Abstract
Inflammation of visceral structures in rats has been shown to produce visceral/somatic hyperalgesia. Our objectives were to determine if trinitrobenzene sulfonic acid (TNBS) induced colitis in rats leads to visceral/somatic hypersensitivity. Male Sprague-Dawley rats (200g–250g) were treated with 20 mg of TNBS in 50% ethanol (n=40) or an equivalent volume of ethanol (n=40) or saline (n=25) via the colon. Colonic distension, Von-Frey, Hargreaves, and tail reflex test were used to evaluate for visceral, mechanical, and thermal sensitivity. The rats demonstrated visceral hypersensitivity at 2–28 days following TNBS (p<0.0001). The ethanol treated rats also demonstrated visceral hypersensitivity that resolved after day 14. TNBS treated rats demonstrated somatic hypersensitivity at days 14–28 (p<0.0001) in response to somatic stimuli of the hind-paw. TNBS colitis is associated with visceral and somatic hypersensitivity in areas of somatotopic overlap. This model of colitis should allow further investigation into the mechanisms of visceral and somatic hypersensitivity.
Keywords: Animal model, TNBS colitis, visceral pain, visceral hypersensitivity, somatic pain, somatic hypersensitivity, viscerosomatic convergence, irritable bowel syndrome
INTRODUCTION
Visceral pain is a common and debilitating disorder. Many common gastrointestinal disorders such as irritable bowel syndrome are characterized by chronic visceral pain. Despite the fact that visceral pain is a common clinical finding, the pathophysiology is still unclear. The mechanism(s) of visceral pain are not as well studied as those which contribute to somatic pain. Patients with visceral pain often exhibit a wide variety of somatic symptoms including back pain, migraine headaches, and muscle pain. These symptoms may be consistent with central sensitization; referral of visceral pain to somatic tissues outside the area of immediate referral [1–5] or neural cross-talk in which afferent activation of one visceral structure influences efferent output in other structures and organs and is mediated by convergence of sensory pathways in the spinal cord [6–10]..
Somatic hypersensitivity may also occur in clinical conditions where visceral pain is directly referred to a corresponding area of somatic tissue. Referred visceral hypersensitivity is most often related to visceral pain being directly referred to the corresponding area of the abdominal wall [2, 11, and 12]. Animal models have been developed to evaluate referred hypersensitivity following a nociceptive visceral stimulus. Uterine inflammation in rats has been shown to increase sensitivity to stimulation of flank muscles [13]. Others have used bladder inflammation or urethral calculus to evaluate hypersensitivity of the paws and tail [11, 14 and 15]. However, to date, no study has demonstrated both visceral and somatic hypersensitivity following colonic inflammation.
Our current study used an animal model of visceral pain to evaluate if trinitrobenzene sulfonic acid (TNBS) induced colitis leads to visceral and somatic hypersensitivity. We monitored the behavioral development in rats at multiple time points following the inflammatory colonic stimulus. We tested visceral and somatic hypersensitivity at 2, 7, 14, 21, and 28 days following TNBS administration. Our overall objective in this study was to determine if visceral pain induced by acute colitis leads to peripheral somatic hypersensitivity. To accomplish this, we performed colorectal distension (CRD) as well as mechanical and thermal cutaneous stimulation to test visceral and somatic hypersensitivity following TNBS administration. Our hypothesis was that TNBS induced colitis produces both visceral hypersensitivity of the colon and somatic hypersensitivity in the hind-paws and tail.
