Abstract
Background
Black patients are at greater of risk of death from bladder cancer than white patients. Potential explanations for this disparity include a more aggressive phenotype and delays in diagnosis resulting in higher stage disease. Alternatively, black patients might receive lower quality of care, which may explain this difference.
Methods
Using SEER-Medicare data for the years 1992–2002, we identified patients with early stage bladder cancer. We fitted multivariable models to measure relationships between race and mortality, adjusting for differences in patients and treatment intensity. Next, we fitted shared frailty proportional hazards models to evaluate whether the disparity is explained by differences in the quality of care provided.
Results
Compared to white patients (n=14,271), black patients (n=342) were more likely to undergo restaging resection (12.0% vs. 6.5%, p<0.01) and urine cytologic evaluation (36.8% vs. 29.7%, p<0.01), and yet received fewer endoscopic evaluations (4.0 vs. 5.0, p<0.01). The use of aggressive therapies (cystectomy, systemic chemotherapy, radiation) was similar among black and white patients (12.0% vs. 10.2%, respectively, p=0.31). Although blacks had a greater risk of death compared to whites (HR 1.23, 95% CI 1.07–1.42), this risk was only modestly attenuated after adjusting for differences in treatment intensity and provider effects (HR 1.22, 95%CI 1.06–1.42).
Conclusions
Although differences in initial treatment are evident, they do not appear to be systematic and are of unclear clinical significance. While black patients are at higher risk of death, this disparity does not appear to be due to differences in the intensity or quality of care provided.
Keywords: disparities, bladder cancer, quality
Introduction
Among those with bladder cancer, black patients have a 70% higher risk of cancer-related death compared to white patients.1 Even among those with localized disease, black patients have significantly worse 10-year disease-specific survival (81% vs. 88%).2 While the underpinnings for this disparity are not entirely clear, plausible explanations include a more aggressive cancer phenotype (i.e., tumor biology), delays in diagnosis resulting in a higher stage disease at presentation, and a greater burden of comorbid diseases. Because early stage (i.e., superficial or non-muscle-invasive) bladder cancer is traditionally thought of as a non-lethal disease, comorbidity may be an important contributor to apparent cancer-related mortality due to difficulties in ascertaining the cause of death (i.e., attribution bias).3
Alternatively, disparities in survival may be the end result of differences in the health care provided to black and white patients. On one hand, the disparity may reflect differential treatment by race. For example, among those with early stage lung cancer, the lower survival rate for black patients appears to be due to the less frequent use of surgery in this group.4 With regard to early stage bladder cancer, differences in the use of surveillance (e.g., endoscopy) and treatment (e.g., intravesical therapy) might underlie the observed survival disparity. On the other hand, survival differences might be attributable to the quality of care provided. Black patients undergoing radical cystectomy are nearly 70% more likely of dying postoperatively compared to white patients,5 a finding which is largely a consequence of the quality of the hospital setting6 where they more commonly receive their care (i.e., low volume with limited access to potentially necessary health services). In the setting of early stage bladder cancer, the physician, rather than the hospital, plays a principal role in determining treatment and outcomes. Because black and white patients generally receive their care by dissimilar physicians,7 differences in the quality of the bladder cancer care provided may explain the observed disparity in mortality.
For this reason, we undertook a study to better understand racial differences in the treatment of patients with early stage bladder cancer. Using national Surveillance, Epidemiology, and End Results (SEER)-Medicare data, we explored the extent to which disparities in mortality are explained by differences in the intensity and quality of the care provided.
Methods
Study Population
We used the SEER-Medicare linked database for the years 1992 through 2002 to identify patients diagnosed with early stage bladder cancer. These files provide information on Medicare patients included in SEER,8 a collection of population-based registries of all incident cancers that comprised approximately 26% of the US population by the end of the study period.9 For each Medicare patient in SEER, the SEER-Medicare linked files contain 100% of Medicare claims from the inpatient, outpatient and national claims history files.
From these files, all fee-for-service Medicare patients aged 65 to 99 with incident cases of bladder cancer were identified by the appropriate code in SEER. We limited our study population to patients with early stage bladder cancer [Ta (noninvasive papillary carcinoma), Tis (carcinoma in situ) and T1 (tumor invades subepithelial connective tissue)]10 using the extent-of-disease codes provided by SEER. All patients were followed using Medicare claims through December 31, 2005.
