Abstract
Anticardiolipin (aCL) autoantibodies are associated with thrombosis, recurrent fetal loss, and thrombocytopenia. Only aCL found in autoimmune disease require the participation of the phospholipid binding plasma protein β2 glycoprotein I (β2GPI) for antibody binding and now are called anti-β2GPI. The antigenic specificity of aCL affinity purified from 11 patients with high titers was evaluated in an effort to better understand the pathophysiology associated with aCL. Seven different recombinant domain-deleted mutants of human β2GPI, and full length human β2GPI (wild-type), were used in competition assays to inhibit the autoantibodies from binding to immobilized wild-type β2GPI. Only those domain-deleted mutants that contained domain 1 inhibited the binding to immobilized wild-type β2GPI from all of the patients. The domain-deleted mutants that contained domain 1 inhibited all aCL in a similar but not identical pattern, suggesting that these aCL recognize a similar, but distinguishable, epitope(s) present on domain 1.
“Antiphospholipid antibodies” is the term generally given to describe autoantibodies that are associated with arterial and venous thromboses, recurrent fetal loss, thrombocytopenia, livedo reticularis, and a biological false positive VDRL test. They may occur alone, as in primary antiphospholipid syndrome, or in association with other autoimmune diseases, such as systemic lupus erythematosus (1, 2). Antiphospholipid antibodies [including anticardiolipin (aCL) antibodies] are detected in many conditions, but only those found in association with autoimmune disease require the presence of the phospholipid binding serum protein β2 glycoprotein I (β2GPI) (3). The exact nature of the antigenic specificity of antiphospholipid autoantibodies is controversial. Initially, the specificity of aCL was thought to be directed solely against anionic phospholipids (4). However, it later was shown that the plasma protein β2GPI, which binds to exposed phospholipids, was the antigenic determinant for these antibodies (5, 6). The precise epitope on β2GPI was not defined. Some groups concluded that these antibodies recognize a complex antigen that includes both β2GPI and anionic phospholipid (6) whereas others have observed aCL binding to β2GPI in the absence of phospholipid (7–14). Others argue that a cryptic epitope, recognized by these antibodies, is generated when β2GPI binds to either cardiolipin-coated or γ-irradiated plastic microplate wells (15). Others have demonstrated that these autoantibodies bind β2GPI in solution in the absence of phospholipid (16–20). These findings strongly support the notion that these autoantibodies recognize epitopes on the native β2GPI molecule. The dichotomy that antiphospholipid antibodies are, in fact, anti-β2GPI antibodies most likely is explained by the observations that autoantibodies to β2GPI are of low affinity (18). The antigen density required for binding of these low-affinity anti-β2GPI autoantibodies is achieved most easily when β2GPI binds to phospholipid-coated polystyrene or irradiated polystyrene. The original nomenclature that called these “aCL antibodies” is a misnomer; these antibodies should be called “anti-β2GPI antibodies”.
β2GPI is composed of five homologous domains numbered 1–5 from the N terminus. Domains 1–4 are composed of ≈60 amino acids (21) that contain a motif characterized by a framework of four conserved cysteine residues, which form two internal disulfide bridges. These repeating motifs were designated sushi domains because of their presumed disk-like shape (22, 23). The fifth domain differs from domains 1–4 in that it contains 82 amino acid residues with six cysteines. The fifth domain contains the phospholipid-binding site (24).
Based on the structural differences between an active form of β2GPI and an inactive form of β2GPI lacking aCL cofactor activity, the putative epitope for anti-β2GPI was proposed to be in the fifth domain of β2GPI (25). This was supported by studies using recombinant β2GPI domain-deleted mutants expressed in bacteria (26). By using recombinant β2GPI domain-deleted mutants (DMs) expressed in insect cells, the epitope for anti-β2GPI was thought to be cryptic, with domain 4 playing a critical role in the exposure of the epitope (27, 28). By contrast, the investigation presented here found that the epitope(s) recognized by 11 of 11 anti-β2GPI tested was located in domain 1.
MATERIALS AND METHODS
Construction, Expression, and Purification of Domain Deletion Mutants.