MATERIALS AND METHODS
Animals Preparation
A total of 105 male adult Sprague-Dawley rats weighing 200–250g were used in this experiment. The rats were housed in pairs under constant temperature and humidity with 12-hour light-dark cycles, and were given free access to food and water. Administration of TNBS with 50% ethanol was used to produce colonic inflammation [16]. Prior to administration of TNBS in the colon, the animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50–90 mg/kg). Using a 5–6 cm of 24 gauge catheter, 20 mg (per rat) of TNBS (Sigma Chemical Co.) in 50% ethanol (total volume, 0.4 ml), was instilled into the lumen of the colon 3–4 cm proximal to the anus (n=40). An equivalent volume of saline (n=25) or ethanol (n=40) was administered into control rats or vehicle rats respectively. Rats were kept in a vertical position for several minutes to avoid leakage of the instilled intracolonic solutions. Rats were monitored daily for changes in body weight, body condition, physical appearance, and behavior following treatment. No adverse effects were observed in any of the rats.
Somatic and visceral pain testing were performed 2, 7, 14, 21, and 28 days following administration of TNBS, ethanol, or saline under blinded conditions and the order of testing was counterbalanced across groups. Behavioral testing was done following a 12 hour fast. The rats were euthanized after all behavior tests were completed and the colon was removed for histopathological study. All procedures were approved by the North Florida/South Georgia Veterans Health System Institutional Animal Care & Use Committee.
Visceral Pain Testing
Colonic Distension
A balloon (3 cm-long, 1.5 cm max diameter) made of polyethylene was secured to tubing attached to an automated distension device (G & J Electronic Inc. Toronto, Canada) was used to perform colonic distension. The balloon was lubricated and placed into the rat’s distal colon so that the tip of the balloon was 1cm from the anus. The rats were allowed 10 minutes to acclimatize before behavior testing began. Using an automated distension device (G & J Electronic Inc. Toronto, Canada) the rats were restrained in a plastic containment device and received phasic distension (0–80 mmHg in 5 mmHg ascending increments) of the colon until the first contraction of the testicles, tail, or abdominal musculature occurred which was defined as the visceral pain threshold indicative of the first nociceptive response as previously described [17, 40]. The colonic distensions were repeated 4 times with 5–10 minute interstimulus intervals and the mean pressures at the nociceptive threshold were recorded for each rat.
Somatic Pain Testing
Mechanical Stimulation
Mechanical hypersensitivity was measured using an automatic Von Frey device (Dynamic Plantar Aesthesiometer; Electronic Unit/Filaments and Calibration Weights, from Ugo Basile S.R.L. Biological Research Apparatus, Italy). Rats were placed on a wire mesh floor in a plastic enclosure. A computer driven filament was then extended up through the mesh floor and exerted an increasing amount of pressure (maximum 50g) onto the rat’s hindpaw. The force in grams required until the rat withdrew its hind-paw was defined as the mechanical pain threshold. Both hind paws were tested in each rat. The stimulus was repeated 4 times following a 5 minute interstimulus interval and the mean was calculated for each rat’s hind paw.
Thermal Stimulation
A thermal stimulus was delivered using the Hargreave’s technique (7371 Plantar Test. From Ugo Basile S.R.L. Biological Research Apparatus, Italy) [18]. Rats were placed in a plastic enclosure on a plexiglass surface and the heat stimulus was applied underneath the plastic chamber. The time in seconds (latency) until the rat withdrew its hind-paw was recorded for each rat. Both hind paws were tested in each rat. The stimulus was repeated 4 times following a 5 minute interstimulus interval and the mean was calculated for each rat’s hind paw.
Tail Flick Reflex
The tail reflex was performed by immersing the rat’s tail 6–7 cm in 50°C water. The length of time in seconds (latency) until the rat withdrew its tail was measured. The stimulus was repeated 4 times following a 5 minute interstimulus interval and the mean was calculated for each rat.
Histopatholgical Evaluation of Colonic Tissues
Immediately following the somatic and visceral pain testing, all rats (8 TNBS; 8 ethanol; and 5 saline per time point: 2, 7, 14, 21, and 28 days; total 40 TNBS; total 40 ethanol; 25 saline) were euthanized using sodium pentobarbital (120mg/kg, IP). Following euthanasia, 3cm of the descending colon was removed and processed for histopathology. The tissue was fixed in formalin and processed using standard techniques for H & E staining. The severity of the lesions in the colon and mucosa were graded using a system previously described [19]. The grades of colitis included: mild (+1) infiltration of a limited number of neutrophils in the lamina propria with minimal interstitial edema; moderate (+2) infiltration of a moderate number of neutrophils in the lamina propria with moderate interstitial edema; severe (+3) diffuse infiltration of neutrophils in the lamina propria with severe interstitial edema.
Statistical Analysis
All statistics were run using Prism version 6. Two way ANOVA following Bonferroni post-test were used to analyze all behavioral test data. Values are expressed as means ± standard deviation (SD).
RESULTS
Histopathology Results
All rats treated with TNBS had colitis (+2–+3) characterized by diffuse infiltration of neutrophils in the lamina propia with severe interstitial edema as previously described (Al Chaer et al., 2000). The colitis was present at all of the time points: 2, 7, 14, 21, and 28 days following TNBS administration each group of rats were euthanized. The ethanol treated colons appeared colitis (+1–+2) characterized by infiltration of a limited or moderated number of neutrophils in the lamina propria with minimal or moderated interstitial edema at days 2 and 7 following ethanol administration. The saline treated colons appeared normal without any pathophysiologic changes.
Behavior Testing
Rats were observed twice daily for 30–60 minutes. TNBS treated rats demonstrated abnormal behavior previously described in acute pain models, including repeated licking of the lower abdomen, testicles, and hind-paw and a hunched position in comparison to control rats (Wesselmann et al., 1998; Laird et al., 2001). This behavior started several hours after TNBS administration and lasted for several days. This abnormal behavior has been previously described in other experimental models of visceral inflammation [17, 40]. In comparison to saline control rats, TNBS treated rats had intermittent runny, loose stool up to day 28 post treatment and exhibited a slight decrease in body weight in comparison to control rats. The difference in body weight was not significant. The ethanol treated rats demonstrated minimal behavior changes after ethanol administration, such as repeated licking of lower abdomen, and testicles areas. These behaviors subsided 5–7 days following.
Colonic Distension
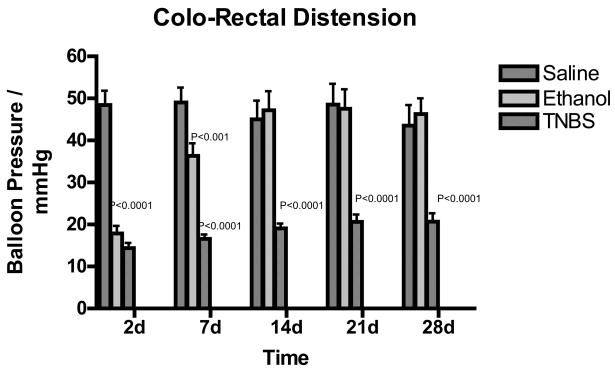
The rats demonstrated visceral hypersensitivity in response to colorectal distension at 2, 7, 14, 21 and 28 days following TNBS treatment (n=40) compared to saline control (n=25) rats. The ethanol treated rats (n=40) also demonstrated visceral hypersensitivity at days 2 and 7, but this hypersensitivity resolved at day 14 following ethanol injection. Two-way analysis of variance indicated p<0.0001. Bonferroni’s post-test revealed that the p< 0.001 at 2, 7, 14, 21, and 28 days after injection of TNBS when compared to saline treated controls (Figure 1).
Figure 1.
Bar graph of colon distension pressures in mmHg vs. days following TNBS treatment. Green indicated Saline, blue indicated ethanol treated rats and red indicated TNBS treated rats. Values are expressed as means ± SEM.