Characterization of treatment
We explored how patients with bladder cancer were managed using International Classification of Diseases, Ninth Revision (ICD-9) procedure and Healthcare Common Procedure Coding System (HCPCS) codes in the Medicare files during the first 2 years after diagnosis. We focused on practices that were relevant to early stage bladder cancer care, including surveillance (endoscopic examination of the bladder, upper urinary tract evaluation, urinary studies, and imaging studies), treatment (intravesical therapy and repeat endoscopic resection within 60 days of the initial resection), and medical services (visits to the urologist and visits to other physicians).
To serve as a proxy for initial treatment intensity, we used early stage bladder cancer expenditures within the first 2-years after diagnosis. Briefly, expenditures were measured at the patient level and included all Medicare payments associated with a primary diagnosis of bladder cancer (ICD-9 codes: 188.x—bladder cancer, 233.7—carcinoma in situ of the bladder, and V105.4—personal history of bladder). Expenditures related to major interventions (cystectomy, chemotherapy, and radiation) were not included. All payments were price-adjusted to 2005 dollars using the Medicare Economic Index and standardized by region.11
Because an objective of our study was to evaluate the extent to which any observed disparity in mortality was attributable to the quality of care provided by the treating physician, it was necessary to assign each patient to a provider. To ascertain the physician primarily responsible for each patient’s bladder cancer care, we first identified all bladder cancer-related procedures, as described by others,12 performed within a 2-year period following their diagnosis. Next, we allocated each patient to the provider with the majority of claims using the Unique Physician Identifier Number. Because it was necessary to obtain reliable estimates of physician’s practice styles, we limited our study to only those physicians with at least 10 patients. Using this method, our final population consisted of 14,613 patients treated by 656 providers.
Outcomes
Our primary outcome measure was mortality, which was assessed from January 1, 1992 thru December 31, 2005. Due to concerns about appropriately assigning the cause of death,3, 13–16 we used all-cause mortality as our primary outcome. However, recognizing that the vast majority of patients with early stage bladder cancer are likely to die from competing causes,17 we also measured bladder cancer-specific mortality using the cause-of-death field available in SEER. We also assessed the use of a major intervention as evidenced by use of radical cystectomy, systemic chemotherapy, and radiation therapy, which could occur at any time during the study period. Further, a composite variable was constructed to reflect the downstream use of any of these therapies. These secondary outcomes were identified by using appropriate ICD-9 and HCPCS codes in the inpatient, national claims history, and outpatient files.
Statistical Analysis
For all of our analyses, our exposure was patient-level race (white, black) as measured by SEER. We first examined differences in patient demographics and disease characteristics according to race. Then, we characterized the extent to which early stage bladder cancer care (surveillance, treatment, and medical services) varied by race. For all of these comparisons, statistical inference was made using chi-square or Kruskal-Wallis tests for categorical and continuous data, respectively.
For the purpose of understanding the relationship between race and mortality, we fitted Cox proportional hazards models adjusting for patient and disease characteristics, including patient age group (5-year intervals), gender, tumor grade (low, medium, high, unknown), and tumor stage (Ta, Tis, T1, and Ta/T1 unspecified).10 Additionally, we adjusted for socioeconomic status using a composite measure assessed at the ZIP code level, as described by Diez-Roux.18 Patient comorbidities were identified using health care encounters in the 12-month period preceding the bladder cancer diagnosis using the well-established methods described by Klabunde and colleagues.19 Next, we evaluated the extent to which variation in the intensity of initial treatment for bladder cancer provided might explain differences in mortality by adjusting for patient-level treatment intensity. Finally, we explored whether the provider contributed to any observed disparities in survival by fitting a shared frailty proportional hazards model, including a provider-level random-effects term.20 This approach accounts for the correlation of mortality outcomes within a provider and for the heterogeneity between providers.
For the secondary outcomes (use of cystectomy, systemic chemotherapy, and/or radiation therapy), we fitted generalized estimating equations to evaluate the relationship between race and each patient-level outcome, adjusting for age, gender, comorbidity, socioeconomic status, tumor grade, and tumor stage. This approach allowed us to account for the potential correlation of our observations (i.e., patients clustered within providers).21 We then used post-estimation commands to predict adjusted percentages for the receipt of each intervention by race.
