Abstract
Smokeless tobacco contains 28 known carcinogens and causes precancerous oral lesions and oral and pancreatic cancer. A recent study conducted by our research team identified 8 different polycyclic aromatic hydrocarbons (PAH) in U.S. moist snuff, encouraging further investigations of this group of toxicants and carcinogens in smokeless tobacco products. In this study, we developed a gas chromatography-mass spectrometry method that allows simultaneous analysis of 23 various PAH in smokeless tobacco after a simple two-step extraction and purification procedure. The method produced coefficients of variation under 10% for most PAH. The limits of quantitation for different PAH varied between 0.3 ng/g tobacco and 11 ng/g tobacco, starting with a 300-mg sample. The recovery of the stable isotope-labeled internal standards averaged 87%. The method was applied to analysis of 23 moist snuff samples that include various flavors of the most popular U.S. moist snuff brands, as well as 17 samples representing the currently marketed brands of spit-free tobacco pouches, a relatively new type of smokeless tobacco. The sum of all detected PAH in conventional moist snuff averaged 11.6 (± 3.7) µg/g dry weight, 20% of this amount being comprised by carcinogenic PAH. The levels of PAH in new spit-free tobacco products were much lower than those in moist snuff, the sum of all detected PAH averaging 1.3 (±0.28) µg/g dry weight. Our findings render PAH one of the most prevalent groups of carcinogens in smokeless tobacco, along with tobacco-specific nitrosamines. Urgent measures are required from the U.S. tobacco industry to modify manufacturing processes so that the levels of these toxicants and carcinogens in the U.S. moist snuff are greatly reduced.
Keywords: PAH, moist snuff, smokeless tobacco, cancer
INTRODUCTION
Investigation of toxic and carcinogenic compounds present in smokeless tobacco is a critical step towards the reduction of harmful health effects associated with its use. The ‘smokeless’ category includes a variety of tobacco products intended for oral or nasal use, including chewing tobacco and moist snuff for oral use, and dry snuff for nasal use. Among these, oral moist snuff is the most popular type of smokeless tobacco in the U.S. (1). This is, to a certain degree, a result of considerable investments made by the tobacco industry into its marketing. Even though overall smokeless tobacco use has declined substantially between 1986 and 2003 (2), accompanied by an 11% decrease in overall smokeless tobacco sales volume (3), the use of moist snuff increased more than 80-fold over the same period (2). In 2005, moist snuff accounted for more than 80% of total sales of smokeless tobacco (1). Since moist snuff is characterized by high moisture and salt content, its use generates excess saliva and, therefore, usually requires spitting, creating a rather unsanitary image. In recent years, the tobacco industry has been also promoting spit-free smokeless tobacco designated for oral use. These new products are sold as small pouches of flavored tobacco with low moisture content, and are marketed to cigarette smokers to be used in situations where smoking is not allowed (4).
Oral smokeless tobacco use can lead to precancerous oral lesions and oral and pancreatic cancer (5–7), and is associated with an increased risk of esophageal cancer (8). These carcinogenic effects are believed to be caused by various carcinogens present in smokeless tobacco (9,10). The International Agency for Research on Cancer (IARC) lists 28 carcinogens present in smokeless tobacco (6). This list includes tobacco-specific N-nitrosamines (TSNA)1, volatile N-nitrosamines, volatile aldehydes, polycyclic aromatic hydrocarbons (PAH), certain lactones, urethane, metals, polonium-210 and uranium-235 and -238 (6,10,11). Among these, TSNA are commonly acknowledged as the most important group from the quantitative point of view (12,13). Over the last 2 decades, TSNA have become the main focus of studies dealing with chemical analysis of various smokeless tobacco products, their levels in a particular product being used as an indicator of its overall carcinogenic potency (14–19). At the same time, there was a common assumption that PAH – ubiquitous environmental carcinogens formed during the incomplete combustion of organic matter – are present in smokeless tobacco only in trace amounts. This assumption was based on the fact that the use of smokeless tobacco does not involve burning. It was also supported by the low amounts of benzo[a]pyrene (BaP) – a representative carcinogenic PAH – quantified in some brands of U.S. moist snuff (20). However, our recent report demonstrated that, in addition to BaP, at least 7 other PAH are present in U.S. smokeless tobacco, some of them at unexpectedly high levels (21). These findings inspired further investigation of the presence of PAH in smokeless tobacco products.
In this study, we developed a gas chromatography-mass spectrometry (GC-MS) method to analyze 23 PAH in smokeless tobacco. We expanded the list of PAH analyzed in smokeless tobacco to include the priority environmental PAH pollutants identified by the U. S. Environmental Protection Agency (EPA), as well as those carcinogenic PAH that, according to IARC, are present in cigarette smoke. The method allows analysis of all of these PAH in a single GC-MS run after a simple two-step extraction and purification procedure. PAH analyzed here include naphthalene (NP), acenaphthylene (ANP), acenaphthene (ANE), fluorine (FLR), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLT), pyrene (PY), benz[a]anthracene (BaA), chrysene (CHR), methylchrysene (MC) isomers, benzo[b]fluoranthene (BbF), benzo[j]fluoranthene (BjF), benzo[k]fluoranthene (BkF), benzo[e]pyrene (BeP), BaP, indeno[1,2,3-cd]pyrene (IcdP), dibenz[a,h]anthracene (DBahA), and benzo[g,h,i]perylene (BghiPy). The method was applied to analysis of 23 moist snuff samples that include various flavors of the most popular U.S. moist snuff brands, as well as 17 samples of spit-free tobacco pouches representing the currently marketed brands of this relatively new type of smokeless tobacco.
MATERIALS AND METHODS
Tobacco Samples
Products collected for analysis represent conventional moist snuff and new smokeless spit-free tobacco products. Conventional moist snuff was obtained from retailers in Minneapolis, MN between July 2007 and July 2009. New spit-free varieties of smokeless tobacco were purchased in retail stores between August 2008 and July 2009. Some of these products were supplied by Dr. Biener (University of Massachusetts, Boston). Triumph Snus, Grand Prix Snus, and Tourney Snus were purchased in Columbus, Ohio. Marlboro Snus was purchased in Indianapolis, Indiana, and Camel Snus and Nordic Ice were procured in Minneapolis.
Chemicals
13C-labeled PAH mix was purchased from Cambridge Isotope Laboratories (Andover, MA). 3MC and 4MC were purchased from the NCI Chemical Carcinogen Reference Standard Repository. 1MC and 6MC were kindly provided by Dr. Amin at the Penn State College of Medicine. Other unlabeled PAH standards were obtained from Cambridge Isotope Laboratories (Andover, MA) and Sigma-Aldrich (Milwaukee, WI). All other chemicals and solvents were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI) or Fisher Scientific (Fairlawn, NJ).
Caution
Many PAH are strong toxicants and carcinogens, and should be handled with extreme care, in a well-ventilated hood and with personal protective equipment.
PAH Analysis
Sample Preparation
Tobacco samples were extracted and purified by a slight modification of a method originally developed by Ding et al. (22) for PAH analysis in cigarette smoke, and later adapted by our group for smokeless tobacco analysis as described elsewhere (21). PAH were extracted by shaking 300 mg tobacco with 1 mL cyclohexane at room temperature for 3 h. The tobacco particles were removed by centrifugation and 500 µL of the extract was mixed with the 13C-labeled internal standard mix. The mixture was loaded on 50-mg BondElut Silica cartridges (Varian) pre-equilibrated with 1 mL cyclohexane. The cartridge was washed with 2 mL cyclohexane, and the eluants from both the load and wash were combined and concentrated under a gentle stream of N2 to a final volume of 200 µL. The concentrated samples were transferred to amber glass microinsert vials. Three µL of the sample were analyzed by GC-MS.
