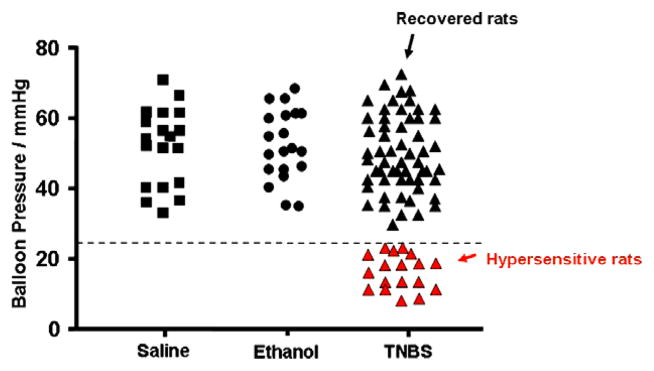
Hypersensitivity to colonic distension (first nociceptive visceral threshold <25 mmHg) was present in 18/75 (24%) of TNBS-treated rats (hypersensitive rats) (Fig. 1). Interestingly, the remaining 57/75 (76%) of TNBS-treated rats (recovered rats) had the same nociceptive visceral threshold as the saline-treated (control) rats and the ethanol-treated (vehicle) rats. One-way ANOVA following Tukey comparison test showed that the p value was <0.001 in the IBS group compared to the other three groups (Table 1).
Fig. 1.
Colonic distension pressures that elicited the nociceptive threshold for each rat. The black squares represent saline controls and the black circles represent the ethanol-treated animals. The TNBS treated animals that recovered without any hypersensitivity are shown as black triangles and the hypersensitive rats are shown as red triangles. All values are means ± standard deviation (see Table 1). The dotted line on the y-axis = 25 mmHg.
3.2.2. Mechanical stimulation
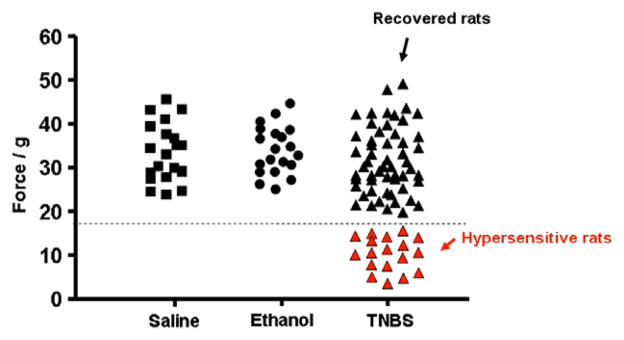
Hypersensitivity to mechanical stimulation (force of <18 g to elicit hind-paw withdrawal) using the von Frey test on hind-paws revealed that mechanical hypersensitivity was present in 18/75 (24%) of TNBS-treated rats (Fig. 2). The rats that had mechanical hypersensitivity were exactly the same rats that exhibited visceral hypersensitivity to colonic distension as described above. The remaining 76% of TNBS-treated rats (recovered rats) had the same nociceptive mechanical threshold as the saline-treated (control) rats and the ethanol-treated (vehicle) rats. One way ANOVA following Tukey comparison test showed that the p value was <0.001 in the IBS group compared to the other three groups (Table 1). However, neither the hypersensitive rats nor recovered rats differed from saline control rats in their mean response to mechanical stimulation of the forepaws. One-way ANOVA following the Tukey comparison test showed a p value greater than 0.05.
Fig. 2.
Mechanical stimulation threshold (g) that elicited hind-paw withdrawal. The black squares represent saline controls and the black circles represent the ethanol-treated animals. The TNBS-treated animals that recovered without any hypersensitivity are shown as black triangles and the hypersensitive rats are shown as red triangles. All values are means ± standard deviation (see Table 1). The dotted line on the y-axis = 18 g.