All analyses were carried out using computerized software (SAS version 9.2 and Stata, version 10). All tests were two-tailed and the probability of Type 1 error was set at 0.05. The study protocol was approved by the institutional review board of the University of Michigan.
Results
Black patients with early stage bladder cancer had significantly lower median overall survival compared to white patients (4.4 vs. 6.5 years, log rank p<0.001). Table 1 illustrates differences in patient and disease characteristics according to race. Importantly, black patients were of lower socioeconomic status and had higher levels of comorbidity (both p<0.01). However, disease severity at diagnosis, as measured by cancer grade and stage, did not vary by race.
Table 1.
Patient characteristics by race
| Characteristic | White | Black | p-value |
|---|---|---|---|
| Number of patients (%) | 14,271 (97.7) | 342 (2.3) | |
| Median treatment intensity, in 2005 Dollars* | $2,778 | $2,768 | 0.61 |
| Age, % | 0.57 | ||
| 65–69 | 14.0 | 13.2 | |
| 70–74 | 24.4 | 24.9 | |
| 75–79 | 25.8 | 29.5 | |
| 80–84 | 20.4 | 19.0 | |
| 85+ | 15.4 | 13.5 | |
| Female Gender, % | 25.4 | 32.7 | 0.003 |
| Socioeconomic status, % | <0.001 | ||
| Low | 29.8 | 74.3 | |
| Medium | 35.7 | 19.0 | |
| High | 34.4 | 6.7 | |
| Comorbidity, % | 0.004 | ||
| 0 | 43.4 | 35.4 | |
| 1 | 30.2 | 30.7 | |
| 2 | 14.8 | 21.6 | |
| 3+ | 11.6 | 12.3 | |
| Tumor grade, % | 0.12 | ||
| Low | 19.5 | 17.8 | |
| Medium | 45.8 | 40.6 | |
| High | 27.6 | 31.3 | |
| Unknown | 7.1 | 10.2 | |
| Tumor stage, % | 0.26 | ||
| Ta | 57.2 | 54.3 | |
| Tis | 6.9 | 8.9 | |
| T1 | 24.6 | 26.7 | |
| Unspecified | 11.3 | 10.2 |
Represent expenditures median per capita expenditures for the first 2-years after diagnosis
The initial treatment of early stage bladder cancer care varied according to race (Table 2). Generally, black patients were more intensively followed with urine cytology (0.80 vs. 0.71 tests, p<0.01). Moreover, black patients were nearly twice as likely to undergo restaging resection of their cancer compared to whites (12.0% vs. 6.5%, p<0.01). In contrast, black patients had fewer endoscopic evaluations of the bladder relative to white patients (4.0 vs. 5.0 studies, p<0.01). The use of intravesical therapy did not vary by race.
Table 2.
Differences in early stage bladder cancer care during the first 2 years after diagnosis
| Category of Care | Process of Care | White | Black | p-value |
|---|---|---|---|---|
| Surveillance-Related | Endoscopic surveillance, mean† | 5.0 | 4.0 | <0.001 |
| Any upper urinary tract evaluation,%‡ | 24.9 | 25.7 | 0.70 | |
| Radiographic imaging studies, mean† | 0.67 | 0.65 | 0.94 | |
| Urinary cytology, mean† | 0.71 | 0.80 | 0.009 | |
| Any urine cytology,%‡ | 29.7 | 36.8 | 0.006 | |
| Urinalysis, mean† | 4.5 | 3.4 | <0.001 | |
| Treatment-related | Intravesical therapy, mean† | 3.5 | 3.3 | 0.65 |
| Induction courses* of intravesical therapy, mean† | 0.30 | 0.26 | 0.51 | |
| Any induction intravesical therapy,%‡ | 26.8 | 25.7 | 0.67 | |
| Repeat endoscopic resection,%‡ | 6.5 | 12.0 | <0.001 | |
| Medical Services | Visits to the urologist, mean† | 3.8 | 3.7 | 0.92 |
| Visits to the other physicians, mean† | 21.7 | 25.5 | <0.001 |
An induction course represents at least 5 instillations within a 45-day period
For continuous measures (e.g., endoscopic surveillance), the mean represents the average number of the service performed for each patient. For example, on average, black patients underwent 4 endoscopic procedures and white patients underwent 5 endoscopic procedures during the first 2 years after diagnosis.