GC-MS Analysis
Separation and analysis of PAH was based on the method originally developed by Ding et al. (23). The analysis was performed on a Thermo Scientific system composed of a TSQ Quantum GC tandem mass spectrometer, coupled with a Trace GC Ultra gas chromatograph and a Tri Plus autosampler. The GC was equipped with a 60-m (0.25 mm internal diameter, 0.25 µm film thickness) DB-5MS fused silica capillary column (J&W Scientific, Folsom, CA) and a 2 m × 0.53 mm deactivated fused silica guard column. The constant flow rate was 1.2 mL/min He, and the splitless injection port temperature was set at 250 °C. The total GC run time was 46.5 min, the oven temperature being programmed as follows: 15 °C/min ramp from 80 °C to 275 °C, then 5 min at 275 °C, then 5 °C/min to 285 °C, then 10 min at 285 °C, then 20 °C/min to 315 °C, and 15 min at 315 °C. The MS was operated in the positive EI mode. The ion source temperature was set at 300 °C, the emission current was 50 µA, scan width was set at m/z 0.100, and scan time was 0.010 sec. The instrument was operated in selected ion monitoring mode (SIM) (Table 1). The filament was turned off for the first 6 min. To assure maximum selectivity and sensitivity, the PAH were divided into 11 SIM groups. The PAH reported here were analyzed in the first 10 SIM groups (Table 1), while the last group was intended to monitor dibenzo[a,e]pyrene and dibenzo[a,i]pyrene, which were not detected under these conditions.
Table 1.
Ions and ion ratios for the analyzed polycyclic aromatic hydrocarbons and corresponding internal standards.
| Analyte | Abbreviation | SIM group (duration, min) |
Quantitation ion (m/z) |
Confirmation ion (m/z) |
Ion ratio |
Internal standard | Quantitation ion (m/z) |
Confirmation ion (m/z) |
Ion ratio |
Response ratioa |
|---|---|---|---|---|---|---|---|---|---|---|
| Naphthalene | NP | 1 (10.00) | 128.2 | 127.2 | 6.27 | 13C6-Naphthalene | 134.2 | 133.2 | 5.35 | 0.88 |
| Acenaphthylene | ANP | 2 (2.25) | 152.1 | 151.1 | 4.54 | 13C6-Acenaphthylene | 158.2 | 157.1 | 3.64 | 0.86 |
| Acenaphthene | ANE | 2 (2.25) | 154.2 | 152.2 | 1.65 | 13C6-Acenaphthene | 160.2 | 158.2 | 1.45 | 0.90 |
| Fluorene | FLR | 3 (1.25) | 166.2 | 165.2 | 0.97 | 13C6-Fluorene | 172.2 | 171.2 | 0.89 | 0.93 |
| Phenanthrene | PHE | 4 (2.00) | 178.2 | 176.2 | 4.59 | 13C6-Phenanthrene | 184.2 | 182.2 | 4.52 | 0.95 |
| Anthracene | ANT | 4 (2.00) | 178.2 | 176.2 | 4.66 | 13C6-Anthracene | 184.2 | 182.2 | 4.50 | 0.92 |
| Fluoranthene | FLT | 5 (3.50) | 202.3 | 200.3 | 4.32 | 13C6-Fluoranthene | 208.3 | 206.3 | 4.67 | 1.03 |
| Pyrene | PY | 5 (3.50) | 202.3 | 200.3 | 4.51 | 13C6-Pyrene | 205.2 | 203.3 | 2.59 | 1.14 |
| Benz[a]anthracene | BaA | 6 (4.00) | 228.3 | 226.3 | 3.47 | 13C6-Benz[a]anthracene | 234.3 | 232.3 | 3.36 | 0.96 |
| Chrysene | CHR | 6 (4.00) | 228.3 | 226.3 | 3.12 | 13C6-Chrysene | 234.3 | 232.3 | 3.04 | 0.84 |
| 1-Methylchrysene | 1MC | 7 (4.00) | 242.3 | 239.3 | 2.97 | 13C6-Chrysene | 234.3 | 232.3 | 3.12 | 1.67 |
| 3-Methylchrysene | 3MC | 7 (4.00) | 242.3 | 239.3 | 2.98 | 13C6-Chrysene | 234.3 | 232.3 | 3.12 | 0.98 |
| 4-Methylchrysene + 6-Methylchrysene | 4MC, 6MC | 7 (4.00) | 242.3 | 239.3 | 2.35 | 13C6-Chrysene | 234.3 | 232.3 | 3.12 | 1.22 |
| 5-Methylchrysene | 5MC | 7 (4.00) | 242.3 | 239.3 | 2.20 | 13C6-Chrysene | 234.3 | 232.3 | 3.12 | 1.32 |
| Benzo[b]fluoranthene + Benzo[j]fluoranthene | BbF, BjF | 8 (2.50) | 252.3 | 250.3 | 3.39 | 13C6-Benzo[b]fluoranthene | 258.3 | 256.3 | 3.54 | 0.97 |
| Benzo[k]fluoranthene | BkF | 8 (2.50) | 252.3 | 250.3 | 3.68 | 13C6-Benzo[k]fluoranthene | 258.3 | 256.3 | 3.72 | 1.01 |
| Benzo[e]pyrene | BeP | 9 (3.50) | 252.3 | 250.3 | 2.89 | 13C4-Benzo[a]pyrene | 256.3 | 254.3 | 3.06 | 0.92 |
| Benzo[a]pyrene | BaP | 9 (3.50) | 252.3 | 250.3 | 3.86 | 13C4-Benzo[a]pyrene | 256.3 | 254.3 | 3.06 | 1.01 |
| Indeno[1,2,3-cd]pyrene | IcdP | 10 (7.00) | 276.3 | 274.3 | 3.84 | 13C6-Indeno[1,2,3-cd]pyrene | 282.3 | 280.4 | 5.01 | 1.08 |
| Dibenz[a,h]anthracene | DBahA | 10 (7.00) | 278.3 | 276.3 | 3.81 | 13C6-Dibenz[a,h]anthracene | 284.3 | 282.4 | 3.41 | 1.01 |
| Benzo[g,h,i]perylene | BghiPy | 10 (7.00) | 276.3 | 274.3 | 3.68 | 13C6-Indeno[1,2,3-cd]pyrene | 282.3 | 280.4 | 5.01 | 0.91 |
Analyte to internal standard peak area ratio in the range of analyte concentrations from 0.01 to 10 ng/µL.
Method characteristics
The accuracy of the method was tested by spiking Hawken Long Cut Wintergreen (which, according to our preliminary experiments, is low in PAH content) with 5 ng, 100 ng, 250 ng, and 500 ng of each PAH. A non-spiked sample of the same product was added to the set. Each sample was analyzed in duplicate. The results at each spiking level were expressed as % of added PAH, after the subtraction of the amount of each PAH measured in the nonspiked sample. The average of these determinations was then calculated for each PAH. The precision of the method for each PAH was determined by calculating the coefficient of variation of 5 measurements obtained from 5 aliquots of a cyclohexane extract of Copenhagen Snuff (a representative moist snuff product). The limit of quantitation was determined as the lowest amount of each PAH in the 300-mg tobacco sample that can be reliably quantified at 10:1 signal to noise ratio for the corresponding quantitation ion. The detection limit of each PAH was determined at 3:1 signal to noise ratio for the corresponding quantitation ion.
Other Analyses
Moisture content was measured via the difference in weight of a tobacco sample before and after drying, as previously described (21).