3.2.3. Thermal stimulation
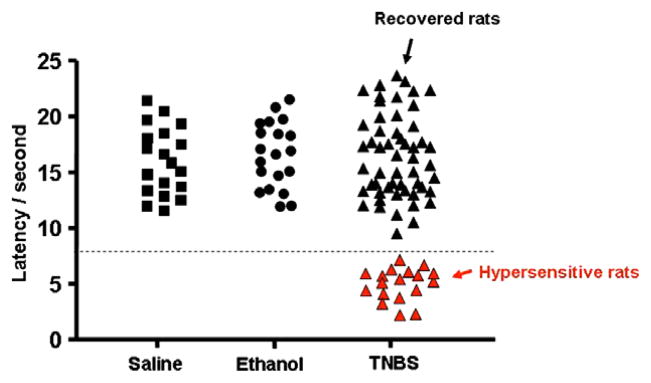
Hypersensitivity to thermal stimulation (latency of <8 s to elicit hind-paw withdrawal) using the Hargreaves technique on the hind-paws was present in 18/75 (24%) of TNBS-treated rats (IBS-rats) (Fig. 3). The rats that had the thermal hypersensitivity were the same rats that exhibited visceral hypersensitivity to colonic distension described above. Again 57/75 (76%) of TNBS-treated rats (recovered rats) recovered and had the same nociceptive thermal threshold as the saline- treated (control) rats and the ethanol-treated (vehicle) rats. One-way ANOVA following Tukey comparison test showed that the p value was <0.001 in the hypersensitive group compared to the other three groups (Table 1). However, neither the hypersensitive rats nor recovered rats differed from saline control rats in their mean response to thermal stimulation of the forepaws. One-way ANOVA following the Tukey comparison test showed a p value greater than 0.05.
Fig. 3.
Thermal stimulation threshold latency (s) that elicited hind-paw withdrawal. The black squares represent saline controls and the black circles represent the ethanol-treated animals. The TNBS-treated animals that recovered without any hypersensitivity are shown as black triangles and the hypersensitive rats are shown as red triangles. All values are means ± standard deviation (see Table 1). The dotted line on the y-axis = 8 s.
3.2.4. Tail flick reflex
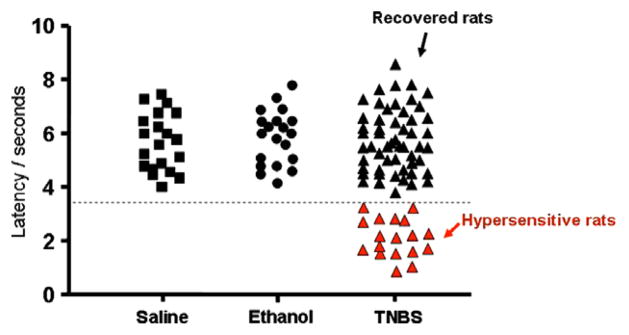
Hypersensitivity to the tail flick reflex (latency of <3.5 s for tail withdrawal) was present in 18/75 (24%) of TNBS-treated rats (Fig. 4). These rats that had the hypersensitivity on the tail reflex test were the same rats that exhibited visceral hypersensitivity to colonic distension described above. A total of 57/75 (76%) of TNBS-treated rats (recovered rats) had the same tail reflex threshold as the saline-treated (control) rats and the ethanol- treated (vehicle) rats. One-way ANOVA following Tukey comparison test showed that the p value was <0.001 in the hypersensitive group compared to the other three groups (Table 1).
Fig. 4.
Tail flick reflect threshold latency (s) that elicited tail withdrawal. The black squares represent saline controls and the black circles represent the ethanol-treated animals. The TNBS-treated animals that recovered without any hypersensitivity are shown as black triangles and the hypersensitive rats are shown as red triangles. All values are means ± standard deviation (see Table 1). The dotted line on the y-axis = 3.5 s.
4. Discussion
The results of our study describe a post-inflammatory animal model in which a subset of rats demonstrates chronic visceral and somatic (thermal and mechanical) hypersensitivity following resolution of TNBS colitis. Thermal hypersensitivity is a unique finding in our current model of visceral and somatic hypersensitivity. Another unique finding is that a distinct subset of rats treated with TNBS displayed chronic somatic and visceral hypersensitivity on all tests that persisted for 16 weeks. Previous studies have evaluated somatic hypersensitivity using only mechanical stimuli (i.e. von Frey, pinch) (Al Chaer et al., 2000; Bourdu et al., 2005) for much shorter time intervals than our study. We characterized somatic hypersensitivity not only to mechanical stimuli (von Frey) but also to nociceptive thermal stimuli to the hind-paws and tail.
Models of colitis have been developed in which colonic irritation in neonatal rats leads to visceral hypersensitivity with characteristics of allodynia and hyperalgesia associated with central sensitization. In one model, rats treated with mustard oil enemas exhibited chronic visceral hypersensitivity, manifested by increased contractility of abdominal muscles and hyperexcitability of viscerosensitive neurons in the lumbosacral cord (L6-S1) in response to colonic distension (Al Chaer et al., 2000). The neurons used for testing of colonic distension were also examined for convergent input from these somatic receptive fields. There was a significant increase in responses of neurons isolated in neonatally treated rats to pinching and deep tissue stimulation of the perineal region, flank, and ipsilateral upper thigh, indicating the possibility of tenderness in the referred field to noxious stimulation. However, when adult rats were treated with colonic irritation in this model, they did not develop visceral and somatic hypersensitivity (Al Chaer et al., 2000) like we have shown in our current study.