For categorical measures (e.g., any upper tract evaluation), the percentage represents the fraction of patients receiving that service. For example, 25.7% of black patients and 24.9% of white patients had any upper tract imaging performed within the first 2 years after diagnosis.
The overall use of downstream major interventions was similar among black and white patients (Figure 1). Although black patients were more likely to undergo radiation therapy (5.6% vs. 3.2%, p=0.02) and systemic chemotherapy (8.1% vs. 5.3%, p=0.04) compared to white patients, the use of radical cystectomy was similar between the two groups. On average, the use of any major intervention did not vary significantly by race with 12.0% of black patients and 10.2% of white patients receiving treatment (p=0.31).
Figure 1.
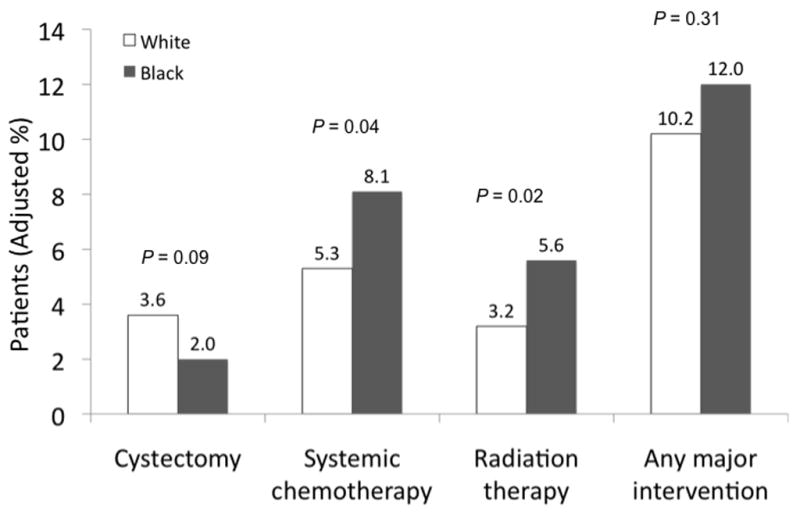
The use of major interventions stratified by race and adjusted for age, gender, socioeconomic status, comorbidity, cancer grade, and cancer stage.
As illustrated in Table 3, black patients had a 23% higher risk of death compared to white patients after adjusting for differences in clinical characteristics. This risk was only modestly attenuated after adjusting for differences in treatment intensity and the effect of the provider (adjusted HR 1.22, 95%CI 1.06–1.42). Similar modest attenuations were evident within stage strata and when using cancer-specific survival as the outcome.
Table 3.
Effects of treatment intensity and provider on risk of mortality
| Model | Category | Adjusted* HR(95% CI) | Adjusted** HR(95% CI) | Adjusted*** HR(95% CI) |
|---|---|---|---|---|
| All-cause Mortality | ||||
| All patients | White | 1.0 | 1.0 | 1.0 |
| Black | 1.23 (1.07–1.42) | 1.22 (1.06–1.42) | 1.22 (1.06–1.42) | |
| Ta | White | 1.0 | 1.0 | 1.0 |
| Black | 1.33 (1.08–1.63) | 1.34 (1.09–1.66) | 1.35 (1.09–1.66) | |
| T1 | White | 1.0 | 1.0 | 1.0 |
| Black | 1.41 (1.10–1.83) | 1.40 (1.09–1.81) | 1.40 (1.08–1.81) | |
| Tis | White | 1.0 | 1.0 | 1.0 |
| Black | 1.03 (0.64–1.65) | 1.04 (0.65–1.66) | 1.05 (0.62–1.76) | |
| Cancer-specific Mortality | ||||
| All patients | White | 1.0 | 1.0 | 1.0 |
| Black | 1.79 (1.30–2.47) | 1.85 (1.35–2.56) | 1.73 (1.23–2.43) | |
| Ta | White | 1.0 | 1.0 | 1.0 |
| Black | 2.28 (1.34–3.87) | 2.37 (1.40–4.02) | 2.34 (1.33–4.10) | |
| T1 | White | 1.0 | 1.0 | 1.0 |
| Black | 1.79 (1.11–2.88) | 1.83 (1.14–2.95) | 1.86 (1.12–3.09) | |
| Tis | White | 1.0 | 1.0 | 1.0 |
| Black | 2.16 (0.75–6.20) | 2.21 (0.76–6.41) | 2.24 (0.72–6.99) | |
Adjusted for age, gender, socioeconomic status, comorbidity, grade, and stage (Note: for stage strata models, stage not included as a covariate)
Adjusted for above and patient-level treatment intensity
Adjusted for above and provider as a random effect
Comment
Black patients diagnosed with early stage bladder cancer are at higher risk of death compared to white patients. However, this disparity does not appear due to the presentation with more severe disease, as measured by bladder cancer stage and grade. Not surprisingly, early stage bladder cancer care varies by race, although the majority of these differences are of questionable clinical significance. Lacking context, these findings might suggest gaps in the quality of care as a principal source for this disparity. However, differences in initial treatment intensity and in the provider responsible for the bladder cancer care failed to account for virtually any of the excess mortality risk. Further, the use of major medical interventions, including radical cystectomy, was similar among black and white patients.