RESULTS
Modification of the method
The method used in our previous study (21) produced satisfactory results, however, the recovery of the higher-molecular weight PAH was somewhat inconsistent. We suggested that some of the PAH were retained on the cartridge and removed only with the last part of the 2-mL cyclohexane volume. In an effort to achieve better efficiency of sample clean-up during solid phase extraction and improve the PAH recovery from the cyclohexane tobacco extracts, we examined sample extraction on 50-mg BondElut Silica cartridges (Varian). This change in the sample purification protocol led to excellent recovery of all 13C-labeled standards (Table 2).
Table 2.
Average recovery of stable isotope-labeled polycyclic aromatic hydrocarbons used as internal standards
| Internal standard | Average recovery, % |
|---|---|
| 13C6-Naphthalene | 76.9 |
| 13C6-Acenaphthylene | 75.6 |
| 13C6-Acenaphthene | 74.7 |
| 13C6-Fluorene | 80.2 |
| 13C6-Phenanthrene | 83.9 |
| 13C6-Anthracene | 80.4 |
| 13C6-Fluoranthene | 90.5 |
| 13C6-Pyrene | 93.0 |
| 13C6-Benz[a]anthracene | 92.4 |
| 13C6-Chrysene | 91.0 |
| 13C6-Benzo[b]fluoranthene | 96.7 |
| 13C6-Benzo[k]fluoranthene | 96.0 |
| 13C4-Benzo[a]pyrene | 98.4 |
| 13C6-Indeno[1,2,3-cd]pyrene | 94.2 |
| 13C6-Dibenz[a,h]anthracene | 88.5 |
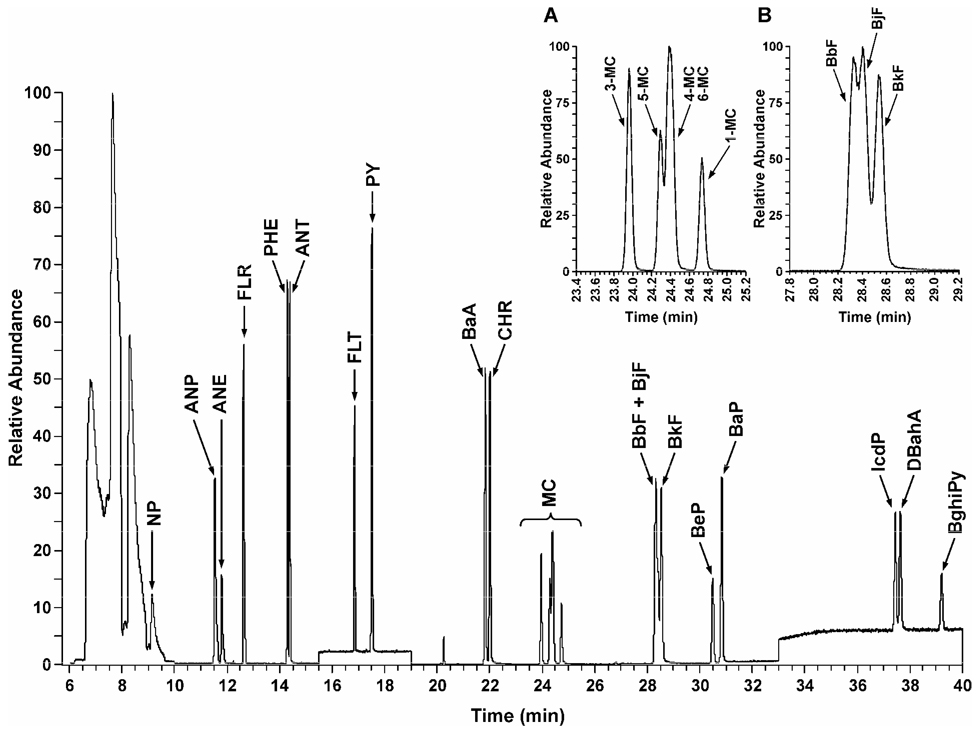
A representative total ion GC-MS trace of a standard mix containing all 23 PAH and 15 stable isotope-labeled internal standards is illustrated in Figure 1. Selected ion chromatograms demonstrated excellent separation for most of the analytes. Two pairs of compounds were not well resolved: BbF co-eluted with BjF, and 4MC co-eluted with 6MC (inserts A and B in Figure 1). The latter two compounds also overlapped with the 5MC peak.
Figure 1.
Typical total ion GC-MS chromatogram obtained upon analysis of a standard PAH mix containing 1 ng/µL each of 23 PAH. All peaks were well resolved with the exception of (A) methylchrysenes and (B) BbF and BjF. For PAH abbreviations, see Table 1.
Characteristics of the method
Accuracy, precision, and limits of quantitation for all PAH are summarized in Table 3. There was a minor contribution of stable isotope-labeled PAH to the ion chromatograms of corresponding unlabeled PAH. This contribution was consistent over the range of labeled PAH concentrations from 0.05 ng/µL to 1 ng/µL, and varied from 0.03% of 13C6-ANT area observed in the ANT ion chromatogram to 6.7% of 13C4-BaP peak area observed in the BaP ion chromatogram. These values were subtracted from peak areas of corresponding PAH in all subsequent calculations. The difference between the PAH content measured in non-spiked and spiked Hawken samples was in good agreement with the amount of PAH added to each sample (Table 3), and the linearity of the method was satisfactory in the studied range of concentrations (R2 > 0.99 for all PAH). Analysis of 5 non-spiked aliquots of Copenhagen Snuff extract produced coefficients of variation under 10% for most PAH (Table 3). The limits of quantitation varied between 0.3 ng/g and 10.9 ng/g tobacco, and the limits of detection varied between 0.1 ng/g and 3.8 ng/g tobacco, starting with a 300-mg sample (Table 3).
Table 3.
Characteristics of the method
| Analyte | Accuracy | Precision | Limit of quantitation, ng/g tobacco |
|---|---|---|---|
| % | % variation (N = 5) |
||
| Naphthalene | 109 | 9.93 | 4.8 |
| Acenaphthylene | 112 | 8.29 | 4.0 |
| Acenaphthene | 105 | 7.89 | 10.9 |
| Fluorene | 118 | 7.98 | 1.6 |
| Phenanthrene | 124 | 7.69 | 2.0 |
| Anthracene | 118 | 9.51 | 2.6 |
| Fluoranthene | 103 | 8.25 | 0.3 |
| Pyrene | 115 | 9.89 | 0.3 |
| Benz[a]anthracene | 109 | 8.03 | 0.9 |
| Chrysene | 108 | 8.15 | 1.0 |
| 1-Methylchrysene | 109 | 8.05 | 2.0 |
| 3-Methylchrysene | 110 | 7.02 | 2.0 |
| 4-Methylchrysene + 6-Methylchrysene | 112 | 9.31 | 2.6 |
| 5-Methylchrysene | 104 | nda | nd |
| Benzo[b]fluoranthene + Benzo[j]fluoranthene | 115 | 6.32 | 1.8 |
| Benzo[k]fluoranthene | 118 | 5.72 | 1.6 |
| Benzo[e]pyrene | 113 | 7.70 | 2.1 |
| Benzo[a]pyrene | 109 | 4.47 | 1.6 |
| Indeno[1,2,3-cd]pyrene | 113 | 7.20 | 2.3 |
| Dibenz[a,h]anthracene | 112 | 10.83 | 3.9 |
| Benzo[g,h,i]perylene | 113 | 12.31 | 2.4 |
nd, not determined (not present in tobacco).