Mechanical Stimulation
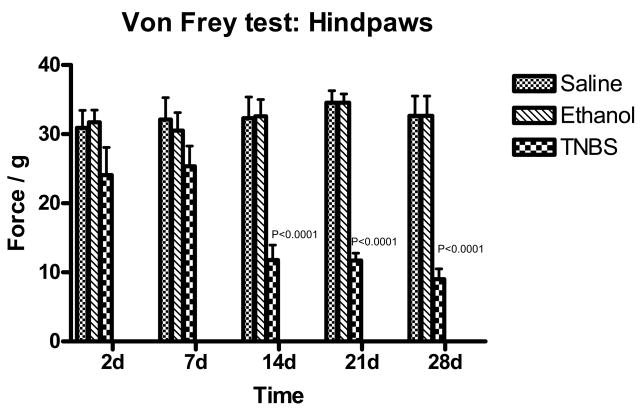
Von-Frey tested on hind-paws revealed hypersensitivity at 14, 21, and 28 days following TNBS administration (Figure 2). Two-way analysis of variance indicated p< 0.0001. Bonferroni’s post-test revealed p<0.001 for 14, 21, and 28 days after administration of TNBS compared to saline and ethanol treated rats.
Figure 2.
Bar graph of mechanical threshold testing on hind-paws in force/g vs. days following TNBS treatment. Green indicated Saline, blue indicated ethanol treated rats and red indicated TNBS treated rats. Values are expressed as means ± SEM.
Thermal Stimulation
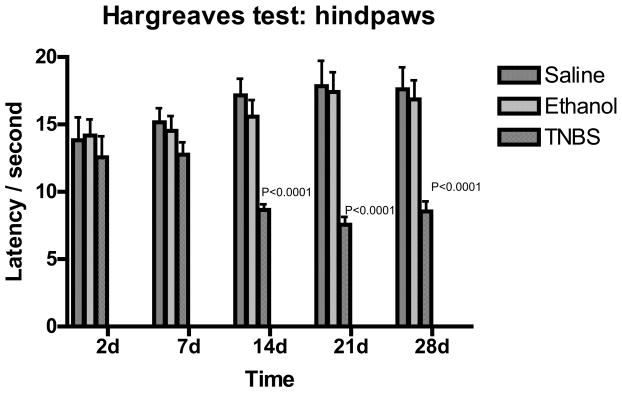
Thermal nociceptive stimuli was applied to hind-paws using Hargreaves’ method [18]. Hypersensitivity as indicated by a reduced latency in response to thermal stimulation was found at 14, 21, and 28 days after TNBS injection (Figure 4). Two-way analysis of variance indicated p< 0.0001. Bonferroni’s post-test revealed p<0.001 for 14, 21, and 28 days after TNBS administration when compared to saline treated control and ethanol treated animals.
Figure 4.
Bar graph of tail flick shown as latency vs. days following TNBS treatment. Green indicated Saline, blue indicated ethanol treated rats and red indicated TNBS treated rats. Values are expressed as means ± SEM.
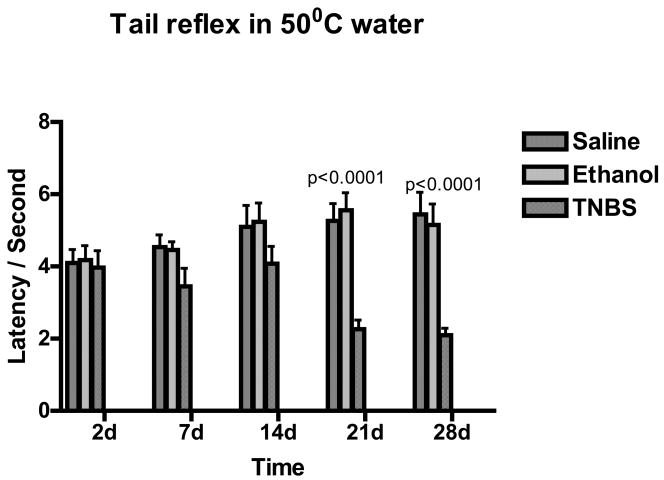
Tail Flick Reflex
Tail reflex test response to 50oC water stimulus, indicated hypersensitivity at 21 and 28 days following TNBS injection (Figure 4). Two-way ANOVA, p value <0.0001; Bonferroni’s post-test indicated p value <0.001 for 21 and 28 days following TNBS administration when compared to saline treated controls and ethanol treated rats.
DISCUSSION
The results of our current study suggest that TNBS colitis in rats produces both visceral and somatic hypersensitivity. The presence of somatic hypersensitivity is a new finding as previous studies using TNBS colitis in rats have only evaluated colonic hypersensitivity [20–24]. There have been several previous studies that have used other visceral cavities (i.e. bladder, uterus) other than the colon to evaluate somatic hypersensitivity [5, 14, and 15, 25–30]. Our current study revealed increased somatic hypersensitivity in the TNBS-induced colitis model. This is in contrast to several other studies that have shown decreased somatic sensitivity in areas outside the region of referred pain following visceral stimulation [31–34]. However, unlike the present study that examined somatic sensitivity over 28 days, these previous studies examined somatic sensitivity for only brief periods after visceral stimulation (e.g. 4 hours [34]. Thus, whereas the immediate effects of noxious visceral stimulation on somatic sensitivity may be inhibitory, the long term effects are facilitatory and more relevant for persistent visceral pain conditions.
Several factors may account for our findings including the time course of the visceral stimulus, the type of inflammatory agent used, and the site of visceral stimulus (colon vs. bladder/uterus). Previous studies tested somatic hypersensitivity within hours after introduction of an inflammatory or nociceptive agent into the viscera [11, 30, 34]. We tested animals up to 4 weeks following the induction of colitis. The lack of somatic hypersensitivity in these earlier studies may be due to the longer time needed to produce sensitization of spinal sensory neurons to somatic stimuli as compared to visceral stimuli. Our results are consistent with this interpretation. Whereas visceral hypersensitivity was present within the spinal cord at our first time of testing (2 days), thermal and mechanical hypersensitivity took several days to develop (Figs. 2–4). Severe colitis was present at all of the time points tested in our model.
From a mechanistic perspective it is interesting that hypersensitivities to different stimuli developed at different time points. Thus, visceral hypersensitivity developed at 2 days, mechanical and thermal hypersensitivity to radiant heat developed at 14 days, and hypersensitivity on the tail reflex test developed at 21 days. Interestingly, these time points of development are the same as the development of increased expression of different types of NMDA receptor subunits between 14 to 28 days following TNBS administration [35]. In contrast to visceral hypersensitivity that is based on immediately inflamed tissues, delays in somatic hypersensitivity may be due to gradual spatial spread of spinal cord neuron sensitization and/or delayed upregulation of different types of glutamate receptors, including NMDA and non-NMDA receptors.
The somatic hypersensitivity present is likely to be a result of central sensitization of spinal dorsal horn neurons that receive somatovisceral convergence [6–10, 36]. An additional factor could be related to neural cross-talk in the pelvis [10]. The persistent colitis may result in the peripheral and/or central release of some excitatory mediator that produces and maintains central sensitization, eventually leading to somatic hypersensitivity.
In our study, there was persistent colitis up to 28 days following TNBS administration. These findings differ from Asfaha et al [37] in which the inflammation peaked at day 3 and then gradually resolved. Our findings may be different as we used Sprague-Dawley rats and not Wistar rats. The intensity and duration of inflammation may very well be strain related. The study by Wells et al [38] used a different concentration of TNBS from our study which makes it difficult to compare the results to our findings. Our TNBS colitis data was most consistent with Morris’ group’s findings [40]. Another likely reason for the differences in the duration of inflammation is the agent used to induce colitis. Mahgoub et al [41] used intracolonic 3% ascetic acid which produced severe colitis 24 hours after administration. Kimball et al [42] used mustard oil that caused a peak inflammation at day 3 and was resolved by day 7. The Natah group found that a quantitative analysis of the endothelial barrier antigen (EBA) after TNBS induced colitis resulted in occluding expression in the frontal cortex. A significant decrease in EBA expression was shown at 2 and 7 days. However, this study was different from our current study, in which we measured somatic behavioral changes after TNBS induced colitis [43].
Although hypersensitivity in chronic visceral pain disorders such as IBS has previously been thought to be limited to the gastrointestinal tract, these patients also exhibit a wide variety of somatic symptoms including back pain, migraine headaches, heartburn, dyspareunia, and muscle pain, consistent with our present results. Collectively, these somatic symptoms are consistent with the possibility that patients with chronic visceral pain disorders may also suffer from central hyperalgesic dysfunction [44]. In fact recent investigations suggest that in both animal models and patients with chronic visceral pain there is evidence of referred somatic hypersensitivity [19, 45–48]. These findings are different from earlier studies which have revealed somatic hyposensitivity in patients with chronic visceral pain disorders such as inflammatory bowel disease and IBS [3, 4, 49–52]. One very plausible explanation for these different findings in humans may be due to the specific type of nociceptive stimuli used. It is possible that previous studies that failed to reveal somatic hypersensitivity in outside referral areas because they did not use nociceptive stimuli that were intense or long enough to stimulate NMDA receptor mechanisms associated with sensitization and somatic hypersensitivity.
In summary, TNBS induced colitis in the rat leads to visceral and somatic hypersensitivity. The somatic hypersensitivity present reflects tonic impulse input from the inflamed colon along with central sensitization. Further studies are needed to determine the duration of visceral and somatic hypersensitivity following resolution of the TNBS colitis. In addition, molecular studies of spinal cord are needed to be done to evaluate potential mediators of colitis-induced visceral and somatic hypersensitivity.
Figure 3.
Bar graph of thermal sensitivity testing on hind-paws shown as latency vs. days following TNBS treatment. Green indicated Saline, blue indicated ethanol treated rats and red indicated TNBS treated rats. Values are expressed as means ± SEM.
Acknowledgments
Dr. Verne is supported by a Merit Review Award (PI: GN Verne) from the Medical Research Service of the Department of Veteran Affairs and a NIH Grant 1-R01-NS053090-01 (PI: GN Verne).
References
- 1.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;1:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 2.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. 1999. [DOI] [PubMed] [Google Scholar]
- 3.Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur JPain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000a;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 7.Janig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst. 1990;30(Suppl):S89–S96. doi: 10.1016/0165-1838(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC, Booth AM, Yoshimura N. Maggi, C A The autonomic nervous system. London, England: Harwood; 1993a. Neurophysiology of micturition and its modification in animal models of human disease; pp. 227–290. [Google Scholar]
- 9.de Groat WC, Roppolo JR, Yoshimura N, Sugaya K. In: Tache Y, Wingate D, Burks T, editors. Neural control of the urinary bladder and colon; Proceedings of the second international symposium on brain-gut interactions.Boca Raton, FL: CRC; 1993b. pp. 167–190. [Google Scholar]
- 10.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Giamberardino MA, Valente R, de Bigontina P, Vecchiet L. Artificial ureteral calculosis in rats: behavioural characterization of visceral pain episodes and their relationship with referred lumbar muscle hyperalgesia. Pain. 1995;61:459–469. doi: 10.1016/0304-3959(94)00208-V. [DOI] [PubMed] [Google Scholar]
- 12.Giamberardino MA, Affaitati G, Lerza R, Vecchiet L. Preemptive analgesia in rats with artificial ureteric calculosis. Effects on visceral pain behavior in the post-operative period. Brain Res. 2000;878:148–154. doi: 10.1016/s0006-8993(00)02728-1. [DOI] [PubMed] [Google Scholar]
- 13.Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998a;246:73–76. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- 14.McMahon SB, Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- 15.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 16.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 17.Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998b;246:73–76. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- 18.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 19.Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:75–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich AE, Gebhart GF. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J Pharmacol Exp Ther. 2000;292:538–544. [PubMed] [Google Scholar]
- 22.Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther. 2002;302:1013–1022. doi: 10.1124/jpet.302.3.1013. [DOI] [PubMed] [Google Scholar]
- 23.Tarrerias AL, Millecamps M, Alloui A, Beaughard C, Kemeny JL, Bourdu S, Bommelaer G, Eschalier A, Dapoigny M, Ardid D. Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain. 2002;100:91–97. doi: 10.1016/s0304-3959(02)00234-8. [DOI] [PubMed] [Google Scholar]
- 24.Fioramonti J, Gaultier E, Toulouse M, Sanger GJ, Bueno L. Intestinal anti-nociceptive behaviour of NK3 receptor antagonism in conscious rats: evidence to support a peripheral mechanism of action. Neurogastroenterol Motil. 2003;15:363–369. doi: 10.1046/j.1365-2982.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 26.Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94:507–513. doi: 10.1097/00000542-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Jaggar SI, Scott HC, James IF, Rice AS. The capsaicin analogue SDZ249–665 attenuates the hyper-reflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain. 2001;89:229–235. doi: 10.1016/s0304-3959(00)00366-3. [DOI] [PubMed] [Google Scholar]
- 28.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 29.Palecek J, Paleckova V, Willis WD. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neuroscience. 2003;116:565–572. doi: 10.1016/s0306-4522(02)00660-7. [DOI] [PubMed] [Google Scholar]
- 30.Kalmari J, Pertovaara A. Colorectal distension-induced suppression of a nociceptive somatic reflex response in the rat: modulation by tissue injury or inflammation. Brain Res. 2004;1018:106–110. doi: 10.1016/j.brainres.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 32.Zhuo M, Gebhart GF. Inhibition of a cutaneous nociceptive reflex by a noxious visceral stimulus is mediated by spinal cholinergic and descending serotonergic systems in the rat. Brain Res. 1992;585:7–18. doi: 10.1016/0006-8993(92)91185-h. [DOI] [PubMed] [Google Scholar]
- 33.Cueva-Rolon R, Gomez LE, Komisaruk BR, Munoz-Martinez EJ. Inhibition of withdrawal responses by pelvic nerve electrical stimulation. Brain Res. 1995;679:267–273. doi: 10.1016/0006-8993(95)00243-j. [DOI] [PubMed] [Google Scholar]
- 34.Traub RJ, Wang G. Colonic inflammation decreases thermal sensitivity of the forepaw and hindpaw in the rat. Neurosci Lett. 2004;359:81–84. doi: 10.1016/j.neulet.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Zhou QQ, Caudle MR, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;17(2):3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol. 1999;276:G703–G710. doi: 10.1152/ajpgi.1999.276.3.G703. [DOI] [PubMed] [Google Scholar]
- 38.Wells RW, Blennerhassett MG. Persistent and selective effects of inflammation on smooth muscle cell contractility in rat colitis. Pflugers Arch. 2004;448:515–524. doi: 10.1007/s00424-004-1286-1. [DOI] [PubMed] [Google Scholar]
- 39.La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudoaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 41.Mahgoub A, El Medany A, Mustafa A, Arafah M, Moursi M. Azithromycin and erythromycin ameliorate the extent of colonic damage induced by acetic acid in rats. Toxicol Appl Pharmacol. 2005;205:43–52. doi: 10.1016/j.taap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Kimball ES, Palmer JM, D’Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- 43.Natah SS, Mouihate A, Pittman QJ, Sharkey KA. Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol Motil. 2005;17:433–446. doi: 10.1111/j.1365-2982.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 44.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 45.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 47.Dunphy RC, Bridgewater L, Price DD, Robinson ME, Zeilman CJ, Verne GN. Visceral and cutaneous hypersensitivity in Persian Gulf war veterans with chronic gastrointestinal symptoms. Pain. 2003;102:79–85. doi: 10.1016/s0304-3959(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 48.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 49.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 50.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 51.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40:819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]
- 52.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000b;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]