Racial disparities in survival after a cancer diagnosis are well described for a variety of malignancies, including bladder cancer.2, 5, 6 In one study, black patients had nearly 70% higher risk of dying from bladder cancer compared to whites.1 However, investigations into the underpinnings of this disparity have largely focused on delayed diagnosis2 or perioperative care following radical cystectomy, which has traditionally had higher operative mortality rates for blacks.5, 6 In one study using national data, black patients were 66% more likely to die perioperatively compared to whites, even after adjusting for patient differences.5 For such high risk procedures, it is generally believed that these disparities are a direct reflection of hospital quality and the fact that minorities generally seek care at lower quality hospitals.22 In fact, white and black patients tend to have similar mortality rates when treated at the same hospital,6 underscoring the importance of the setting and the provider to high risk operations.
Importantly, our study illustrates that racial disparities in mortality are equally evident for those with early stage bladder cancer. When considering patients diagnosed with bladder cancers of all stages, black patients are diagnosed with more advanced disease,23 a discrepancy that has generally been thought to underlie the observed survival differences. However, in this study of patients with early stage disease, we did not observe a predilection for more aggressive phenotypes (e.g., T1 bladder cancer) by race. That is, racial differences in survival in this population were not secondary to higher grade and stage bladder cancer. Further, in contrast to the literature surrounding radical cystectomy, we observed no protective effect of the provider on survival differences. Indeed, our data indicate that the mortality disparity is persistent and equally robust even after adjusting for differences in the provider and the treatment intensity.
A principal limitation of our analysis relates to unmeasured patient differences that may confound relationships between race and mortality, an important consideration given the relatively small number of black patients in the study. In particular, black patients may have more aggressive bladder cancer and medical diseases that explain disparities in mortality risk. We addressed this well-described limitation of observational data24, 25 in several ways. First, we used a clinical registry to ascertain cancer stage and grade, arguably the most important determinants of death in the bladder cancer population.26, 27 Second, we ascertained comorbid conditions using a well-described methodology19 incorporating data from both inpatient and outpatient claims. Due to entitlement issues, our comorbidity assessment (using 12 months of data preceding diagnosis) may underestimate the disease burden among 65-year old patients, who would have more limited claims information. However, these patients had a median entitlement period of approximately 8 months (range 45 to 365 days), so the effects of such underestimation are likely limited. Further, while more detailed measures of patients’ health status may improve our ability to risk adjust, such would require a large detailed clinical registry, which is not possible for practical reasons (e.g., cost, sample size). Although we accounted for additional demographic differences using a composite measure for socioeconomic status,18 a well-described predictor of long-term mortality,28 we recognize that race and class are complex constructs that can not always be comprehensively captured in administrative data.
As with any observational study, there are additional limitations to consider. Since we used national SEER-Medicare data, these findings may not be generalizable to patients under the age of 65. However, because nearly three-quarters of bladder cancer cases occur within the Medicare population,9 extrapolation of our findings to a broader cohort appears reasonable. Finally, we acknowledge that race, as captured in SEER data, represents a constellation of constructs as described by others,29 including acculturation, education, socioeconomic class, and socialization. Future work should seek to disentangle this complicated web and evaluate these relationships in other minority populations.
Compared to whites with early stage bladder cancer, black patients are at significantly greater risk of death. This disparity is not attributable to the diagnosis with more aggressive disease, the initial treatment intensity or the quality of care provided by the urologist. Eliminating disparities in mortality for this chronic disease will likely require looking beyond factors pertaining to health care delivery alone. Such factors, including behavior modification (e.g., smoking cessation) and greater use of other preventive services, may inevitably lie upstream from the diagnosis of bladder cancer imparting significant, but not insurmountable, challenges for future research.