Analysis of moist snuff and spit-free tobacco pouches
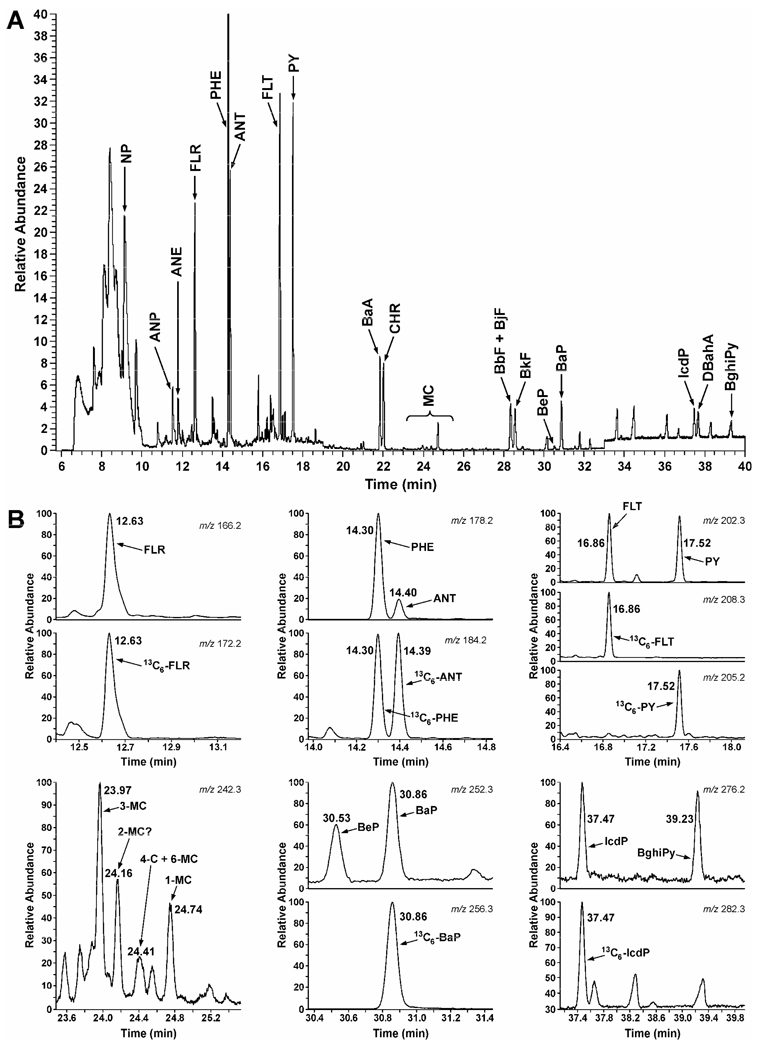
The method was used to analyze PAH in various smokeless tobacco products marketed and test-marketed in the U.S. Twenty three moist snuff samples and 17 spit-free tobacco products were included in the analysis. A typical GC-MS trace obtained upon analysis of conventional moist snuff is illustrated in Figure 2. The results are summarized in Table 4.
Figure 2.
Typical GC-MS chromatogram obtained upon analysis of a moist snuff sample: A, total ion chromatogram; B, some examples of individual ion chromatograms. For PAH abbreviations, see Table 1.
Table 4.
Levels of polycyclic aromatic hydrocarbons in smokeless tobacco marketed in the U.S.
| Sample content | PAHa, ng / g dry weight | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moist Snuff | ||||||||||||||||||||
| Skoal Long Cut Straight | 54.4 | 1990 | 132 | 126 | 783 | 4250 | 712 | 1180 | 1020 | 149 | 195 | 79 | 76 | 15 | 45 | 53 | 43 | 23 | 6.8 | 10900 |
| Skoal Long Cut Mint | 53.8 | 1940 | 143 | 163 | 945 | 5030 | 852 | 1460 | 1250 | 198 | 254 | 102 | 281 | 17 | 80 | 56 | 33 | 21 | 8.2 | 12800 |
| Copenhagen Snuff | 54.7 | 1860 | 85 | 112 | 709 | 4960 | 784 | 1650 | 1420 | 220 | 269 | 95 | 278 | 26 | 102 | 60 | 27 | 26 | LOQb | 12700 |
| Copenhagen Long Cut | 54.6 | 2020 | 96 | 120 | 878 | 6030 | 1050 | 1790 | 1490 | 222 | 293 | 95 | 186 | 28 | 70 | 68 | 31 | 27 | LOQ | 14500 |
| Kodiak Straight | 56.2 | 2210 | 114 | 165 | 1110 | 6340 | 1070 | 2010 | 1880 | 346 | 462 | 217 | 189 | 32 | 111 | 102 | 40 | 34 | 10 | 16400 |
| Kodiak Wintergreen | 55.1 | 2100 | 174 | 200 | 1440 | 8660 | 1440 | 2540 | 2250 | 328 | 477 | 139 | 139 | 37 | 89 | 86 | 49 | 41 | 11 | 20200 |
| Grizzly Snuff | 52.7 | 1330 | 140 | 83 | 840 | 4230 | 835 | 975 | 1090 | 154 | 153 | 69 | 61 | 16 | 35 | 40 | 13 | 14 | LOQ | 10100 |
| Grizzly Pouches Straight | 27.1 | 1240 | 107 | 53 | 482 | 2860 | 535 | 999 | 1060 | 178 | 177 | 86 | 73 | 20 | 40 | 46 | 16 | 16 | LOQ | 7990 |
| Grizzly Pouches Mint | 60.7 | 1990 | 113 | 46 | 782 | 3840 | 701 | 785 | 853 | 112 | 109 | 48 | 41 | 12 | 28 | 27 | 8.9 | 12 | LOQ | 9500 |
| Kayak Long Cut Straight | 53.2 | 2120 | 56 | 22 | 609 | 4170 | 651 | 989 | 1070 | 139 | 130 | 64 | 48 | 11 | 31 | 30 | 10 | 13 | LOQ | 10200 |
| Kayak Long Cut Mint | 51.2 | 2270 | 37 | 26 | 496 | 3800 | 567 | 961 | 1090 | 149 | 148 | 71 | 56 | 14 | 34 | 30 | 11 | 13 | LOQ | 9780 |
| Kayak Long Cut Wintergreen | 52.5 | NQc | 53 | 27 | 534 | 3610 | 607 | 870 | 910 | 120 | 114 | 57 | 41 | 11 | 27 | 26 | 7.9 | 11 | LOQ | 7020 |
| Timber Wolf Fine Cut Natural | 48.3 | 1530 | 138 | 156 | 1090 | 5970 | 1100 | 1940 | 1640 | 244 | 340 | 108 | 127 | 22 | 60 | 70 | 19 | 20 | LOD | 14600 |
| Timber Wolf Long cut Straight | 53.6 | 2010 | 141 | LOQ | 1140 | 5700 | 1070 | 1900 | 1670 | 244 | 360 | 105 | 110 | 21 | 47 | 74 | LODd | LOD | 6.7 | 14600 |
| Timber Wolf Long cut Wintergreen | 53.8 | 886 | 153 | 136 | 1100 | 5600 | 1090 | 1760 | 1580 | 235 | 237 | 101 | 103 | 21 | 47 | 73 | 20 | 11 | 6.5 | 13100 |
| Timber Wolf Fine cut Wintergreen | 54.0 | 1300 | 121 | 125 | 903 | 4220 | 850 | 1340 | 1160 | 169 | 170 | 78 | 77 | 16 | 34 | 57 | 14 | 8.2 | LOQ | 10600 |
| Timber Wolf Long cut Mint | 53.9 | 1810 | 147 | 127 | 970 | 4990 | 1000 | 1610 | 1430 | 219 | 260 | 86 | 102 | 20 | 46 | 71 | 20 | 15 | 7.3 | 12900 |
| Timber Wolf Apple | 49.7 | 1590 | 122 | 128 | 897 | 5140 | 981 | 1820 | 1560 | 245 | 247 | 106 | 115 | 22 | 53 | 74 | 20 | 17 | 8.8 | 13100 |
| Timber Wolf Peach | 50.5 | 1530 | 96 | 120 | 841 | 4540 | 845 | 1520 | 1330 | 197 | 283 | 77 | 95 | 18 | 45 | 61 | 16 | 15 | 6.8 | 11600 |
| Red Seal Natural | 55.0 | 1860 | 111 | 153 | 924 | 5670 | 976 | 1800 | 1580 | 229 | 231 | 102 | 105 | 21 | 49 | 69 | 20 | 16 | 7.0 | 13900 |
| Red Seal Long cut Wintergreen | 51.9 | 1610 | 89 | 117 | 781 | 4560 | 768 | 1350 | 1190 | 163 | 247 | 57 | 79 | 14 | 35 | 53 | 16 | 9.8 | 6.2 | 11100 |
| Longhorn Long Cut Wintergreen | 64.9 | NQ | 173 | 105 | 761 | 3890 | 935 | 1000 | 1140 | 188 | 175 | 96 | 70 | 17 | 40 | 45 | 12 | 15 | LOQ | 8670 |