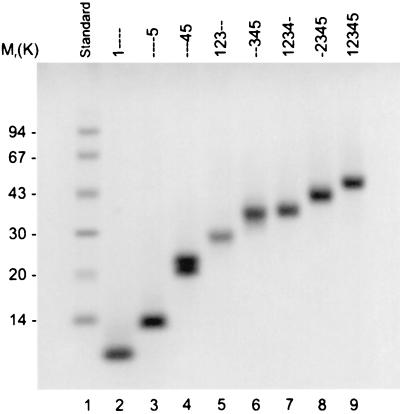
The starting point for the construction of β2GPI DMs was the full length cDNA clone of human β2GPI (29) cloned into pBacPAK9 (a gift from S. Krilis, St. George Hospital, Kogarah, Australia). Mutagenesis was performed by using single-stranded phagemid DNA as described by Kunkel et al. (30). The initial mutagenesis added a glyhis6 immediately after the C-terminal Cys. DMs of β2GPI were made from the construction containing the glyhis6 by using the same method originally described by Koike and colleagues (27). A summary of the relevant data for each is shown in Table 1. DNA coding for the desired DM of β2GPI was transfected into Sf9 insect cells by using BaculoGold (PharMingen) linearized baculovirus DNA. High titer virus was used to infect TN5 insect cells. Approximately 48 h after infection, the his6 mutant β2GPI protein was purified from the medium by nickel chelation chromatography (Qiagen, Valencia, CA). To assess purity, the first five amino acids of the DMs were determined by N-terminal microsequencing (Argo BioAnalytica, Morris Plains, NJ). Protein concentration was determined by amino acid analysis (Peptide Technologies, Gaithersburg, MD). Recombinant proteins then were analyzed by SDS/PAGE (Fig. 1). HPLC analysis has confirmed that preparations are routinely >95% pure (data not shown).
Table 1.
Summary of construction of deletion mutants of β2GPI
| Domain(s) | Construction* | N-terminal 5-aa protein sequence |
|---|---|---|
| 1- - - - | B2del(65-326) | GRTCP |
| 123- - | B2del(182-326) | GRTCP |
| 1234- | B2del(242-326) | GRTCP |
| -2345 | B2del(3-60) | GRTPR |
| - -345 | B2del(3-120) | GRIIC |
| - - -45 | B2del(3-182) | GREVK |
| - - - -5 | B2del(3-242) | GRASC |
| 12345 | B2del (0) | GRTCP |
The numbers in the construction refer to the amino acids that were deleted. For example, the domain 5 deletion mutant, B2del(3-242), has had amino acids 3 through 242 deleted.
Figure 1.
SDS/PAGE analysis of the DMs used. Lane 1 is molecular mass markers (97, 67, 43, 30, 20.1, and 14 kDa). Purified recombinant β2GPI and DMs are in lanes 2–9. Lanes: 2, 1----; 3, ----5; 4, ---45; 5, 123--; 6, --345; 7, 1234-; 8, -2345; 9, 12345. The gel was loaded with 2 μg of protein per lane.
Anti-β2GPI Antibody (aCL) Purification from Patient Plasma or Serum.
The clinical synopses of patients who provided plasma or serum for affinity purification of anti-β2GPI antibody are summarized in Table 2. No patient selection criteria were applied other than availability of sufficient volumes and titer to yield sufficient amount of affinity-purified antibody to carry out the inhibition and/or the direct binding studies with all eight recombinant β2GPIs. GPL (a standardized score for IgG anticardiolipin antibodies) scores were determined with commercial calibrators (APL Diagnostic, Louisville, KY) used in a standard aCL ELISA at plasma dilution of 1:50 (31). All subsequent measurements of anti-β2GPI activity with affinity-purified antibodies used human and not bovine β2GPI. The method used for purification of anti-β2GPI antibody followed two previously published reports (7, 32) that used β2GPI bound to cardiolipin-containing multilamellar dispersions as an affinity matrix to bind anti-β2GPI. The washed liposome pellet with bound anti-β2GPI was dissolved in 1 ml of 2% (wt/vol) solution of n-octyl-B-d-glucopyranoside in TBS (50 mM tris/150 mM NaCl, pH 7.5) and was applied to a 0.6-ml protein A agarose (Repligen) column that had been prewashed with 15 bed volumes of 1 M acetic acid and had been equilibrated with 15 bed volumes of TBS. The antibody-protein A agarose column was washed with 40 bed volumes of 2% n-octyl-B-d-glucopyranoside to remove lipids, followed by extensive washings with TBS until the A280 of the eluate approached baseline. The bound antibody was eluted with 1 M acetic acid. One-milliliter fractions were collected, were neutralized immediately with 3 M Tris, and were kept in an ice bath. Based on A280 readings, fractions containing antibody were pooled, concentrated, and washed 4 times with TBS in Centricon-30 concentrators (Amicon) per the manufacturer’s protocol. The yield ranged from 10 to 190 μg antibody per 1 ml of starting patient plasma/serum. It was imperative, for the assays that follow, that the affinity purification scheme used yield a true representation of the specificity’s present in the plasma. Plasma samples were tested for anti-β2GPI activity after adsorption to ensure that all of the anti-β2GPI antibodies were captured by the affinity matrix used. The purified antibody was tested for anti-β2GPI activity and was checked for purity by SDS/PAGE. Western blotting with anti-β2GPI showed no detectable contamination with β2GPI.
Table 2.
Summary profile of patients
| Identification no. | 6203 | 6501 | 6626 | 6632 | 6641 | 6644 | 6701 | 7008 | 7015 | 7101 | 7104 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPL | 131 | 151 | 141 | 93 | 482 | 329 | 102 | 461 | 60 | 70 | 200 |
| Age, sex | 41, F | 64, F | 36, M | 26, F | 51, F | 35, M | 70, F | 40, F | ?, F | 30, F | 57, M |
| Diagnosis | CI | SLE | PAPS | ? | SLE | PAPS | PAPS | PAPS | PAPS | SLE | PAPS |
| Clinical manifestations | IS, TIA | RFL | VT | TP, CVA | AIHA | AT | CVA, TIA | RFL, TP | RFL | AT | TIA |
AIHA, autoimmune hemolytic anemia; AT, arterial thrombosis; CI, cerebral infarct; CVA, cerebrovascular accident; IS, ischemic stroke; PAPS, primary antiphospholipid syndrome; RFL, recurrent fetal loss; SLE, systemic lupus erythematosus; TIA, transient ischemic attacks; TP, thrombocytopenia; VT, venous thrombosis; M, male; F, female.
Competitive Inhibition ELISA.
Microplates (MaxiSorp, Nunc) were coated with 50 μl of full length recombinant β2GPI at 5 μg/ml in 0.1 M bicarbonate (pH 9.5), were incubated overnight at 4°C, were washed three times with 0.15 M PBS (pH 7.2), and were blocked for 1 hour at room temperature with 75 μl of 2% nonfat dried milk in PBS (2% NFDM). Each antibody preparation was titered to determine the concentration required to give ≈50% maximum binding. The antibodies were used at concentrations between 2.5 and 10 μg/ml in both the inhibition assays and direct binding assays. Test inhibitors were diluted in 2% NFDM, and 25 μl of each dilution or NFDM alone was added to coated wells. Affinity purified anti-β2GPI antibody was diluted in 2% NFDM, and 25 μl of a constant concentration was added to the wells. The contents of the wells were mixed, and the plates were incubated at 37°C for 1 hour. After the plates were washed three times with PBS, 50 μl of alkaline phosphatase conjugated anti-human IgG, γ-chain specific (Zymed) and diluted appropriately in 2% NFDM, was added and incubated at 37°C for 1 hour. After the plates were washed three times with PBS, 50 μl of alkaline phosphatase chromogenic substrate was added, and the plates were incubated for 30 minutes at 20°C. The A550 was measured in a microplate autoreader (Bio-Tek, Burlington, VT). The percent inhibition was determined as follows: [(mean A550 obtained from the control wells without inhibitor less A550 of background) minus (A550 obtained in the presence of inhibitor less A550 of background) divided by (mean A550 obtained from the control wells without inhibitor less A550 of background)] times 100.
Direct binding ELISA of Recombinant β2GPI and Deletion Mutants.
Nickel chelate-coated microwell plates (Xenopore, Hawthorne, NJ) were coated with 50 μl of serial dilutions of recombinant β2GPI-his6 or DMs in PBS at 20°C for 2 hours. The plates were washed three times with PBS and were blocked with 75 μl of a 1% gelatin (Sigma) in PBS for 1 hour at 20°C. After the plates were washed three times with PBS, 50 μl of affinity purified anti-β2GPI antibody (at a concentration that had been shown to give ≈50% of maximum binding) or rabbit polyclonal anti-β2GPI were added, and plates were incubated at 37°C for 1 hour. The plates were washed three times with PBS and 50 μl of alkaline phosphatase conjugated anti-Ig [anti-human IgG, γ-chain specific (Zymed) or anti-rabbit IgG (Zymed)] diluted appropriately in 1% gelatin was added and incubated at 37°C for 1 hour. After the plates were washed three times with PBS, 50 μl of alkaline phosphatase chromogenic substrate was added, and the plates were incubated for 30 minutes at 20°C. The A550 was measured in a microplate autoreader (Bio-Tek).
RESULTS
Analysis of Purified β2GPI Recombinant Proteins.
The objective of this investigation was to determine which domain(s) on β2GPI was recognized by anti-β2GPI antibodies. To this end, β2GPI mutant genes were made by deleting certain domains from the complete β2GPI gene. A his6 tag was added to the carboxy terminal end of each to aid in their purification. Each DM plus the full length β2GP1 were prepared in insect cell cultures. Each of the purified recombinant human β2GPI DMs was resolved on 10% SDS/PAGE (Fig. 1) and was shown to be essentially pure. In addition, all DMs were analyzed by N-terminal microsequencing of the first five amino acids, and each was shown to contain a single N-terminal amino acid and the expected sequence (Table 1). A summary of the relevant data for the DMs is shown in Table 1.
Inhibition Studies.
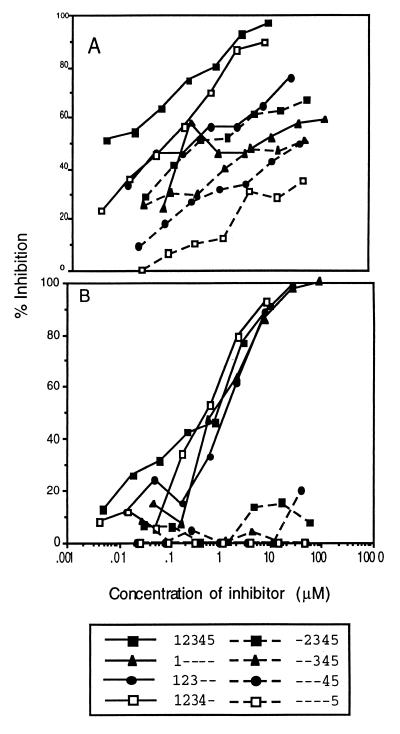
Eight different recombinant β2GPI mutant proteins were used to determine the antigenic specificity of affinity purified anti-β2GPI preparations from 11 different patients with various manifestations of the disease (Table 2). Each mutant recombinant β2GPI protein was tested, in a dose-dependent fashion, for its ability to inhibit affinity purified anti-β2GPI antibody from binding to full length recombinant β2GPI (Fig. 2A). All β2GPI constructs inhibited the binding of rabbit polyclonal anti-β2GPI to purified β2GPI immobilized on ELISA plates. By contrast, only those constructs that contained domain 1 inhibited patient 7104 affinity-purified anti-β2GPI from binding to β2GPI (Fig. 2B). To extend this observation, other affinity-purified anti-β2GPI antibodies from patients with high GPL scores were analyzed similarly (Table 3). As shown in Table 3, the anti-β2GPI binding of all 11 patients was inhibited by β2GPI constructs that contained domain 1. IC50 values for mutants containing domain 1 ranged from 0.1 to 10 μM. By contrast, those constructs in which domain 1 had been deleted did not effectively inhibit anti-β2GPI binding to β2GPI. Mutants lacking domain 1 inhibited only 5–20% of anti-β2GPI binding with IC50 values generally >40 μM. Thus, domain 1 is required to bind anti-β2GPI when competing in solution for purified β2GPI bound on ELISA plates.
Figure 2.
Competitive inhibition of anti-β2GPI from binding to β2GPI adsorbed on microtiter wells, by recombinant β2GPI and DMs. A constant amount of antibody was mixed with varying concentrations of inhibitor in wells coated with β2GPI. Recombinant β2GPI and DMs were used as inhibitors. In A are results obtained with rabbit anti-β2GPI. In B are results obtained with anti-β2GPI 7104. Solid line, inhibitors that contain domain 1; broken lines, inhibitors that do not contain domain 1.
Table 3.
Competitive inhibition assays using 11 different aCL antibody preparations with indicated recombinant B2GP1 and deletion mutants
| Antibody no. | 12345*
|
1- - - -
|
123- -
|
1234-
|
-2345
|
- -345
|
- - -45
|
- - - -5
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max† | 50%‡ | Max | 50% | Max | 50% | Max | 50% | Max | 50% | Max | 50% | Max | 50% | Max | 50% | |
| 7104 | 90 | 0.8 | 90 | 1 | 98 | 1 | 90 | 0.7 | 10 | >57 | 20 | >50 | 5 | >40 | 0 | >47 |
| 6203 | 75 | 0.8 | 20 | >10 | 75 | 1 | 30 | >8 | 10 | >57 | 10 | >50 | 10 | >40 | 5 | >47 |
| 7008 | 90 | 0.2 | 40 | >10 | 80 | 0.4 | 50 | 8 | 20 | >57 | 20 | >50 | 20 | >40 | 20 | >47 |
| 6501 | 80 | 0.2 | 30 | >10 | 85 | 0.8 | 30 | >8 | 20 | >57 | 15 | >50 | 15 | >40 | 10 | >47 |
| 6626 | 80 | 0.3 | 50 | 10 | 90 | 0.8 | 40 | >8 | 18 | >57 | 20 | >50 | 15 | >40 | 10 | >47 |
| 6632 | 90 | 0.8 | 70 | 3 | 90 | 0.2 | 60 | 2 | 20 | >57 | 20 | >50 | 20 | >40 | 10 | >47 |
| 6644 | 90 | 0.2 | 45 | >10 | 90 | 0.7 | 50 | 8 | 10 | >57 | 10 | >50 | 10 | >40 | 10 | >47 |
| 7015 | 90 | 0.2 | 30 | >10 | 90 | 0.7 | 50 | 8 | 10 | >57 | 10 | >50 | 10 | >40 | 10 | >47 |
| 7101 | 80 | 0.8 | 20 | >10 | 70 | 3 | 20 | >8 | 5 | >57 | 5 | >50 | 5 | >40 | 5 | >47 |
| 6701 | 100 | 0.1 | 80 | 4 | 95 | 0.3 | 30 | >8 | 10 | >16 | 20 | >15 | 15 | >5 | 10 | >47 |
| 6641 | 96 | 0.1 | 98 | 10 | 60 | 4 | 60 | 2 | 20 | >16 | 10 | >15 | 20 | >5 | 10 | >47 |
>, Highest concentration tested.
Domains included in construct.
Maximum Inhibition observed at concentrations tested.
Concentration (micromolar) to give 50% inhibition.
Direct Binding of Recombinant Mutant β2GPI Proteins by Anti-β2GPI Antibodies.
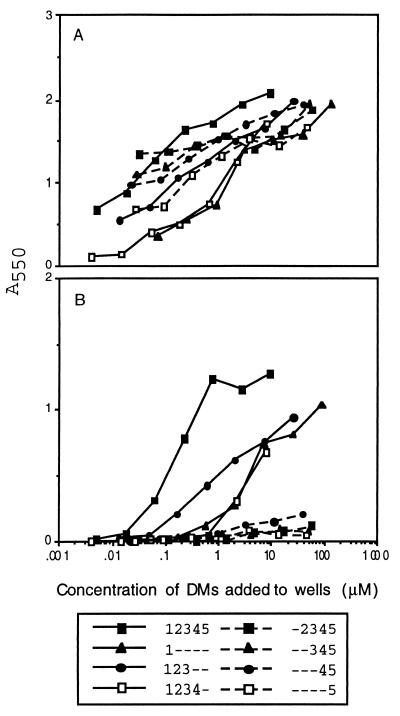
The competitive inhibition assays clearly show that domain 1, in solution, inhibits anti-β2GPI antibodies from binding to β2GPI immobilized on plastic. To demonstrate that the anti-β2GPI from the patients recognized domain 1 of β2GPI and not a neoantigen expressed by the interaction of β2GPI bound to plastic or anionic phospholipid, the β2GPI mutant proteins were bound directly to nickel chelate wells via their his6 tag in the absence of such interactions. Each mutant recombinant β2GPI protein was tested, in a dose-dependent fashion, with affinity-purified anti-β2GPI antibody preparations from 10 different patients. All eight recombinant mutant β2GPI proteins bound rabbit anti-β2GPI, showing that they were accessible to antibody (Fig. 3A). The results from a typical binding experiment using anti-β2GPI from patient 6203 showed that only those proteins containing domain 1 bound affinity purified anti-β2GPI antibody (Fig. 3B). The results from assays of a battery of 10 affinity-purified anti-β2GPI antibodies is summarized in Table 3. All anti-β2GPI bound strongly to domain 1–5 β2GPI. Binding to other domain 1-containing constructs was significant, although less robust, and displayed higher degrees of variability than did the intact β2GPI. By contrast, those constructs in which domain 1 was deleted showed little, if any, specific binding of anti-β2GPI. Thus, anti-β2GPI recognizes domain 1 when β2GPI is immobilized in the absence of any neoantigen that might be created when it is bound to plastic or anionic phospholipid.
Figure 3.
Direct binding of anti-β2GPI to recombinant β2GPI and DMs. Different concentrations of recombinant β2GPI and DMs were immobilized on nickel chelate microtiter wells. A constant amount of antibody was added, incubated, and washed, and the amount of antibody bound was detected by using an alkaline phosphatase conjugated second antibody. In A are results obtained with rabbit anti-β2GPI. In B are results obtained with anti-β2GPI 6203. Solid line, inhibitors that contain domain 1; broken lines, inhibitors that do not contain domain 1.
DISCUSSION
Identification of the antigenic site on β2GP1 that is recognized by anti-β2GPI antibodies has been controversial. The antigenic specificity of anti-β2GPI antibody has been reported to be in domain 5 (25, 26), but it also has been reported that domain 4 plays a critical role in the exposure of a cryptic epitope (27, 28). Both the inhibition studies (Fig. 2) and the direct binding studies (Fig. 3) clearly show that the antigenic specificity of the battery of 11 anti-β2GPI antibody preparations studied in this report are directed toward an epitope that is contained in domain 1 of the β2GPI molecule. It should be noted that antibodies from all 11 patients were inhibited by fluid-phase recombinant β2GPI. This strongly supports the observations of others (16–20) that anti-β2GPI antibodies recognize epitopes present on the native molecule and are not specific for cryptic or neoepitopes present only when β2GPI is bound to phospholipid or irradiated polystyrene. The full length construct completely inhibited the binding of the anti-β2GPI in an almost identical pattern. The DMs that contained domain 1 inhibited in a similar but not identical pattern among the various anti-β2GPI that were examined. This suggests that these antibodies recognize a comparable, but distinguishable, epitope(s) present on domain 1. Domain 1 may have different conformational states when present alone or in constructs containing more domains. For example, some antibodies recognized domain 1 equally well by itself or in a construct that contained more domains. Other antibodies did not recognize domain 1 equally well by itself as compared to multidomain constructs. Thus, these antibodies may recognize an epitope(s) on domain 1 that is affected by the presence of additional domains.
Reports that β2GP1 played a role, as a cofactor, in the binding of aCL antibody in conjunction with some reports that aCL antibodies could bind β2GPI itself has led to conflicting interpretations as to the nature of the antigenic site. McNeil et al. (6) showed that aCL recognized β2GPI when it was bound to anionic phospholipid but not when the β2GPI was bound to heparin. This led them to suggest that both the phospholipid and β2GPI comprised the antigenic epitope being recognized. A number of investigators (7–14) reported that aCL antibodies could recognize β2GPI in the absence of phospholipid, implying that β2GPI was itself the target of these antibodies. aCL antibodies also have been reported to bind to β2GPI adsorbed to microtiter plates under certain conditions. Matsuura et al. (15) demonstrated that aCL antibodies could bind β2GPI adsorbed to irradiated plates but not to nonirradiated plates. They suggested that a cryptic epitope was expressed by a conformational change occurring when β2GPI interacted with some surfaces, such as irradiated plates or cardiolipin, but not when it interacted with other surfaces, such as nonirradiated plates. Roubey et al. (18) reported similar findings but concluded that irradiated plates bound more β2GPI than did nonirradiated plates, which favored the binding of low affinity anti-β2GPI via bivalent attachment.
The data presented in this report offer an explanation to the conflicting interpretations outlined above. Under certain conditions, β2GPI is bound to solid phase supports in such a way as to allow the antigenic epitope on domain 1 to be freely accessible to anti-β2GPI antibodies. These would include irradiated plates, cardiolipin coated plates, Nunc microtiter plates, and nickel chelate plates in the case of the recombinant β2GPI proteins that contain a his6 tag. However, when β2GPI is bound to other surfaces, such as nonirradiated plates or other brands of microtiter plates, its adsorption to the plate may not favor antibody accessibility to domain 1 (data not shown). These inhibition studies confirm reports by others (7, 14) that anti-β2GPI can bind β2GPI in the absence of phospholipid. Four of the eight β2GPI constructs contain domain 1. Only these constructs inhibited all 11 anti-β2GPI from binding to immobilized wild-type β2GPI. The same domain 1-containing constructs, when attached to nickel chelate wells, supported direct binding of all 11 anti-β2GPI. On the other hand, the remaining constructs that do not contain domain 1 neither inhibited any of the anti-β2GPI nor did they support direct binding of these antibodies. The small number of subjects examined in this study is a limitation, and further analysis is under way to determine the extent to which these findings can be generalized.
Table 4.
Direct binding of affinity-plurified aCL to nickel-chelated wells charged with recombinant deletion-mutant B2GP1 protein
| Antibody no. | 12345* | 1- - - - | 123- - | 1234- | -2345 | - -345 | - - -45 | - - - -5 |
|---|---|---|---|---|---|---|---|---|
| 6501 | 1.772† | 0.911 | 0.909 | 0.628 | 0.018 | 0.030 | 0.086 | 0.004 |
| 6626 | 1.527 | 0.560 | 1.250 | 0.563 | 0.008 | 0.022 | 0.086 | 0.028 |
| 6652 | 0.640 | 0.262 | 0.320 | 0.135 | 0.008 | 0.016 | 0.013 | 0.012 |
| 6632 | 1.419 | 0.351 | 0.121 | 0.003 | 0.031 | 0.004 | 0.000 | 0.013 |
| 7008 | 1.380 | 0.195 | 0.360 | 0.149 | 0.019 | 0.018 | 0.030 | 0.007 |
| 6701 | 0.948 | 0.388 | 0.841 | 0.715 | 0.002 | 0.002 | 0.000 | 0.000 |
| 6203 | 1.270 | 1.029 | 0.938 | 0.668 | 0.074 | 0.072 | 0.142 | 0.044 |
| 6641 | 2.555 | 0.252 | 0.530 | 0.145 | 0.045 | 0.019 | 0.112 | 0.018 |
| 6644 | 1.848 | 0.493 | 1.020 | 0.768 | 0.041 | 0.048 | 0.151 | 0.017 |
| 7101 | 1.257 | 0.804 | 0.951 | 0.843 | 0.056 | 0.062 | 0.142 | 0.059 |
| 7015 | 1.864 | 1.102 | 1.160 | 0.454 | 0.114 | 0.042 | 0.167 | 0.078 |
| Rabbit anti-B2 | 2.065 | 1.9737 | 1.971 | 1.708 | 1.873 | 1.933 | 1.941 | 1.663 |
Domains included in constructs.
Maximum OD for each recombinant deletion-mutant:antibody combination.
Acknowledgments
We thank Miss Jackie Crisologo, Miss Darlene Tuyay, and Mr. Eric Smith for expert technical assistance, Dr. Kim Victor for assistance with the construction of deletion mutants, and Richard Jack and Matthew Linnik for helpful comments on the manuscript. We also thank Ware Branch, M.D., Richard Furie, M.D., Kenneth Herbst, M.D, David Hess, M.D., Steven Levine, M.D., and Michael Weisman, M.D. for the plasma samples.
ABBREVIATIONS
- β2GPI
β2 glycoprotein I
- aCL
anticardiolipin
- NFDM
nonfat dried milk
- DM
domain-deleted mutant
References
- 1.Harris E N, Gharavi A E, Bowey M L, Patel B M, Mackworth-Young C G, Loizou S, Hughes G R. Lancet. 1983;ii:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 2.Lockshin M D, Druzin M L, Goei S, Qamar T, Magid M S, Jovanovic L, Ferenc M. N Engl J Med. 1985;313:152–156. doi: 10.1056/NEJM198507183130304. [DOI] [PubMed] [Google Scholar]
- 3.Vaarala O, Palosuo T, Kleemola M, Aho K. Clin Immunol Immunopathol. 1986;41:8–15. doi: 10.1016/0090-1229(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 4.Gharavi A E, Harris E N, Asherson R A, Hughes G R V. Ann Rheum Dis. 1987;46:1–6. doi: 10.1136/ard.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermylen J, Arnout J. J Lab Clin Med. 1992;120:10–12. [PubMed] [Google Scholar]
- 6.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli M, Comfurius P, Massen H, Hemker M C, DeBaets M H, van Breda-Vriesman P J C, Barbui T, Zwaal R F A, Bevers E M. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 8.Arvieux J, Roussel B, Jacob M C, Colomb M G. J Immunol Methods. 1991;143:223–229. doi: 10.1016/0022-1759(91)90047-j. [DOI] [PubMed] [Google Scholar]
- 9.Roubey R A S, Maldonado M A, Byrd S N, Winfield J B. Arthritis Rheum. 1994;37:S298. [Google Scholar]
- 10.Viard J P, Amoura Z, Bach J F. Am J Med. 1992;93:181–186. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 11.Cabral A R, Cabiedes J, Alarcon-Segovia D. J Rheumatol. 1995;22:1894–1898. [PubMed] [Google Scholar]
- 12.Cabiedes J, Cabral A, Alarcon-Segovia D. J Rheumatol. 1995;22:1899–1906. [PubMed] [Google Scholar]
- 13.Martinuzzo M E, Forastriero R R, Carreras L O. Br J Haematol. 1995;89:397–402. doi: 10.1111/j.1365-2141.1995.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 14.Keeling D M, Wilson A J G, Makie I J, Machin S J, Isenberg D A. Br J Haematol. 1992;82:571–574. doi: 10.1111/j.1365-2141.1992.tb06469.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura E, Igarashi Y, Yashada T, Koike T, Triplett D A. J Exp Med. 1994;179:457–462. doi: 10.1084/jem.179.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roubey R A. Blood. 1994;84:2854–2867. [PubMed] [Google Scholar]
- 17.Arvieux J, Roussel B, Colomb M G. Ann Biol Clin (Paris) 1994;52:381–385. [PubMed] [Google Scholar]
- 18.Roubey R A S, Eisenberg R A, Harper M F, Winfield J B. J Immunol. 1995;154:954–960. [PubMed] [Google Scholar]
- 19.Balestrieri G, Tincani A, Spatola L, Allegri F, Prati E, Cattaneo R, Valesini G, Del Papa N, Meroni P L. Lupus. 1995;4:122–130. doi: 10.1177/096120339500400208. [DOI] [PubMed] [Google Scholar]
- 20.Sheng Y, Kandiah D A, Krills S A. J Immunol. 1998;161:2038–2043. [PubMed] [Google Scholar]
- 21.Lozier J, Takahashi N, Putnam F W. Proc Natl Acad Sci USA. 1984;81:3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid K B M, Day A J. Immunol Today. 1989;10:177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- 23.Ichinose A, Bottenus R E, Davie E W. J Biol Chem. 1990;265:13411–13414. [PubMed] [Google Scholar]
- 24.Hunt J E, Simpson R J, Krilis S A. Proc Natl Acad Sci USA. 1993;90:2141–2145. doi: 10.1073/pnas.90.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt J, Krilis S A. J Immunol. 1994;152:653–659. [PubMed] [Google Scholar]
- 26.Yang C D, Chen S L, Shen N, Pei J, Xue F, Lu Y, Gu W, Bao C D. J Rheumatol. 1997;1:96–100. [Google Scholar]
- 27.Igarashi M, Matsuura E, Igarashi Y, Nagae H, Ichikawa K, Triplett D A, Koike T. Blood. 1996;87:3262–3270. [PubMed] [Google Scholar]
- 28.George J, Gilburd B, Hojnik M, Levy Y, Langevitz P, Matsuura E, Koike T, Shoenfeld Y. J Immunol. 1998;160:3917–3923. [PubMed] [Google Scholar]
- 29.Steinkasserer A, Estaller C, Weiss E H, Sim R B, Day A J. Biochem J. 1991;277:387–391. doi: 10.1042/bj2770387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 31.Harris E N. Br J Haematol. 1990;74:1–9. doi: 10.1111/j.1365-2141.1990.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 32.Pengo V, Thiagarajan P, Shapiro S S, Heine M J. Blood. 1987;70:69–76. [PubMed] [Google Scholar]