Acknowledgments
Dr. Hollenbeck was supported by the American Cancer Society Pennsylvania Division Dr. William and Rita Conrady Mentored Research Scholar Grant (MSRG-07-006-01-CPHPS), the American Urological Association Foundation, and Astellas Pharma US, Inc. Dr. Birkmeyer was supported by the National Cancer Institute (R01 CA098481-01 A1 and K05 CA115571-01 A2).
Reference List
- 1.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 2.Lee CT, Dunn RL, Williams C, Underwood W., 3rd Racial disparity in bladder cancer: trends in tumor presentation at diagnosis. Journal of Urology. 2006;176(3):927–33. doi: 10.1016/j.juro.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Medical Care. 2002;40(8 Suppl):IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. New England Journal of Medicine. 1999;341(16):1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 5.Taub DA, Hollenbeck BK, Cooper KL, Dunn RL, Miller DC, Taylor JM, et al. Racial disparities in resource utilization for cystectomy. Urology. 2006;67(2):288–93. doi: 10.1016/j.urology.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Annals of Surgery. 2006;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. New England Journal of Medicine. 2004;351(6):575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program
- 10.Greene FL, Page EL, Fleming ID. AJCC Cancer Staging Manual. New York: Springer-Verley; 2002. [Google Scholar]
- 11.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Medical Care. 2002;40(8 Suppl):IV-104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 12.Schrag D, Hsieh LJ, Rabbani F, Bach PB, Herr H, Begg CB. Adherence to surveillance among patients with superficial bladder cancer. Journal of the National Cancer Institute. 2003;95(8):588–97. doi: 10.1093/jnci/95.8.588. [DOI] [PubMed] [Google Scholar]
- 13.Hoel DG, Ron E, Carter R, Mabuchi K. Influence of death certificate errors on cancer mortality trends. Journal of the National Cancer Institute. 1993;85(13):1063–8. doi: 10.1093/jnci/85.13.1063. [DOI] [PubMed] [Google Scholar]
- 14.Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. Journal of the National Cancer Institute. 1999;91(12):1025–32. doi: 10.1093/jnci/91.12.1025. [DOI] [PubMed] [Google Scholar]
- 15.Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? Journal of the National Cancer Institute. 2001;93(23):1822–23. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock MA, Reynes JF. Validation of cause-of-death certification for outpatient cancers: the contrasting cases of melanoma and mycosis fungoides. American Journal of Epidemiology. 1998;148(12):1184–6. doi: 10.1093/oxfordjournals.aje.a009607. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. Journal of Urology. 2000;163(1):60–61. [PubMed] [Google Scholar]
- 18.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Wolfe RA, Huang X. Shared frailty models for recurrent events and a terminal event. Biometrics. 2004;60(3):747–56. doi: 10.1111/j.0006-341X.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 21.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Annals of Internal Medicine. 2003;139(8):658–65. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 22.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, et al. Disparities in the utilization of high-volume hospitals for complex surgery. Jama. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 23.Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: early-versus late-stage cancer diagnosis. Health Affairs. 2009;28(1):160–8. doi: 10.1377/hlthaff.28.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radford MJ, Foody JM. How do observational studies expand the evidence base for therapy? JAMA. 2001;286(10):1228–30. doi: 10.1001/jama.286.10.1228. [DOI] [PubMed] [Google Scholar]
- 26.Heney NM, Nocks BN, Daly JJ, Prout GR, Jr, Newall JB, Griffin PP, et al. Ta and T1 bladder cancer: location, recurrence and progression. British Journal of Urology. 1982;54(2):152–57. doi: 10.1111/j.1464-410x.1982.tb13538.x. [DOI] [PubMed] [Google Scholar]
- 27.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. Journal of Clinical Oncology. 2001;19(3):666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 28.Anderson RT, Sorlie P, Backlund E, Johnson N, Kaplan GA. Mortality effects of community socioeconomic status. Epidemiology. 1997;8(1):42–7. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Medical Care. 2006;44(11 Suppl 3) doi: 10.1097/01.mlr.0000245427.22788.be. [DOI] [PubMed] [Google Scholar]