| Hawken Long Cut Wintergreen | 25.86 | 1050 | LOD | LOD | 4.8 | 58 | 8.6 | 45 | 46 | 5.3 | 7.8 | LOD | 7.4 | LOQ | LOQ | 13 | LOQ | LOQ | LOQ | 1250 |
| Average for moist snuff | 52.6 | 1730 | 111 | 105 | 827 | 4700 | 844 | 1400 | 1290 | 194 | 232 | 93 | 107 | 20 | 52 | 56 | 21 | 18 | 7.5 | 11600 |
| STD | 7.0 | 392 | 43 | 54 | 287 | 1570 | 278 | 537 | 429 | 71 | 110 | 35 | 70 | 6.6 | 24 | 22 | 12 | 8.3 | 1.9 | 3700 |
| Spit-free tobacco pouches | ||||||||||||||||||||
| Marlboro Snus Rich | 20.8 | 865.9 | LOQ | NQ | LOQ | 13.5 | LOQ | 9.0 | 9.0 | 1.7 | 1.9 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | 901.0 |
| Marlboro Snus Mild | 11.9 | 722.4 | LOQ | NQ | LOQ | 9.7 | LOQ | 6.9 | 7.6 | 1.1 | 1.3 | LOQ | LOQ | LOD | LOQ | LOQ | LOQ | LOQ | LOQ | 749.0 |
| Marlboro Snus Spearmint | 13.4 | 1065 | LOQ | NQ | LOQ | 10.0 | LOQ | 5.7 | 7.0 | LOQ | 1.4 | LOQ | LOQ | LOD | LOQ | LOQ | LOQ | LOQ | LOD | 1091 |
| Marlboro Snus Peppermint | 13.0 | 1225 | 8.1 | NQ | LOQ | 9.4 | LOQ | 5.6 | 6.0 | 1.1 | 1.5 | LOQ | LOQ | LOD | LOQ | LOQ | LOQ | LOQ | LOQ | 1257 |
| Camel Snus Original | 31.2 | 1108 | LOQ | LOQ | 9.0 | 68.0 | 6.9 | 60.1 | 46.5 | 5.9 | 13.3 | LOD | 38.8 | 3.1 | 23.2 | 15.2 | LOQ | LOQ | 19.8 | 1428 |
| Camel Snus Spice | 31.5 | 1084 | 9.0 | LOQ | 11.8 | 79.4 | 8.1 | 59.7 | 45.4 | 5.5 | 9.1 | LOD | 30.2 | 3.1 | LOQ | 15.0 | LOQ | LOQ | 15.2 | 1375 |
| Camel Snus Frost | 30.9 | 1073 | LOQ | LOQ | 9.7 | 68.7 | 6.9 | 60.5 | 46.3 | 5.4 | 12.4 | LOD | 31.5 | 3.1 | 21.5 | 14.9 | LOQ | LOQ | 8.8 | 1363 |
| Camel Snus Mellow | 33.6 | 1056 | LOQ | NQ | LOQ | 44.8 | 6.1 | 33.0 | 25.3 | 2.5 | 3.2 | LOQ | LOQ | LOD | LOQ | LOQ | LOQ | LOQ | LOD | 1173 |
| Tourney Original | 17.6 | 1061 | LOQ | 17.7 | 5.2 | 36.2 | LOQ | 8.8 | 17.3 | 2.6 | 5.6 | LOD | 6.6 | LOQ | LOQ | 12.1 | LOQ | LOQ | LOQ | 1173 |
| Tourney Spearmint | 22.6 | 1129 | LOQ | 19.5 | 5.6 | 41.9 | LOQ | 23.7 | 28.2 | 5.5 | 9.9 | LOD | 9.8 | 3.4 | LOQ | 14.5 | 4.2 | LOQ | LOQ | 1296 |
| Tourney Wintergreen | 20.1 | 992.6 | LOQ | 20.7 | 5.9 | 34.9 | LOQ | 19.4 | 25.4 | 5.1 | 8.7 | LOD | 9.9 | 3.2 | LOQ | 14.0 | 4.0 | LOQ | 3.6 | 1148 |
| Grand Prix Original | 25.1 | 1103 | LOQ | 129.1 | 6.1 | 42.4 | LOQ | 10.9 | 18.5 | 2.9 | 5.1 | LOD | 7.6 | LOQ | LOQ | 13.3 | LOQ | LOQ | 4.2 | 1344 |
| Grand Prix Spearmint | 23.7 | 1100 | LOQ | 16.0 | 6.4 | 43.2 | LOQ | 37.0 | 35.4 | 7.1 | 11.1 | LOD | 11.9 | 3.6 | 3.2 | 15.0 | 4.4 | LOQ | 3.7 | 1298 |
| Grand Prix Wintergreen | 21.3 | 932.2 | LOQ | LOQ | 5.7 | 51.7 | LOQ | 36.4 | 37.5 | 7.6 | 11.5 | LOD | 12.6 | 4.4 | 2.9 | 15.6 | LOD | LOQ | LOQ | 1123 |
| Triumph Original | 54.0 | 1561 | LOQ | LOQ | 9.2 | 65.5 | LOQ | 53.1 | 48.5 | 5.3 | 11.7 | LOD | 45.1 | LOQ | 75.9 | 0.0 | LOQ | LOQ | 68.7 | 1944 |
| Triumph Mint | 49.5 | 1513 | LOQ | LOQ | 7.4 | 44.4 | LOQ | 33.6 | 36.1 | 4.1 | 8.6 | LOD | 21.2 | LOQ | 32.4 | 5.9 | LOQ | LOQ | 8.6 | 1715 |
| Nordic Ice Snus | 21.6 | 1314 | LOQ | NQ | 2.6 | 36.7 | 5.7 | 15.6 | 17.5 | 3.2 | 3.7 | 3.4 | 2.9 | LOQ | LOQ | LOQ | LOQ | LOQ | LOD | 1408 |
| Average for spit-free tobacco | 21.5 | 1112 | 8.5 | 35.4 | 6.3 | 41.2 | 6.7 | 28.2 | 26.9 | 4.0 | 7.1 | 3.4 | 19.0 | 2.8 | 23.0 | 12.3 | 4.4 | LOQ | 16.6 | 1281 |
| STD | 12.7 | 207.0 | 0.7 | 46.1 | 3.1 | 21.6 | 0.9 | 20.4 | 15.0 | 2.1 | 4.3 | nae | 14.0 | 1.4 | 26.2 | 4.9 | 0.4 | na | 21.8 | 275.7 |
With the exception of Hawken Long Cut Wintergreen, the levels of individual PAH were very similar across various brands of conventional moist snuff. Average amounts of detected PAH in these products ranged from 7.5 ng/g dry weight for DBahA to 4700 ng/g tobacco for PHE. Total MC in Table 4 represent the calculated sum of 1 MC, 3MC, 4MC, and 6MC. 5MC was not detected in any of the tested products. We also consistently observed an additional peak which, based on its spectrum and retention time (24.16 min, Figure 2), could be 2-methylchrysene (34). However, we did not have a corresponding standard to confirm its identity. The sum of all detected PAH (total PAH) in 23 samples of conventional moist snuff averaged 11639 (± 3702) ng/g dry weight. Hawken Long Cut Wintergreen was relatively low in total PAH content – only 1253 ng/g dry weight.
The levels of PAH in the new spit-free tobacco products were much lower than those in moist snuff (Table 4), total PAH averaging 1281 (± 275.7) ng/g tobacco. The levels of individual PAH in these products were not as consistent across different brands as in moist snuff: PHE varied from 9.4 ng/g dry weight in Marlboro Snus Peppermint to 79.4 ng/g dry weight in Camel Snus Spice; FLT content in the same products was 5.6 ng/g and 59.7 ng/g dry weight, respectively, and BaP content was below the limit of quantitation and 15.0 ng/g dry weight, respectively (Table 4). None of the tested new products had detectable levels of BghiPy, and only Nordic Ice Snus had quantifiable levels of MC isomers.
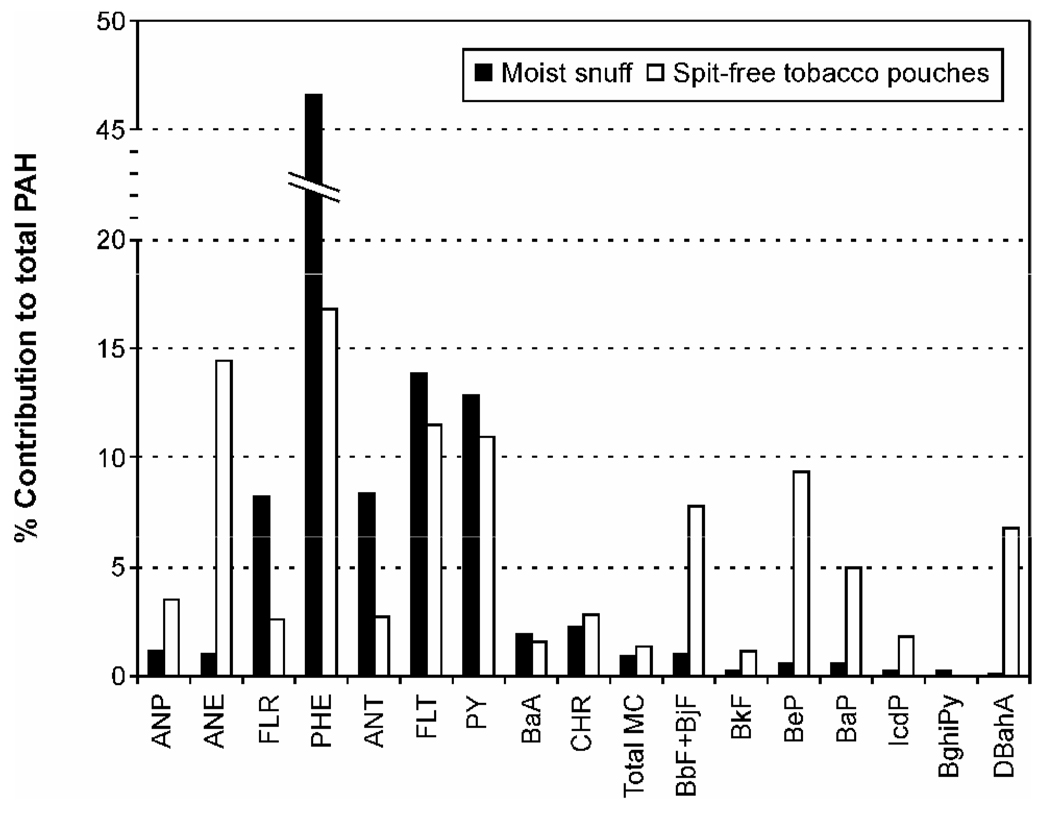
The only PAH that was present in both types of smokeless tobacco in comparable amounts was NP: 1726 (± 392.3) ng/g dry weight in moist snuff and 1112 (± 207.0) ng/g dry weight in the spit-free products, accounting for 15% and 87%, respectively, of average total PAH in these products. Average relative contributions of other individual PAH to the sum of 22 PAH (excluding NP) are illustrated in Figure 3.
Figure 3.
Comparison of average relative contributions of individual PAH to total PAH content in moist snuff (solid bars) and spit-free tobacco pouches (open bars). Naphthalene, which accounted for 87% of total PAH in spit-free tobacco products, is excluded from this chart. For PAH abbreviations, see Table 1.
DISCUSSION
A recent study conducted by our research team identified 8 PAH in moist snuff, encouraging further investigations of this group of toxicants and carcinogens in smokeless tobacco products. In this study, we used gas chromatography-mass spectrometry to analyze 23 different PAH in some U.S. moist snuff brands, as well as in some new spit-free smokeless tobacco products currently marketed and test-marketed in the U.S. This is the first study to report the presence of naphthalene, acenaphthene, fluorene, benz[a]anthracene, chrysene, 1-methylchrysene, 3-methylchrysene, 4-methylchrysene, 6-methylchrysene, benzo[j]fluoranthene, benzo[e]pyrene, indeno[1,2,3-cd]pyrene, benzo[g,h,i]perylene, and dibenz[a,h]anthracene in U.S. moist snuff. Our findings render PAH one of the most prevalent groups of carcinogens in smokeless tobacco.
The sum of NP, PHE, FLT, and PY accounted for about 78% of total PAH in moist snuff. Of these, NP – a bicyclic aromatic hydrocarbon – is an IARC group 2B carcinogen (24) (Table 5), and FLT is a weak lung tumorigen in mice (25). The sum of FLR, ANT, and IARC group 2B carcinogens BaA and CHR in moist snuff accounted for an additional 18% of total PAH. The remaining 4% were comprised of ANP, ANE, MC isomers, BbF, BjF, BkF, BaP, BeP, IcdP, BghiPy, and DBahA. Overall, of the 22 PAH detected in moist snuff, 9 are classified by IARC as group 1, 2A, or 2B carcinogens (Table 5), their sum averaging 2.36 µg/g dry weight, or 20% of total PAH. This is the first study to demonstrate that the sum of carcinogenic PAH in U.S. moist snuff reaches microgram per g levels. The relatively high levels of FLT and PY in moist snuff are also disturbing, as these PAH act as co-carcinogens when applied to mouse skin along with, or prior to, BaP treatment (26–28). Co-application of FLT or PY with BaP on mouse skin also results in a 56% and 66% increase, respectively, in the levels of BaP-derived DNA adducts (29). Co-carcinogenicity with BaP has been also reported for BeP and BghiPy (26,27). Some of the PAH found in smokeless tobacco, however, were reported to inhibit carcinogenicity of other PAH. Thus, NP and BaA inhibit carcinogenicity of BaP (30,31), while BeP inhibits carcinogenicity of DBahA (32).
