Abstract
Background
Data on the quality of life (QOL) of children with Nonalcoholic fatty liver disease (NAFLD) are needed to estimate the true burden of illness in children with NAFLD.
Aim
To characterize QOL and symptoms of children with NAFLD, and to compare QOL in children with NAFLD to a sample of healthy children.
Methods
QOL and symptoms were assessed in children with biopsy-proven NAFLD enrolled in the NASH Clinical Research Network. PedsQL scores were compared to scores from healthy children. For children with NAFLD, between groups comparisons were made to test associations of demography, histologic severity, symptoms and QOL.
Results
239 children (mean age 12.6 years) were studied. Children with NAFLD had worse total (72.8 vs. 83.8, p<.01), physical (77.2 vs. 87.5, p<.01) and psychosocial health (70.4 vs. 81.9, p<.01) scores compared to healthy children. QOL scores did not significantly differ by histological severity of NAFLD. Fatigue, trouble sleeping, and sadness accounted for almost half of the variance in QOL scores. Impaired QOL was present in 39% of children with NAFLD.
Conclusions
Children with NAFLD have a decrement in QOL. Symptoms were a major determinant of this impairment. Interventions are needed to restore and optimize QOL in children with NAFLD.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, quality of life, fatigue, children, adolescents, liver biopsy
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), characterized by the accumulation of large droplets of triglyceride within hepatocytes in the absence of chronic alcohol consumption is the most common cause of pediatric liver disease.1 The high prevalence of NAFLD and its risk for future morbidity have resulted in the recognition of NAFLD as an important disease entity in children.2–6 While the hepatic, cardiovascular, and endocrine effects of NAFLD are evident,7 NAFLD may also be associated with effects on quality of life (QOL).8
QOL, a subjective measure of a disease’s overall impact from an individual’s perspective, is an important component to the complete understanding of disease burden. Traditional indicators of disease burden such as mortality and objective measures of morbidity may underestimate the impact of disease on an individual. Symptoms have the potential for direct effects on QOL. However, the range of symptoms in children with NAFLD is not well characterized. In some cases, NAFLD may be associated with symptoms such as abdominal pain or fatigue. Recent data demonstrate that fatigue is highly prevalent in adults with NAFLD,9 and fatigue is independently associated with lower QOL in adults with other types of chronic liver disease.10 In addition to the potential for symptoms to directly effect QOL, indirect effects such as the emotional influence of worrying about one’s disease may also decrease QOL. Emotional effects of chronic illnesses are often greater than physical effects on the QOL of children11,12 and are only quantifiable using a validated QOL instrument. Thus, QOL measurement provides information on disease-related burden of illness that may otherwise be missed if relying solely on standard clinical measures.
The study aim was to describe the QOL of children with NAFLD using baseline data from a large, multi-center sample of NAFLD. In order to place the findings in a meaningful context, we compared the QOL of children with NAFLD to a reference sample of healthy children. A secondary aim was to characterize the range and severity of symptoms in children with NAFLD.
METHODS
Subjects
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) was established in 2002 to assess the natural history, pathogenesis, and therapy of NAFLD in the United States.13,14 Data for this study came from two NASH CRN studies: (1) an observational cohort study, the NAFLD Database, and (2) a randomized, placebo-controlled pediatric treatment trial entitled ‘Treatment of Nonalcoholic Fatty Liver Disease in Children’ (TONIC) (Clinical Trial #NCT00063635). These studies were conducted by 8 Clinical Centers and a central Data Coordinating Center (see acknowledgments for roster). Study protocols were approved by all participating center institutional review boards. Participant selection criteria for inclusion in the NASH CRN have been previously reported.15 Inclusion criteria for the present analysis were age 5–17 years and biopsy-proven NAFLD. Participants with < 5% steatosis or missing histology or QOL data were excluded.
Measures
Anthropometrics
Height and weight (without shoes or heavy clothing) were measured. Body Mass Index (BMI) was calculated as weight (kg) / [height (m)]2. BMI z-scores and percentiles were calculated using data provided on the CDC web site.16 Obesity was defined as a BMI ≥ the age- and gender-specific 95th percentile from the 2000 CDC BMI Charts. Overweight was defined as BMI ≥ the 85th but < the 95th percentiles.
Histology
The NASH CRN Pathology Committee developed a feature-based histological scoring system that encompasses the spectrum of lesions of NAFLD.17 Liver biopsy slides were rigorously evaluated through a central reading by the Pathology Committee during which biopsies were according to the published system.17 Steatosis was scored according to the percentage of hepatocytes containing fat droplets. A diagnosis of NAFLD required the presence of ≥ 5% steatosis. Fibrosis was staged as follows: stage 1a (mild) and 1b (moderate) zone 3 perisinusoidal fibrosis; stage 1c--portal fibrosis only; stage 2--zone 3 perisinusoidal plus periportal fibrosis; stage 3--bridging fibrosis; and stage 4--definite or probable cirrhosis. Each biopsy was classified into one of the following diagnostic categories: not steatohepatitis, borderline steatohepatitis (zone 3 or zone 1 pattern), and definite steatohepatitis.
Quality of life
The PedsQL 4.0 (Pediatric Quality of Life Inventory™ Version 4.0) is a general quality of life measure comprised of 23 items that make up 4 core scales: Physical Functioning (8 items), Emotional Functioning (5 items), Social Functioning (5 items), and School Functioning (5 items).18,19 The PedsQL was self-administered for parents and for children ages 8 to 17 and interview administered for children ages 5 to 7, in their preferred language (Spanish or English). The PedsQL uses a 5-point likert scale (0=never a problem; 4=almost always a problem). Items are reverse-scored and linearly transformed to a zero to 100 scale (0=100, 1=75, 2=50, 3=25, 4=0), so that higher scores indicate better health-related QOL. A total QOL score (derived by the mean of all 23 items), a physical health summary score (mean of items in the physical health subscale) and a psychosocial health summary score (mean of items in the emotional, social, and school functioning subscales) are calculated to provide a summary of the child or adolescent’s health-related QOL. Children with NAFLD were also categorized as having impaired QOL and not impaired QOL in order to examine factors associated with impaired QOL. Impaired QOL was defined using established criteria20 as being ≥1 standard deviations below the mean total QOL score of the healthy reference population (i.e., a score of ≤ 71.2). QOL was quantified as not impaired if the total QOL score was ≥ the mean total QOL score of the healthy reference population (i.e., ≥ 83.8).
Symptoms, Frequency and Severity
Participants self-reported the presence of the following symptoms using the NIDDK Symptoms of Liver Disease questionnaire: Neuropsychological: fatigue, irritability, sadness, trouble concentrating, and trouble sleeping; Physical: diarrhea, nausea, swelling of abdomen, swelling of ankles; and Pain: headache, liver pain, muscle aches/cramps. Participants reported whether they were bothered by the symptom in the past month: not at all (none), a little bit (mild), medium (moderate), quite a bit or extremely (severe).
Data analysis
Descriptive statistics (mean, median, standard deviation, frequencies, and percentages) were used to characterize the sample population. A student’s t-test (continuous data) or chi-square test (categorical data) was used to compare characteristics between boys and girls. A student’s t-test was used to compare PedsQL scores between boys and girls and between the NASH CRN sample and the established reference sample of healthy children.19 The healthy reference sample included slightly more boys than girls and had a similar age range (5 to 18) and ethnic distribution (Hispanic 62%) as the NASH CRN sample. Analysis of variance (ANOVA) was used to compare PedsQL scores by degree of fibrosis. Multiple regression analysis was conducted to identify factors independently associated with PedsQL score. Factors included in the multiple regression models were: gender, age (<10 years, 10–13 years, and 14–17 years), race (non-Hispanic White, Non-Hispanic, non-White or non-Hispanic, multi-ethnic, and Hispanic), BMI z-score, and presence of each symptom (yes/no). Clinical site was also included in the multivariate analyses to account for potential confounding. Dummy variables were created for categorical variables with more than two levels. In all multiple regression models, multicollinearity among variables was assessed by examining Tolerance and Variance-inflation factor values (multicollinearity defined as Tolerance < .20 and/or Variance-inflation factor ≥4). Homoscedasticity was assessed by simple regression plots, and outliers were assessed by examining standardized residuals [outlier defined as standardized residuals greater than 3.3). A p-value of <.05 was considered statistically significant. To examine determinants of impaired QOL, children who met the definition of impaired QOL were compared to children who did not have impaired QOL. Logistic regression was conducted to identify factors independently associated with impaired QOL.
RESULTS
Participants Baseline Characteristics
Of the 331 children enrolled in the NASH CRN as of May 2007, 239 met inclusion and exclusion criteria and were included in this analysis (Table 1). Participants were from the following clinical centers: Case Western Reserve University (n=14), Indiana University (n=31), Johns Hopkins (n=16), Saint Louis University (n=19), University of California, San Diego (n=124), University of California, San Francisco (n=10), Virginia Commonwealth University (n=7), University of Washington (n=18). Children from UC San Diego were significantly more likely to be Hispanic than children from centers, but did not differ with respect to the distribution of age or gender. The mean age of participants was 12.6 ± 2.5 years. Consistent with the epidemiology of pediatric NAFLD, there were more boys than girls.1,21 The mean BMI of participants was 32.8 ± 5.6 Kg/m2. The distribution of disease severity was: NAFLD but not NASH 23% (54/239), borderline NASH 38% (91/239), and definite NASH 39% (94/239). There were no significant differences between boys and girls for presence of NASH or stage of fibrosis (Table 1).
Table 1.
Baseline Characteristics of Children Enrolled in the NASH CRN
| Total N=239 |
Boys N=174 |
Girls N=65 |
p | |
|---|---|---|---|---|
| Age, mean (SD), y | 12.6 ± 2.5 | 12.6 ± 2.4 | 12.5 ± 2.7 | 0.683 |
| Age Category, n (%) | ||||
| <10 years | 24 (10.0) | 14 (8.0) | 10 (15.4) | 0.089 |
| 10 –13 years | 131 (54.8) | 102 (58.6) | 29 (44.6) | |
| 14 – 17 years | 84 (35.1) | 58 (33.3) | 26 (40.0) | |
| Race, n (%) | ||||
| Hispanic | 148 (61.9) | 106 (60.9) | 42 (64.6) | |
| White, Non-Hispanic | 75 (31.5) | 59 (33.3) | 16 (24.6) | 0.229 |
| Non-White, Non-Hispanic1 | 16 (6.7) | 9 (5.7) | 7 (10.8) | |
| Weight, mean (SD), Kg | 85.6 ± 23.3 | 87.4 ± 23.0 | 81.8 ± 25.4 | 0.050 |
| Height, mean (SD), m | 1.60 ± .140 | 1.62 ± .142 | 1.56 ± .115 | 0.001 |
| BMI | ||||
| mean (SD), Kg/m2 | 32.8 ± 5.6 | 32.8 ± 5.2 | 33.0 ± 6.9 | 0.945 |
| Z-score, mean (SD) | 2.32 ± 0.35 | 2.35 ± 0.35 | 2.24 ± 0.37 | 0.055 |
| Percentile, mean (SD) | 98.5 ± 1.7 | 98.6 ± 1.8 | 98.2 ± 1.8 | 0.160 |
| BMI percentile category, n(%) | ||||
| Overweight (85th – <95th %) | 10 (4) | 7 (4) | 3 (5) | 0.545 |
| Obese (>=95th %) | 228 (95) | 166 (96) | 62 (95) | |
| Type 2 Diabetes, n(%) | ||||
| Yes | 9 (4) | 2 (1) | 7 (11) | 0.002 |
| No | 230 (96) | 172 (99) | 58 (89) | |
| NAFLD pattern, n(%) | ||||
| NAFLD but not NASH | 54 (23) | 36 (21) | 18 (28) | 0.425 |
| Borderline NASH | 91 (38) | 66 (38) | 25 (38) | |
| Definite NASH | 94 (39) | 72 (41) | 22 (34) | |
| Fibrosis stage, n(%) | ||||
| Stage 0 | 68 (29) | 47 (27) | 21 (32) | 0.534 |
| Stage 1a, 1b, 1c | 98 (41) | 74 (43) | 24 (37) | |
| Stage 2 | 39 (16) | 28 (16) | 11 (17) | |
| Stage 3 – 4 | 34 (14) | 25 (14) | 9 (14) | |
Category includes 4 multi-racial subjects (non-Hispanic), and 1 subject missing race
Symptom Frequency and Severity
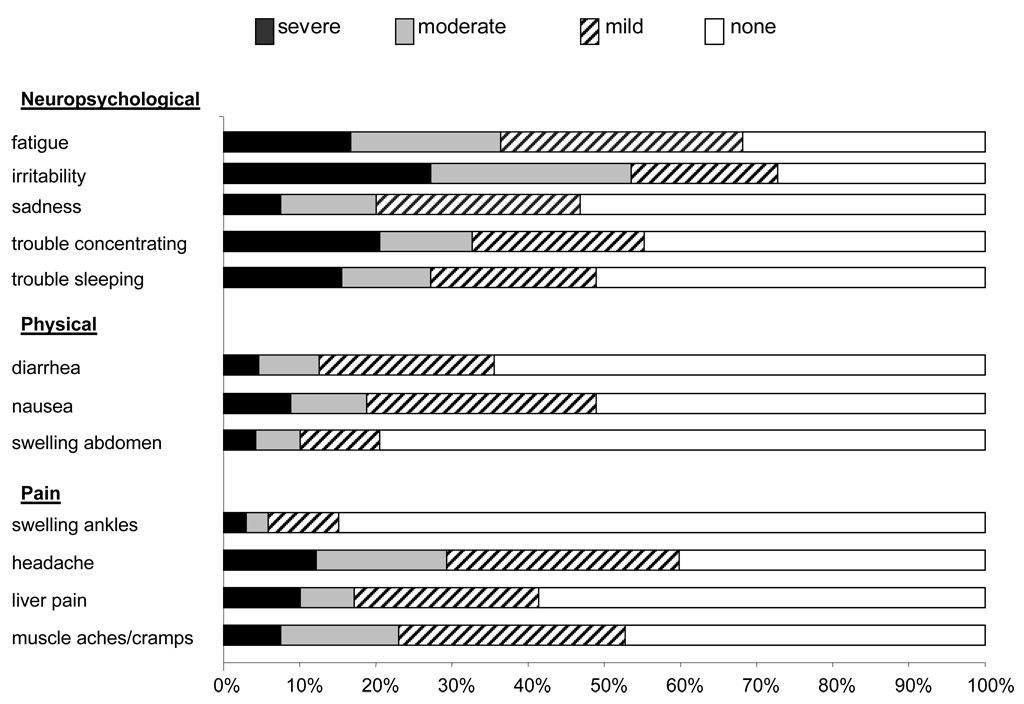
Figure 1 shows the frequency and severity of reported symptoms. The presence of multiple symptoms was common, with 50% of participants reporting having 5 or more symptoms. Irritability was the most common individual symptom (reported by 73% of children). Other common symptoms were fatigue (68%), headache (60%), trouble concentrating (55%), and muscle aches or cramps (53%). The other symptoms were reported as being present by less than half of the children. In addition to being the most frequently reported symptom, irritability was the most severe symptom, with 65 of 239 children (27%) reporting severe irritability. Other symptoms frequently rated as severe were trouble concentrating (21%) and fatigue (17%).
Figure 1.
Frequency and severity of symptoms in children with NAFLD
QOL of Children with NAFLD versus Healthy Children
The mean total QOL score for children with NAFLD was 72.7 ± 15.5 (Table 2). There were no significant differences in QOL between participants with NAFLD but not NASH, borderline NASH, or definite NASH (p=0.49). Similarly, there were no significant differences in QOL by degree of fibrosis (p=0.71).
Table 2.
Comparison of QOL Scores between Children with NAFLD and Healthy Children
| Self-report NAFLD N=240 |
Healthy Children N=5480 |
Parents of NAFLD Children Proxy Report N=240 |
Parents of Healthy Children Proxy Report N=9430 |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-Value1 | Mean (SD) | Mean (SD) | P-Value2 | |
| Total Score | 72.7 (15. 5) | 83.8 (12.7) | <0.001 | 64.8 (18.0) | 82.7 (15.4) | <0.001 |
| Physical Health Score | 77.1 (17.6) | 87.5 (13.5) | <0.001 | 67.0 (22.2) | 84.5 (19.5) | <0.001 |
| Psychosocial health score | 70.3 (16.7) | 81.9 (14.1) | <0.001 | 63.7 (18.8) | 81.7 (15.2) | <0.001 |
| Emotional | 68.5 (20.6) | 79.3 (18.2) | <0.001 | 63.6 (22.1) | 81.3 (16.5) | <0.001 |
| Functioning | ||||||
| Social Functioning | 77.0 (20.8) | 81.1 (16.8) | <0.001 | 67.0 (24.9) | 83.7 (19.4) | <0.001 |
| School Functioning | 65.4 (19.7) | 81.1 (16.5) | <0.001 | 60.3 (22.7) | 78.8 (19.6) | <0.001 |
Comparison is between children with NAFLD and Healthy Children; Healthy reference sample from (19)
Comparison is between parent proxy report of children with NAFLD and parent proxy report of healthy children. (19)
As shown in Table 2, children with NAFLD reported significantly (p<0.01) lower QOL (72.7) compared to healthy children (83.8). Physical health and psychosocial health were also significantly (p<0.01) lower for children with NAFLD. The greatest discrepancy in scores between children with NAFLD and healthy children was in psychosocial health. The largest contributor to poor psychosocial health in children with NAFLD was school functioning.
Comparison of QOL between Boys and Girls with NAFLD
Boys reported significantly (p=0.02) higher total QOL (74.0) than girls (69.0). The difference in total QOL was primarily due to the large difference in physical health for boys (79.1) compared to girls (71.9), p=0.005. Psychosocial health scores did not significantly differ between boys and girls (Table 3).
Table 3.
QOL Scores Stratified by Sex
| Boys N=174 |
Girls N=65 |
||
|---|---|---|---|
| Mean (SD) | Mean (SD) | P | |
| Total Score | 74.0 (16.1) | 69.0 (12.9) | 0.02 |
| Physical health score | 79.1 (17.7) | 71.9 (16.4) | 0.005 |
| Psychosocial health score | 71.4 (17.5) | 67.5 (14.2) | 0.11 |
| Emotional Functioning | 69.9 (20.8) | 64.6 (19.8) | 0.08 |
| Social Functioning | 77.6 (21.6) | 75.3 (18.5) | 0.45 |
| School Functioning | 66.6 (19.8) | 62.5 (19.2) | 0.15 |
Factors Associated with QOL in Participants with NAFLD
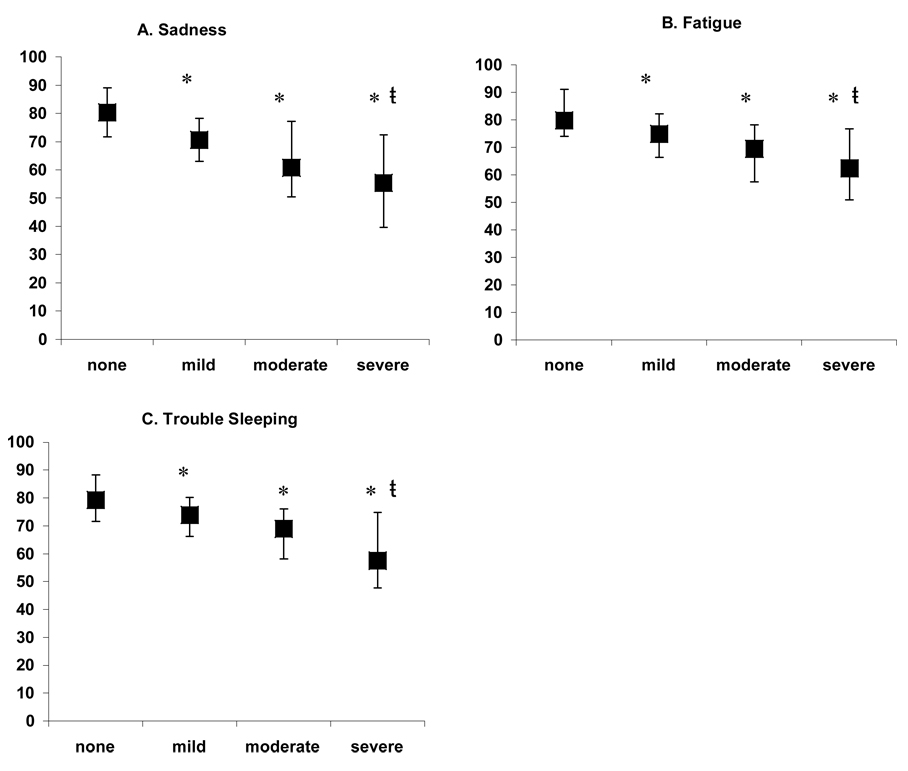
QOL scores of participants from UC San Diego were compared to participants from the remaining 7 centers. Participants from UC San Diego were found to have a significantly (p=0.04) higher mean QOL score (74.7) compared to participants from the other centers (70.5). Therefore in addition to demography, histology, obesity, and symptoms; clinical site was included in the multiple regression models. Symptoms were the only factors independently associated with total QOL, as well as for the subscales of physical and psychosocial health. Symptoms significantly associated with lower QOL included: fatigue (p=0.030), sadness (p=0.002), and trouble sleeping p=0.010) (model R2 = 0.45). Fatigue (p=0.026), sadness (p=0.027), and trouble sleeping (p=0.017) were also significantly associated with lower physical health (model R2 = 0.29). Lower psychosocial health was associated with sadness (p=0.002), trouble sleeping (p=0.029), nausea (p=0.005), diarrhea (p=0.025), muscle aches or cramps (p=0.016), and trouble concentrating (p=0.037) (model R2 = 0.47). Based upon the presence or absence of each symptom, the 3 symptoms that had the strongest association with QOL were fatigue, trouble sleeping, and sadness. Therefore we examined the relationship between the severity of each of these symptoms and QOL. As shown in Figure 2, there was a significant (p < 0.01) inverse relationship between symptom severity and overall QOL.
Figure 2.
Total QOL score by severity of (A) sadness, (B) fatigue, and (C) trouble sleeping. ANOVA demonstrated significant (p<0.001) overall differences for each symptom. Tukey’s post-hoc test demonstrated that: * significantly (p<0.01) different from category none; ŧ significantly (p≤0.01) different from category mild. Boxes=median scores; whiskers=interquartile range
Impaired QOL was noted in 39% of children. Children with impaired QOL had a mean QOL score of 57.6 ± 12.3. Sex and ethnicity were both associated with impaired QOL. Girls, 54% (35/65) had a significantly (p <0.001) higher frequency of impaired QOL than boys, 33% (58/175). Children of Hispanic ethnicity, 32% (47/148) were significantly (p < 0.01) less likely to have impaired QOL than children of non-Hispanic ethnicity (48%, 36/75). No measure of obesity (weight [p=0.61], BMI percentile [p=0.19], or BMI Z score [p=0.19]) had a significant association with impaired QOL. Additionally there was no association between impaired QOL and age, study site, diagnosis of NASH, or stage of fibrosis. As shown in Table 4, children who had impaired QOL were significantly (p<.05) more likely to report having more symptoms than children without impaired QOL. Impaired QOL, after adjustment for sex, ethnicity, and each symptom, had a significant independent association with 4 symptoms: fatigue (β= −1.52, p=0.04), trouble concentrating (β= −1.83, p=.01), sadness (β= −1.82, p=.04), and nausea (β= −1.67, p=.03).
Table 4.
Baseline Characteristics of Children Enrolled in the NASH CRN with Impaired Versus not impaired QOL
| Symptoms Present, n(%) | Total (n=239) |
Impaired (n=93) |
Not Impaired (n=50) |
p |
|---|---|---|---|---|
| Neuropsychological | ||||
| Fatigue | 163 (68) | 83 (89) | 18 (36) | <0.001 |
| Irritability | 174 (73) | 79 (85) | 24 (48) | <0.001 |
| Sadness | 112 (47) | 65 (70) | 4 (8) | <0.001 |
| Trouble concentrating | 132 (55) | 68 (73) | 11 (22) | <0.001 |
| Trouble sleeping | 118 (49) | 64 (69) | 9 (18) | <0.001 |
| Physical | ||||
| Diarrhea | 85 (35) | 50 (54) | 5 (10) | <0.001 |
| Nausea | 117 (49) | 65 (70) | 12 (24) | <0.001 |
| Swelling abdomen | 49 (20) | 29 (31) | 7 (14) | 0.027 |
| Swelling ankles | 36 (15) | 23 (24) | 6 (12) | 0.083 |
| Pain | ||||
| Headache | 143 (60) | 66 (71) | 16 (32) | <0.001 |
| Liver pain | 99 (41) | 55 (59) | 9 (18) | <0.001 |
| Muscle aches/cramps | 126 (53) | 64 (69) | 17 (34) | <0.001 |
DISCUSSION
In a large, multi-center study, children with biopsy-proven NAFLD reported lower QOL scores than a reference sample of healthy children. Furthermore, nearly 40% of children with NAFLD had impaired QOL. Among children with NAFLD, QOL did not differ by histological severity of disease. There was a high rate of symptoms reported by children with NAFLD. The symptoms of fatigue, trouble sleeping, and sadness accounted for almost half of the variance in QOL scores in children with NAFLD.