Another recent study by Adam et al., 2006 evaluated visceromotor response to colorectal distension with histological evaluation of colonic inflammation and measurement of pro-inflammatory cytokines following TNBS colitis in male Lewis rats. Increased serum cytokine levels of IL-2 and IL-6 were associated with an increase in the visceromotor response to colorectal distension and a dose-dependent increase in the severity of inflammation with increasing doses of TNBS/ethanol (Adam et al., 2006). Another recent study by La et al. (2003) revealed persistent hypersensitivity following intracolonic instillation of 4% acetic acid in male Sprague–Dawley rats. Finally, Bourdu et al. (2005) described a model of colonic hypersensitivity and referred cutaneous lumbar hyperalgesia in female rats following administration of butyrate solution twice daily for 3 days.
Similar to the above studies, our study evaluated visceral hypersensitivity to colonic distension. However, our study differs from previous studies in several aspects. First, we used a high dose of TNBS which produces an intense colitis that involves all muscle layers of the colon and most closely mimics post-infectious IBS following Salmonella or Campylobacter enteritis. Except for Adam et al., 2006, the other studies discussed above used mustard oil, acetic acid or butyrate solution. Adam et al. used a much lower dose of TNBS in Lewis rats unlike our study that used a high dose in Sprague–Dawley rats. Second, our study evaluated animals up to 16 weeks following induction of colitis suggesting that the hypersensitivity developed is chronic. The three studies above only evaluated animals out 7–42 days following colonic irritation.
Although visceral afferents may develop acute mechanosensitization after acute injury as discussed above, there is increasing evidence that persistent hyperalgesia may be a consequence of chronic tissue injury. Inflammatory injury to the colon may sensitize visceral afferents and lead to a chronic hypersensitivity state in which the visceral afferents respond to stimuli that are non-noxious (Habler et al., 1990; Sengupta and Gebhart, 1998). Somatic hypersensitivity may result from tonic input from the colon that sensitizes spinal cord neurons that have viscerosomatic convergence, as we have suggested previously (Zhou et al., 2006b). Consequently, somatic stimuli may cause enhanced responses in these neurons. This tonic input may also dynamically maintain hypersensitivity to both somatic and visceral nociceptive stimulation. This explanation is supported by the current finding that a subset of rats (18/75) treated with TNBS exhibited visceral hypersensitivity and showed somatic hypersensitivity on all tests. The hypersensitivity persisted despite complete histological resolution of colitis, similar to patients with post-infectious IBS (Gwee et al., 1996; Spiller, 2003).
The mechanisms of visceral hyperalgesia are not as well studied as those contributing to somatic hyperalgesia. Although previous studies suggest certain triggering events may lead to chronic somatic and visceral hypersensitivity, little is known about the specific peripheral and central afferents that are sensitized and lead to chronic hypersensitivity. Hyperalgesia in IBS includes both central and peripheral nervous system mechanisms (Bouin et al., 2002; Moshiree et al., 2006). The primary visceral afferents within the gut are known to have an important role in chronic visceral hypersensitivity. The function of the visceral afferents is well defined and conveys gut sensation from the viscera to the central nervous system. The majority of the input from the gastrointestinal tract is not consciously perceived. However, in response to a triggering event such as inflammation, trauma or environmental stress, the gut becomes sensitized to luminal distension and visceral hypersensitivity develops as is seen in patients with IBS (Mayer and Gebhart, 1994). Primary visceral afferents are found in the serosa, muscle, and mucosa of the gut. Visceral afferents respond to mechanical (distension) stimuli and local luminal and chemical stimuli. An earlier study by our laboratory has shown neuronal plasticity in the colonic myenteric plexus during TNBS-colitis (Zhou et al., 2006a). Understanding of the physiology of primary visceral afferents has led to better understanding of the mechanisms that lead to chronic altered sensations from the viscera. These states of abnormal sensations (visceral hypersensitivity) include: interstitial cystitis, functional bowel disorders (i.e., IBS), and ureteric colic (Su and Gebhart, 1998; Gebhart, 2000).
Although our current subset of hypersensitive TNBS rats parallels many functional gastrointestinal disorders, it also has many characteristics seen in patients with post-infectious IBS. For example, the hypersensitivity in IBS patients has previously been thought to be limited to the gastrointestinal tract. However, many patients with IBS also exhibit a wide variety of extra-intestinal symptoms including back pain, migraine headaches, heartburn, dyspareunia, and muscle pain. Collectively, these extra-intestinal symptoms are consistent with the possibility that patients with IBS may also suffer from central hyperalgesic dysfunction (Dubner, 1991; Kajander and Bennett, 1992). The role of central sensitization in IBS patients is now further supported by data that show that IBS patients have hypersensitivity in response to experimental nociceptive cutaneous stimuli in addition to visceral hypersensitivity. The spatial distribution of hyperalgesia in IBS patients shows a gradient, wherein the most pronounced hyperalgesia occurs at lumbosacral levels similar to that seen in response to somatic testing in our hypersensitive rats. Rectal and foot nociceptive afferents are most likely to converge on common spinal neuron at these levels (i.e., viscerosomatic convergence) (Moshiree et al., 2006).
The somatic hypersensitivity is likely to at least partly result from present may be a result of peripheral sensitization, central sensitization and involve dorsal horn mechanisms. Coffin and colleagues demonstrated enhanced spinal cord processing in IBS patients in studies that analyzed enhanced effects of rectal distension on electromyographic recording of the somatic nociceptive flexion reflex (R-III) (Coffin et al., 2004). Sensitization of spinal neurons of human IBS-patients and animal IBS-rats may result from one or more of these factors: (1) facilitatory mechanisms confined within the brain; (2) a spinal sensitization maintained by tonic impulse input from the rectum and/or colon or (3) a mechanism of descending facilitation from the brain to the spinal cord and/or gut. These three possibilities are not mutually exclusive (Moshiree et al., 2006; Price et al., 2006).
In summary, we have demonstrated a model of persistent visceral and somatic hypersensitivity in a subset of rats following transient TNBS colitis that persists to 16 weeks. In addition, our model is the first to demonstrate thermal hypersensitivity of the hind-paw and tail following colonic injury. The somatic hypersensitivity present is at least partly the result of central sensitization that is induced and maintained by tonic nociceptive input from the colon. This animal model will allow the mechanisms of visceral/somatic hypersensitivity in patients with functional gastrointestinal pain to be further elucidated. It can be used to study etiological factors leading to chronic visceral pain as well as molecular mechanisms in the colonic myenteric plexus and spinal cord that lead to persistent hypersensitivity. Future studies are needed to study visceral and somatic hypersensitivity in this model at longer time points (i.e. >16 weeks) following colitis induction; in different species of rats and in female rats.
Acknowledgments
G.N. Verne is supported by a Merit Review Award (PI: G.N. Verne) from the Medical Research Service of the Department of Veteran Affairs and NIH Grant 1-R01-NS053090-01 (PI: G.N. Verne).
References
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–86. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–85. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P, et al. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology. 2005;128:1996–2008. doi: 10.1053/j.gastro.2005.03.082. [DOI] [PubMed] [Google Scholar]
- Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–7. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- Coffin B, Bouhassira D, Sabate JM, Barbe L, Jian R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut. 2004;53:1465–70. doi: 10.1136/gut.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R. Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: Bond M, Charlton E, Woolf CJ, editors. Proceedings of the 6th World Congress on Pain, Pain Res Clin Manage. Vol. 5. 1991. pp. 263–76. [Google Scholar]
- Dunphy RC, Bridgewater L, Price DD, Robinson ME, Zeilman CJ, Verne GN. Visceral and cutaneous hypersensitivity in Persian Gulf War veterans with chronic gastrointestinal symptoms. Pain. 2003;102:79–85. doi: 10.1016/s0304-3959(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–8. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhea. Lancet. 1996;347:150–3. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–62. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1998;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–44. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- La J-H, Kim T-W, Sung T-S, Kang J-W, Kim H-J, Yang I-S. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–5. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99:1688–704. doi: 10.1016/0016-5085(90)90475-g. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–93. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Moshiree B, Zhou Q, Price DD, Verne GN. Central sensitization in visceral pain disorders. Gut. 2006;55:905–8. doi: 10.1136/gut.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–12. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distention as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudoaffective reflexes in the rat. Brain Res. 1998;450:153–69. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contribution to hyperalgesia in irritable bowel syndrome. Pain. 2006;7:529–35. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. The sensory innervation of the colon. Curr Opin Gastroenterol. 1998;14:15–20. [Google Scholar]
- Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–71. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol. 1998;80:2632–44. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998;246:73–6. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Caudle MR, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006a;17:2–3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Stamm P, Price DD, Verne GN. Somatic hypersensitivity in an animal model of IBS. J Pain. 2006b;7:S7. [Google Scholar]