Table 5.
Carcinogenic polycyclic aromatic hydrocarbons analyzed in U.S. smokeless tobacco products.
| PAH | Structure | IARC groupa |
|---|---|---|
| Naphthalene | 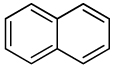 |
2B |
| Benz[a] anthracene | 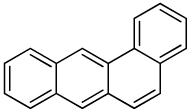 |
2B |
| Chrysene | 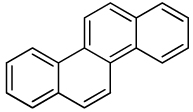 |
2B |
| Benzo[b]fluoranthene | 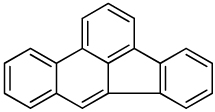 |
2B |
| Benzo[j]fluoranthene | 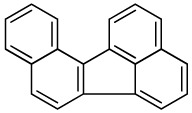 |
2B |
| Benzo[k]fluoranthene | 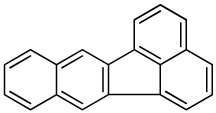 |
2B |
| Benzo[a]pyrene | 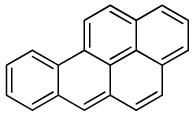 |
1 |
| Indeno [1,2,3-cd]pyrene | 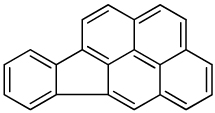 |
2B |
| Dibenz[a,h]anthracene | 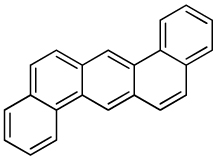 |
2A |
International Agency for Research on Cancer (IARC) classification of carcinogens: group 1 – carcinogenic to humans; group 2A – probably carcinogenic to humans; group 2B – possibly carcinogenic to humans.
Comparison to the levels of PAH reported for cigarette smoke can be useful for a better understanding of the relative amounts of this group of chemicals found in U.S. moist snuff. In the report by Ding et al, the sums of 14 PAH in the smoke of full flavor varieties of the U.S. cigarettes Marlboro, Camel, and Newport were 1.15 µg/cigarette, 1.29 µg/cigarette, and 1.15 µg/cigarette, respectively (23). The reported PAH included NP, ANP, ANE, FLR, PHE, ANT, FLT, PY, BaA, CHR, BbF, BkF, BeP, and BaP. In our study, the sum of the same 14 PAH and BjF (which could not be separated from BbF, but accounted for only about 1% of the sum) in one gram of product averaged 5.41 (±1.66) µg. Thus, an average single portion of traditional moist snuff (1–2 g) can contain about 5 times higher amounts of the sum of these 14 PAH than the smoke of a single cigarette. This is mostly due to the presence in moist snuff of relatively high levels of NP, PHE, FLT, and PY (Table 4). Of these, only NP is considered a group 2B carcinogen (Table 5).
Among moist snuff products analyzed here, Hawken Long Cut Wintergreen is drastically different from the rest of the products, the sum of all PAH in this product being only about 11% of the average total PAH in the rest of moist snuff brands. Apparently, the tobacco processing method used in the manufacturing of Hawken does not lead to its contamination with PAH. It is worth mentioning that Hawken brand has low prevalence of use, while high-PAH products such as Skoal, Copenhagen, and Grizzly, are among the most popular smokeless tobacco brands in the U.S.(33)
Of the 5 analyzed MC isomers, 5MC exhibits high carcinogenic activity similar to that of BaP (34). None of the products analyzed here had detectable levels of this isomer. We were not able to determine individual amounts of 4MC and 6MC, because these two peaks co-eluted (Figure 2). The identity of 2MC could not be confirmed because of the lack of the standard compound. The average contributions of 1MC and 3MC to the total amount of MC isomers identified in moist snuff were 40% and 42%, respectively, while the sum of 4MC and 6MC accounted for 18% of the total amount. These relative contributions are different from those observed in cigarette smoke, where MC are formed as a result of tobacco pyrolysis (34). Among the detected methylchrysenes, 3MC is a strong tumor initiator and shows some carcinogenic activity in animal studies (34). The amount of 3MC in moist snuff averaged 39.0 ng/g dry weight – comparable to an average 55.8 ng/g dry weight for BaP.
In agreement with our previous results (21), the levels of PAH in spit-free tobacco pouches were very low. Similarly to Hawken, the sum of all PAH in these products was only about 11% of the average total PAH in moist snuff. In our previous study we were not able to detect ANT, BbF, BkF, and BaP in most of the similar new smokeless tobacco products (21). In this study, probably due to the use of a more sensitive instrument and changes in the sample preparation procedure, we were able to detect these and other PAH, including carcinogenic NP, BaA, CHR, IcdP, and DBahA, in many new spit-free tobacco samples. The sum of carcinogenic PAH in these products averaged 1.18 µg/g dry weight, which is somewhat similar to moist snuff. However, this amount was mainly due to high naphthalene content, which was quite uniform across the studied new brands and similar to that of moist snuff (Table 4). When NP was excluded from the calculations, the sum of the remaining carcinogenic PAH in spitless tobacco was about 10 % of that in moist snuff (0.066 µg/g dry weight vs. 0.64 µg/g dry weight, respectively). Moreover, NP content was the major contributor to the sum of all PAH detected in newer brands, accounting for more than 80% of total PAH; the sum of all other PAH in this category was about 2% of that in moist snuff (0.25 µg/g dry weight vs. 10.1 µg/g dry weight, respectively).
The differences in PAH levels, as well as the differing patterns in the relative contribution of individual PAH to the total PAH content between conventional moist snuff and spit-free tobacco pouches, reflect the differences in the tobacco processing techniques used in the manufacturing of these two categories of smokeless tobacco. Tobacco used in moist snuff undergoes fire-curing – the process that includes direct contact with the smoke of smoldering hardwoods, a rich source of PAH. Moreover, the high chloride content in moist snuff (21) could possibly facilitate the absorption of PHE, FLT, and PY upon curing, leading to the high levels of these PAH in moist snuff. Thus, a study of atmospheric PAH in an urban location in U.K. suggested that salt used to treat roads during the winter efficiently absorbs vehicular emissions of PHE, FLT, and PY (35). Tobacco used in the manufacturing of spit-free smokeless products is pasteurized. Since there is no direct contact with the wood smoke, the major source of contamination of these products with PAH is probably their absorption from the air by tobacco leaves or processed tobacco. Spit-free smokeless tobacco is also low in salt content (21). As mentioned earlier, the only similarity between these two types of products was NP content, suggesting that the sources of NP contamination could be common for moist snuff and spit-free tobacco manufacturing.
In summary, our findings demonstrate that PAH are one of the most prevalent groups of carcinogens in moist snuff, and that the use of moist snuff can be considered an important source of human exposure to PAH, along with smoking. The low amounts of PAH in the brand Hawken and in various new spit-free smokeless brands represent direct and strong evidence that their amounts in moist snuff can be also brought to trace levels. Urgent measures are required from the U.S. tobacco industry to modify manufacturing processes so that the levels of these toxicants and carcinogens in U.S. moist snuff products are greatly reduced.
Acknowledgements
We thank Dr. Shantu Amin at the Penn State College of Medicine for generously sharing 1-methylchrysene and 6-methylchrysene, Dr. Lois Biener at the Center for Survey Research (University of Massachusetts, Boston) for providing most of the new spit-free tobacco products, and Bob Carlson for editorial assistance. This study was supported by NIH grants P50 DA-13333 as part of the Tobacco Harm Reduction Network and CA-81301.
Footnotes
Abbreviations: acenaphthene (ANE), acenaphthylene (ANP), anthracene (ANT), BaA, benz[a]anthracene; BbF, benzo[b]fluoranthene; BjF, benzo[j]fluoranthene; BkF, benzo[k]fluoranthene; BaP, benzo[a]pyrene; BeP, benzo[e]pyrene; BghiPy, benzo[g,h,i]perylene; chrysene (CHR), DBahA, dibenz[a,h]anthracene; fluorine (FLR), fluoranthene (FLT), GC-MS, gas chromatography-mass spectrometry; IcdP, indeno[1,2,3-cd]pyrene; methylchrysene (MC), naphthalene (NP), phenanthrene (PHE), pyrene (PY), TSNA, tobacco-specific nitrosamines.
References
- 1.U.S.Federal Trade Commission. Smokeless tobacco reports for the years 2002–2005. Washington: Federal Trade Commission; 2007. [Google Scholar]
- 2.Nelson DE, Mowery P, Tomar S, Marcus S, Giovino G, Zhao L. Trends in smokeless tobacco use among adults and adolescents in the United States. Am. J. Public Heal. 2006;96:897–905. doi: 10.2105/AJPH.2004.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxwell JC., Jr . The Maxwell Report: The Smokeless Tobacco Industry in 2003. Richmond, Va: John C. Maxwell Jr; 2004. [Google Scholar]
- 4.Hatsukami DK, Feuer RM, Ebbert JO, Stepanov I, Hecht SS. Changing smokeless tobacco products: new tobacco delivery systems. Am. J. Prev. Med. 2007;33:S368, S378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46:4162–4166. [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 41–417. [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 37. Lyon, FR: IARC; 1985. Tobacco habits other than smoking: betel-quid and areca-nut chewing and some related nitrosamines; pp. 31–202. [PubMed] [Google Scholar]
- 8.Boffetta P, Hecht SS, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2007;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 9.Brunnemann KD, Qi J, Hoffmann D. Chemical profile of two types of oral snuff tobacco. Food Chem. Toxicol. 2002;40:1699–1703. doi: 10.1016/s0278-6915(02)00134-5. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Smoking and Tobacco Control Monographs. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1992. Smokeless Tobacco or Health, An International Perspective; pp. 96–106. [Google Scholar]
- 11.Hoffmann D, Djordjevic MV. Chemical composition and carcinogenicity of smokeless tobacco. Adv. Dent. Res. 1997;11:322–329. doi: 10.1177/08959374970110030301. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 14.Brunnemann KD, Genoble L, Hoffmann D. N-Nitrosamines in chewing tobacco: an international comparison. J. Agric. Food Chem. 1985;33:1178–1181. [Google Scholar]
- 15.Hoffmann D, Djordjevic MV, Fan J, Zang E, Glynn T, Connolly GN. Five leading U.S. commercial brands of moist snuff in 1994 - Assessment of carcinogenic N-nitrosamines. J. Natl. Cancer Inst. 1995;87:1862–1869. doi: 10.1093/jnci/87.24.1862. [DOI] [PubMed] [Google Scholar]
- 16.Osterdahl BG, Jansson C, Paccou A. Decreased levels of tobacco-specific N-nitrosamines in moist snuff on the Swedish market. J Agric. Food Chem. 2004;52:5085–5088. doi: 10.1021/jf049931a. [DOI] [PubMed] [Google Scholar]
- 17.Richter P, Hodge K, Stanfill S, Zhang L, Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob. Res. 2008;10:1645–1652. doi: 10.1080/14622200802412937. [DOI] [PubMed] [Google Scholar]
- 18.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob. Res. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 19.Djordjevic MV, Brunnemann KD, Hoffmann D. The need for regulation of carcinogenic N-nitrosamines in oral snuff. Food Chem. Toxicol. 1993;31:497–501. doi: 10.1016/0278-6915(93)90109-c. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann D, Adams JD, Lisk D, Fisenne I, Brunnemann KD. Toxic and carcinogenic agents in dry and moist snuff. J. Natl. Cancer Inst. 1987;79:1281–1286. [PubMed] [Google Scholar]
- 21.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding YS, Ashley DL, Watson CH. Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J. Agric. Food Chem. 2007;55:5966–5973. doi: 10.1021/jf070649o. [DOI] [PubMed] [Google Scholar]
- 23.Ding YS, Trommel JS, Yan XJ, Ashley D, Watson CH. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environ. Sci. Technol. 2005;39:471–478. doi: 10.1021/es048690k. [DOI] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 82. Lyon, FR: IARC; 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene; pp. 367–435. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JS, Busby WF., Jr Induction of lung and liver tumors by fluoranthene in a preweanling CD-1 mouse bioassay. Carcinogenesis. 1993;14:1871–1874. doi: 10.1093/carcin/14.9.1871. [DOI] [PubMed] [Google Scholar]
- 26.Van Duuren BL, Katz C, Goldschmidt BM. Brief communication: cocarcinogenic agents in tobacco carcinogenesis. J. Natl. Cancer Inst. 1973;51:703–705. [PubMed] [Google Scholar]
- 27.Van Duuren BL, Goldschmidt BM. Cocarcinogenic and tumor-promoting agents in tobacco carcinogenesis. J. Natl. Cancer Inst. 1976;56:1237–1242. doi: 10.1093/jnci/56.6.1237. [DOI] [PubMed] [Google Scholar]
- 28.Slaga TJ, Jecker L, Bracken WM, Weeks CE. The Effects of Weak or Non-Carcinogenic Polycyclic Hydrocarbons on 7,12-Dimethylbenz[a]anthracene and Benzo[a]pyrene Skin Tumor-Initiation. Cancer Lett. 1979;7:51–59. doi: 10.1016/s0304-3835(79)80076-2. [DOI] [PubMed] [Google Scholar]
- 29.Rice JE, Hosted TJ, LaVoie EJ. Fluoranthene and Pyrene Enhance Benzo[a]pyrene-DNA Adduct Formation in vivo in Mouse Skin. Cancer Lett. 1984;24:327–333. doi: 10.1016/0304-3835(84)90030-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmeltz I, Tosk J, Hilfrich J, Hirota N, Hoffmann D, Wynder E. Bioassays of naphthalene and alkylnaphthalenes for co-carcinogenic activity. Relation to tobacco carcinogenesis. In: Jones PW, Freudenthal RI, editors. Carcinogenesis - A Comprehensive Survey, v.3: Polynuclear Aromatic Hydrocarbons. New York: Raven Press; 1978. pp. 47–60. [Google Scholar]
- 31.Hoffmann D, Wynder EL. Studies on gasoline engine exhaust. J. Air Pollut. Contr. Ass. 1963;13:322–327. doi: 10.1080/00022470.1963.10468184. [DOI] [PubMed] [Google Scholar]
- 32.DiGiovanni J, Rymer J, Slaga TJ, Boutwell RK. Anticarcinogenic and cocarcinogenic effects of benzo[e]pyrene and dibenz[a,c]anthracene on skin tumor initiation by polycyclic hydrocarbons. Carcinogenesis. 1982;3:371–375. doi: 10.1093/carcin/3.4.371. [DOI] [PubMed] [Google Scholar]
- 33.U.S.Department of Health and Human Services. Results from the National Survey on Drug Use and Health: 2005 Detailed Tables, Tobacco Brands. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. [Google Scholar]
- 34.Hecht SS, Bondinell WE, Hoffmann D. Chrysene and methylchrysenes: presence in tobacco smoke and carcinogenicity. J. Natl. Cancer Inst. 1974;53:1121–1133. doi: 10.1093/jnci/53.4.1121. [DOI] [PubMed] [Google Scholar]
- 35.Harrison RM, Smith DJT, Luhana L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Brimingham, UK. Environ. Sci. Technol. 1996;30:825–832. [Google Scholar]