The current data advance the understanding of QOL in children with NAFLD. The QOL scores reported by children with NAFLD were comparable with the range of scores reported by children with other chronic diseases such as asthma or diabetes19,22. Because the majority of children with NAFLD are obese, careful consideration is warranted regarding the relationship between obesity and QOL. There is a large body of literature showing lower QOL in obese children compared to children who are not obese.19,20, 23–27 In such studies, the severity of obesity as measured by BMI or BMI z-score has a significant negative correlation with QOL.20,24,28 Notably, in the current study of children with NAFLD BMI z-score was not associated with QOL score nor the category of impaired QOL. One important difference is that children studied from obesity clinics typically have much higher BMIs (i.e. BMI of 35 to 50)20,23,29 than children with NAFLD. In order to fully evaluate the effect of NAFLD on QOL separate from obesity a study of obese children without NAFLD is required. Such a study could only be done utilizing magnetic resonance to accurately classify obese children as having or not having NAFLD.
Beyond NAFLD, the study of QOL in pediatric liver disease is relatively nascent. The majority of studies have focused on QOL after liver transplantation,30–34 and have demonstrated impaired QOL compared to healthy reference groups. Three studies have reported QOL data for children with chronic viral hepatitis. Initially, a small study of 19 children with chronic hepatitis C showed lower QOL compared to a normative sample.35 Conversely, the multi-center PEDS-C study found no difference between children with chronic hepatitis C and a normative sample of children.36 While another study found a decrease in QOL for children with chronic hepatitis C or hepatitis B only during treatment with alpha interferon.37 These studies used a different QOL instrument than used in the current study so no direct comparison can be made. In one study of adults, QOL was lower in adults with NAFLD than in adults with hepatitis B.38 Lastly, the only previous data on QOL in children with NAFLD was from a small pilot study of children with biopsy-proven NASH and it showed significant impairment in QOL prior to treatment.8 A recent study from the NASH CRN found that in adults with NAFLD, QOL was low compared to healthy peers with the decrement particularly attributable to physical health.39 When taken in aggregate, these limited studies, using different instruments, demonstrate marked impairment of QOL in children following liver transplantation, moderate impairment in children with NASH, and inconsistent findings for children with chronic viral hepatitis.
NAFLD is not as asymptomatic as previously thought; the number and severity of symptoms reported by children with NAFLD was higher than anticipated. To what extent these symptoms are a direct result of NAFLD is unclear. Symptoms, in particular the symptom of fatigue, were associated with reduced QOL in children with NAFLD. Fatigue was independently associated with both QOL score and impaired QOL. Fatigue is associated with an imbalance of sleep, stress, and/or psychological coping skills and has a profound negative effect on one’s well-being. For many chronic conditions, fatigue is regarded as one of the most debilitating symptoms experienced.40–42 In adults with NAFLD, fatigue was similar to that of adults with primary biliary cirrhosis.9 While fatigue has been described as occurring in children and adolescents with NAFLD,43 the frequency and potential effects of fatigue had not previously been assessed.
The frequency of fatigue in children with NAFLD, 68% in the NASH CRN, appears to be greater than in healthy children. In a nationally representative sample, fatigue was reported in 15% of boys and 25% of girls.44 Although the majority of children with NAFLD are obese, obesity cannot be assumed to be the cause of fatigue in children with NAFLD. Prospective studies in children and adolescents have shown that obesity is not independently associated with either incident or persistent fatigue.45,46 Moreover, in the current sample, the severity of obesity as measured by BMI z-score was not associated with the symptoms experienced. Thus while the cause of fatigue remains to be determined, it is important to appreciate that fatigue was very common in children with NAFLD and was associated with decreased QOL.
Fatigue may be thought of as biopsychosocial complex of conditions with many components beyond general fatigue. Viewed in this framework many symptoms reported by children with NAFLD may not be unrelated but rather fit into a rubric as has been previously proposed for fatigue.47–49 Thus, the symptoms in addition to fatigue that were significantly associated with QOL and impaired QOL can be classified as neuropsychological (trouble sleeping, trouble concentrating, sadness) and physical (nausea) symptoms associated with fatigue. In adults, structured exercise programs have decreased fatigue associated with medical conditions as well as fatigue experienced by healthy individuals. Structured exercise may also be beneficial for fatigue in children with NAFLD, and exercise is already recommended in the treatment of NAFLD. Whether fatigue is a meaningful deterrent to exercise in children with NAFLD is unknown; future studies should consider this possibility as well as test exercise as a treatment for fatigue and QOL in children with NAFLD.
The multi-center design of the NASH CRN was a key strength of this study. Although the ascertainment of participants from the 8 clinical sites was not equal the sample was of sufficient size and diversity as to provide the best representation to date of the QOL of children and adolescents with NAFLD. However, the timing of the development of NAFLD, the development of symptoms, and the impairment of QOL cannot be determined from a cross-sectional study. Despite the large sample size, the smaller number of girls was also an important limitation. While this is to be expected given the epidemiology of pediatric NAFLD, future studies should endeavor to include a larger number of girls in order to characterize NAFLD in girls in more detail.
In summary, children with NAFLD have a decrement in QOL relative to healthy children. Given the high prevalence of pediatric NAFLD, this finding portends a potentially large number of children at risk for poor QOL. Symptoms, particularly fatigue, are common in children with NAFLD, and are a major determinant of both QOL score and impaired QOL. It remains unclear whether fatigue in children with NAFLD is related to liver disease itself or some other aspect of the metabolic syndrome phenotype. The care of children with NAFLD should be broadened to include evaluation of both symptoms and QOL. Interventions are needed to restore and optimize QOL in children with NAFLD.
Acknowledgments
The following members of the Nonalcoholic Steatohepatitis Clinical Research Network have been instrumental in the design and conduct of the NAFLD Database and TONIC trial.
Case Western Reserve University Clinical Centers, Cleveland, OH: MetroHealth Medical Center: Carol Hawkins, RN; Yao-Chang Liu, MD; Margaret Stager, MD Cleveland Clinic Foundation: Arthur McCullough, MD; Srinivasan Dasarathy, MD; Ruth Sargent, LPN
Seattle Children’s Hospital & Research Institute, WA: Melissa Coffey; Karen Murray, MD; Melissa Young
Children’s National Medical Center, Washington DC: Parvathi Mohan, MD; Kavita Nair
Duke University Medical Center, Durham, NC: Manal Abdelmalek, MD; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Paul Killenberg, MD (2004–2008); Samantha Kwan; Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith
Indiana University School of Medicine, Indianapolis, IN: Prajakta Bhimalli; Naga Chalasani, MD; Oscar W. Cummings, MD; Lydia Lee, Linda Ragozzino, Raj Vuppalanchi, MD
Riley Hospital for Children, Indianapolis, IN: Elizabeth Byam; Ann Klipsch, RN; Jean Molleston, MD; Girish Subbarao, MD
Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer; Ann Scheimann, MD; Michael Torbenson, MD
Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD (2007-); Joyce Hoffmann; Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Lisa Clark, PhD, MPH; Janis Durelle; Tarek Hassanein, MD; Joel E. Lavine, M.D., Ph.D.; Susana Mendoza; Jeffrey B. Schwimmer, M.D.; Claude Sirlin, M.D.; Tanya Stein, MD; Zobeida Palomares
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD; Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, MD; Raphael Merriman, MD (2002–2007); Mark Pabst; Monique Rosenthal; Philip Rosenthal, MD; Tessa Steel (2006–2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Bimalijit Sandhu, MD; Arun J. Sanyal, MD; Carol Sargeant, RN, MPH; Kimberly Selph; Melanie White, RN
Virginia Mason Medical Center, Seattle, WA1: Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Ackermann; Cheryl Saunders, MPH; Vy Trinh; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD; Terry TK Huang, PhD, MPH
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Edward Doo, MD; James Everhart, MD, MPH; Jay Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (Project Scientist); Leonard Seeff, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS; Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson; Wana Kim; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH; Laura Miriel; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Laura Wilson, ScM; Katherine Yates, ScM
Footnotes
- Authors' declaration of personal interests: None of the authors have any conflict of interests.
-
Declaration of funding interests:The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) was funded in full by the National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713, and the National Institute of Child Health and Human Development. This study was also supported in part by the Intramural Research Program of the National Cancer Institute. Other grant support included the following National Institutes of Health General Clinical Research Centers or Clinical and Translational Science Awards: UL1RR024989, M01RR000750, RR02413101, M01RR000827, UL1RR02501401, and M01RR000065.NAFLD Database and TONIC trial are supported by the National Institute of Diabetes and Digestive and Kidney Disease. Vitamin E softgels and matching placebo used in the TONIC trial are provided by the Pharmavite, LLC through a Clinical Trials Agreement (CTA) with the National Institutes of Health.
original grant with University of Washington
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. Expert committee. [DOI] [PubMed] [Google Scholar]
- 5.Kwiterovich PO. Primary and secondary disorders of lipid metabolism in pediatrics. Pediatr Endocrinol Rev. 2008;5:727–738. [PubMed] [Google Scholar]
- 6.Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:67–72. doi: 10.1007/s11894-008-0011-1. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein E, Lavine JE, Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:380–385. doi: 10.1055/s-0028-1091982. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 9.Newton JL, Jones DE, Henderson E, et al. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57:807–813. doi: 10.1136/gut.2007.139303. [DOI] [PubMed] [Google Scholar]
- 10.Sogolow ED, Lasker JN, Short LM. Fatigue as a major predictor of quality of life in women with autoimmune liver disease: the case of primary biliary cirrhosis. Womens Health Issues. 2008;18:336–342. doi: 10.1016/j.whi.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Janse AJ, Uiterwaal CS, Gemke RJ, Kimpen JL, Sinnema G. A difference in perception of quality of life in chronically ill children was found between parents and pediatricians. J Clin Epidemiol. 2005;58:495–502. doi: 10.1016/j.jclinepi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Loonen HJ, Derkx BH, Griffiths AM. Pediatricians Overestimate Importance of Physical Symptoms Upon Children’s Health Concerns. Med Care. 2002;40:996–1001. doi: 10.1097/00005650-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Nonalcoholic Steatohepatitis Clinical Research Network. Hepatology. 2003;37:244. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 14.Lavine J, Schwimmer J for NASH CRN. J Pediatr Gastroenterol Nutr. 2003;37:220–221. doi: 10.1097/00005176-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Patton HM, Lavine JE, Van Natta ML, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [accessed Jan 2008]; http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
- 17.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Rode CA. The PedsQL™: Measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 21.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:561–565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 22.Naughton MJ, Ruggiero AM, Lawrence JM. Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus. Arch Pediatr Adolesc Med. 2008;162:649–657. doi: 10.1001/archpedi.162.7.649. [DOI] [PubMed] [Google Scholar]
- 23.Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117:1155–1161. doi: 10.1542/peds.2005-1141. [DOI] [PubMed] [Google Scholar]
- 24.Pinhas-Hamiel O, Singer S, Pilpel N, Fradkin A, Modan D, Reichman B. Health-related quality of life among children and adolescents: associations with obesity. Int J Obes (Lond) 2006;30:267–272. doi: 10.1038/sj.ijo.0803107. [DOI] [PubMed] [Google Scholar]
- 25.Fallon EM, Tanofsky-Kraff M, Norman AC, et al. Health-related quality of life in overweight and nonoverweight black and white adolescents. J Pediatr. 2005;147:443–450. doi: 10.1016/j.jpeds.2005.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams J, Wake M, Hesketh K, Maher E, Waters E. Health-related quality of life of overweight and obese children. JAMA. 2005;293:70–76. doi: 10.1001/jama.293.1.70. 5. [DOI] [PubMed] [Google Scholar]
- 27.Swallen KC, Reither EN, Haas SA, Meier AM. Overweight, obesity, and health-related quality of life among adolescents: the National Longitudinal Study of Adolescent Health. Pediatrics. 2005;115:340–347. doi: 10.1542/peds.2004-0678. [DOI] [PubMed] [Google Scholar]
- 28.de Beer M, Hofsteenge GH, Koot HM, Hirasing RA, Delemarre-van de Waal HA, Gemke RJ. Health-related-quality-of-life in obese adolescents is decreased and inversely related to BMI. Acta Paediatr. 2007;96:710–714. doi: 10.1111/j.1651-2227.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 29.Modi AC, Loux TJ, Bell SK, Harmon CM, Inge TH, Zeller MH. Weight-specific Health-related Quality of Life in Adolescents With Extreme Obesity. Obesity. 2008;16:2266–2271. doi: 10.1038/oby.2008.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Midgley DE, Bradlee TA, Donohoe C, et al. Health-related quality of life in long-term survivors of pediatric liver transplantation. Liver Transpl. 2000;6:333–339. doi: 10.1053/lv.2000.6139. [DOI] [PubMed] [Google Scholar]
- 31.Bucuvalas JC, Britto M, Krug S, et al. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transpl. 2003;9:62–71. doi: 10.1053/jlts.2003.50012. [DOI] [PubMed] [Google Scholar]
- 32.Alonso EM, Neighbors K, Mattson C, et al. Functional outcomes of pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2003;37:155–160. doi: 10.1097/00005176-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Manificat S, Dazord A, Cochat P, et al. Quality of life of children and adolescents after kidney or liver transplantation: child, parents and caregiver’s point of view. Pediatr Transplant. 2003;7:228–235. doi: 10.1034/j.1399-3046.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 34.Alonso EM, Neighbors K, Barton FB, et al. Health-related quality of life and family function following pediatric liver transplantation. Liver Transpl. 2008;14:460–468. doi: 10.1002/lt.21352. [DOI] [PubMed] [Google Scholar]
- 35.Nydegger A, Srivastava A, Wake M, et al. Health-related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol. 2008;23:226–230. doi: 10.1111/j.1440-1746.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigue JR, Balistreri W, Haber B, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48:341–347. doi: 10.1097/MPG.0b013e318185998f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iorio R, Pensati P, Botta S, et al. Side effects of alpha-interferon therapy and impact on health-related quality of life in children with chronic viral hepatitis. Pediatr Infect Dis J. 1997;16:984–990. doi: 10.1097/00006454-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Dan AA, Kallman JB, Wheeler A, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26:815–820. doi: 10.1111/j.1365-2036.2007.03426.x. [DOI] [PubMed] [Google Scholar]
- 39.David K, Kowdley KV, Unalp A, et al. Quality of Life in Adults with Nonalcoholic Fatty Liver Disease: Baseline Data from the NASH CRN. Hepatology. 2009;49:1904–1912. doi: 10.1002/hep.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zifko UA. Management of fatigue in patients with multiple sclerosis. Drugs. 2004;64:1295–1304. doi: 10.2165/00003495-200464120-00003. [DOI] [PubMed] [Google Scholar]
- 41.Richardson A. Fatigue in cancer patients: a review of the literature. Eur J Cancer Care. 1995;4:20–32. doi: 10.1111/j.1365-2354.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 42.Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52:2531–2539. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- 43.Schwimmer JB. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 2007;27:312–318. doi: 10.1055/s-2007-985075. [DOI] [PubMed] [Google Scholar]
- 44.Rhee H, Miles MS, Halpern CT, Holditch-Davis D. Prevalence of recurrent physical symptoms in U.S. adolescents. Pediatr Nurs. 2005;31:314–319. 350. [PubMed] [Google Scholar]
- 45.Viner RM, Clark C, Taylor SJ, et al. Longitudinal risk factors for persistent fatigue in adolescents. Arch Pediatr Adolesc Med. 2008;162:469–475. doi: 10.1001/archpedi.162.5.469. [DOI] [PubMed] [Google Scholar]
- 46.ter Wolbeek M, van Doornen LJ, Kavelaars A, