Abstract
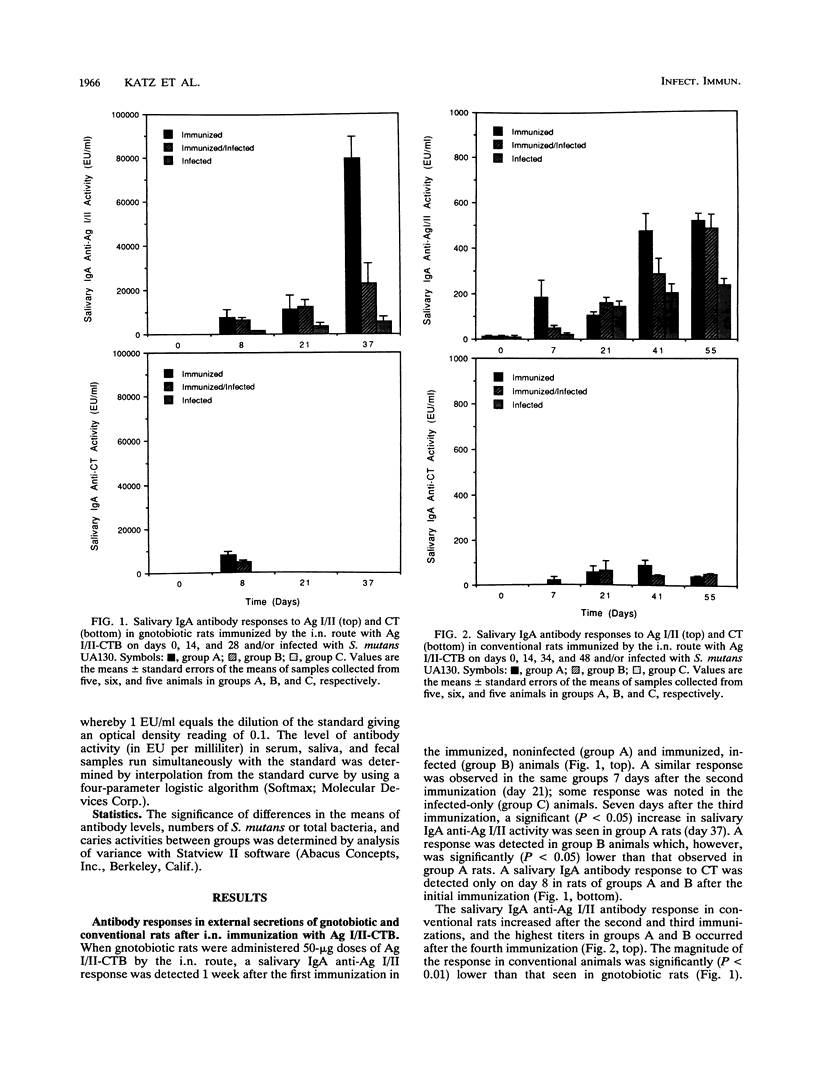
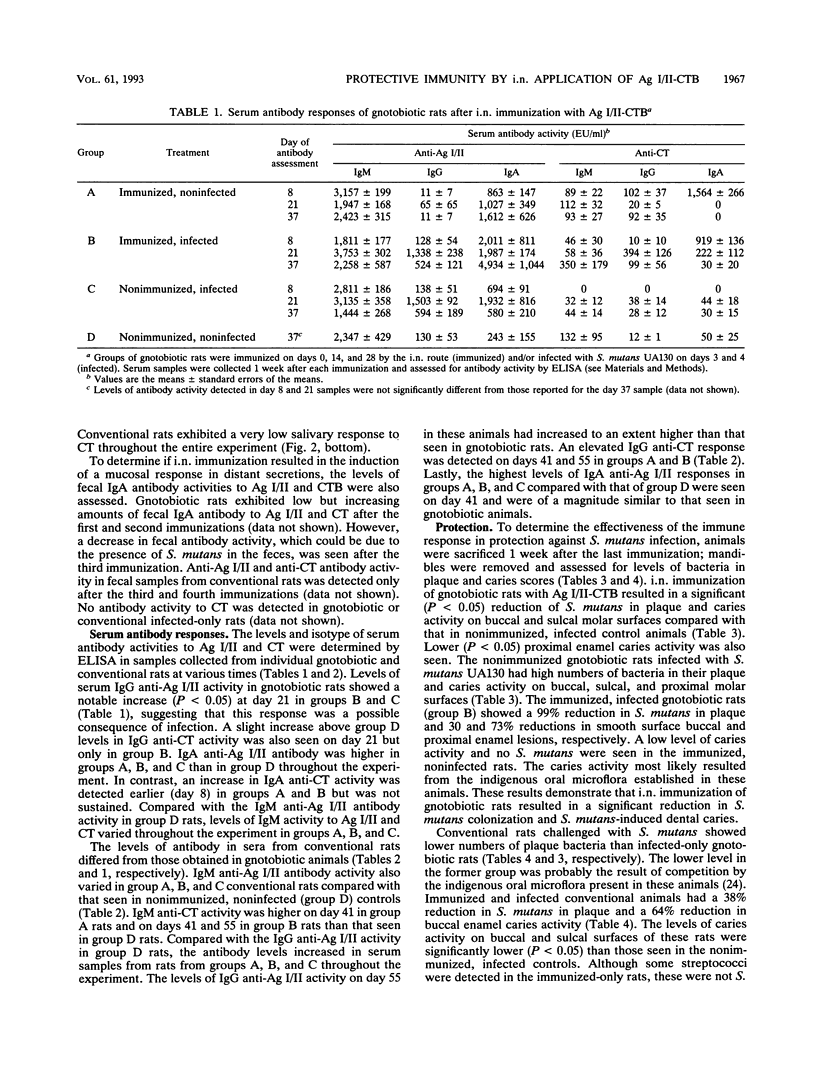
The B subunit of cholera toxin (CTB) has been shown to augment mucosal responses to microbial virulence antigens, including those of Streptococcus mutans, which is the principal etiologic agent of dental caries. In the present study, the surface fibrillar protein antigen of S. mutans, antigen I/II (Ag I/II), was chemically coupled to CTB (Ag I/II-CTB), and the conjugate was examined for its effectiveness in inducing salivary immune responses protective against S. mutans infection. Weanling Fischer rats were given Ag I/II-CTB (50 micrograms) by the intranasal route and then orally infected with a virulent strain of S. mutans. Gnotobiotic or conventional rats were given two or three additional immunizations, respectively, at about 2-week intervals. One week after each immunization, individual serum, saliva, and fecal samples were collected and stored frozen until assayed for antibody activity to Ag I/II and cholera toxin (CT) by an enzyme-linked immunosorbent assay. The rats were sacrificed 1 week after the last immunization, when mandibles were also collected from individual rats for assessment of S. mutans levels in plaque and caries activity. Rats immunized only or both immunized and infected showed a salivary immunoglobulin A (IgA) anti-Ag I/II response which reached significantly (P < 0.05) higher levels than those seen in nonimmunized, infected controls. A salivary IgA anti-Ag I/II response was also seen in rats infected only with S. mutans. Essentially no salivary antibody activity to CT was detected. Some serum anti-Ag I/II and anti-CT responses were seen in immunized animals. Serum IgG anti-Ag I/II responses were seen in immunized, infected rats and also in infected-only rats, suggesting that the responses were a result of infection with S. mutans. The immunized and infected rats had significantly (P < 0.05) lower levels of S. mutans in plaque and lower caries activity than nonimmunized, infected rats. These results indicated that intranasal immunization of rats with Ag I/II-CTB induced a protective salivary immune response which was associated with a reduction in S. mutans colonization and S. mutans-induced dental caries.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayakawa G. Y., Boushell L. W., Crowley P. J., Erdos G. W., McArthur W. P., Bleiweis A. S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh M. T., Peterson D. L., Macrina F. L. Cholera toxin B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect Immun. 1990 Jan;58(1):70–79. doi: 10.1128/iai.58.1.70-79.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O. Cholera toxin and its subunits as potential oral adjuvants. Curr Top Microbiol Immunol. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Gizurarson S., Tamura S., Aizawa C., Kurata T. Stimulation of the transepithelial flux of influenza HA vaccine by cholera toxin B subunit. Vaccine. 1992;10(2):101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- Gizurarson S., Tamura S., Kurata T., Hasiguchi K., Ogawa H. The effect of cholera toxin and cholera toxin B subunit on the nasal mucosal membrane. Vaccine. 1991 Nov;9(11):825–832. doi: 10.1016/0264-410x(91)90220-z. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G., Nikolova E., Russell M. W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992 Dec;60(12):5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameleers D. M., van der Ven I., Biewenga J., Sminia T. Mucosal and systemic antibody formation in the rat after intranasal administration of three different antigens. Immunol Cell Biol. 1991 Apr;69(Pt 2):119–125. doi: 10.1038/icb.1991.18. [DOI] [PubMed] [Google Scholar]
- Hameleers D. M., van der Ven I., Sminia T., Biewenga J. Anti-TNP-forming cells in rats after different routes of priming with TNP-LPS followed by intranasal boosting with the same antigen. Res Immunol. 1990 Jul-Aug;141(6):515–528. doi: 10.1016/0923-2494(90)90020-y. [DOI] [PubMed] [Google Scholar]
- Harris G. S., Michalek S. M., Curtiss R., 3rd Cloning of a locus involved in Streptococcus mutans intracellular polysaccharide accumulation and virulence testing of an intracellular polysaccharide-deficient mutant. Infect Immun. 1992 Aug;60(8):3175–3185. doi: 10.1128/iai.60.8.3175-3185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y., Kurata H., Funato H., Nagamine T., Aizawa C., Tamura S., Shimada K., Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990 Jun;8(3):243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958 Nov-Dec;37(6):1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- Katz J., Michalek S. M. A method for generating antigen-specific rat T helper cell clones. J Immunol Methods. 1991 Apr 8;138(1):77–86. doi: 10.1016/0022-1759(91)90066-o. [DOI] [PubMed] [Google Scholar]
- Koornstra P. J., de Jong F. I., Vlek L. F., Marres E. H., van Breda Vriesman P. J. The Waldeyer ring equivalent in the rat. A model for analysis of oronasopharyngeal immune responses. Acta Otolaryngol. 1991;111(3):591–599. doi: 10.3109/00016489109138388. [DOI] [PubMed] [Google Scholar]
- Kuper C. F., Koornstra P. J., Hameleers D. M., Biewenga J., Spit B. J., Duijvestijn A. M., van Breda Vriesman P. J., Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992 Jun;13(6):219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Kiyono H., Michalek S. M., Babb J. L., Rosenstreich D. L., Mergenhagen S. E. Lipopolysaccharide (LPS) regulation of the immune response: T lymphocytes from normal mice suppress mitogenic and immunogenic responses to LPS. J Immunol. 1980 Apr;124(4):1603–1611. [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975 Jul;12(1):69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R. Virulence of Streptococcus mutans: an antibiotic-suppressed rat model for studies of pathogenesis. J Dent Res. 1977 Mar;56(3):205–211. doi: 10.1177/00220345770560030301. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Morisaki I., Harmon C. C., Hamada S., McGhee J. R. Effective immunity to dental caries: gastric intubation of Streptococcus mutans whole cells or cell walls induces protective immunity in gnotobiotic rats. Infect Immun. 1983 Feb;39(2):645–654. doi: 10.1128/iai.39.2.645-654.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W. Immunization against dental caries. Curr Opin Dent. 1992 Sep;2:72–80. [PubMed] [Google Scholar]
- Russell M. W., Mestecky J. Induction of the mucosal immune response. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S440–S446. doi: 10.1093/cid/10.supplement_2.s440. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Wu H. Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991 Nov;59(11):4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Wu H. Y., White P. L., Takahashi I., Okahashi N., Koga T. Peroral immunization with a cholera toxin-linked bacterial protein antigen and synthetic peptide. Adv Exp Med Biol. 1992;327:199–207. doi: 10.1007/978-1-4615-3410-5_22. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Kanamoto T., Asakawa H., Koga T. Intranasal immunization of mice with recombinant protein antigen of serotype c Streptococcus mutans and cholera toxin B subunit. Arch Oral Biol. 1990;35(6):475–477. doi: 10.1016/0003-9969(90)90211-r. [DOI] [PubMed] [Google Scholar]
- Tamura S. I., Samegai Y., Kurata H., Kikuta K., Nagamine T., Aizawa C., Kurata T. Enhancement of protective antibody responses by cholera toxin B subunit inoculated intranasally with influenza vaccine. Vaccine. 1989 Jun;7(3):257–262. doi: 10.1016/0264-410x(89)90240-5. [DOI] [PubMed] [Google Scholar]
- Tamura S., Ito Y., Asanuma H., Hirabayashi Y., Suzuki Y., Nagamine T., Aizawa C., Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992 Aug 1;149(3):981–988. [PubMed] [Google Scholar]
- Tilney N. L. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971 Sep;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Tlaskalová-Hogenová H., Sterzl J., Stepánkova R., Dlabac V., Veticka V., Rossmann P., Mandel L., Rejnek J. Development of immunological capacity under germfree and conventional conditions. Ann N Y Acad Sci. 1983 Jun 30;409:96–113. doi: 10.1111/j.1749-6632.1983.tb26862.x. [DOI] [PubMed] [Google Scholar]
- Wilson A. D., Clarke C. J., Stokes C. R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990 Apr;31(4):443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993 Jan;61(1):314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deVos T., Dick T. A. A rapid method to determine the isotype and specificity of coproantibodies in mice infected with Trichinella or fed cholera toxin. J Immunol Methods. 1991 Aug 9;141(2):285–288. doi: 10.1016/0022-1759(91)90155-9. [DOI] [PubMed] [Google Scholar]



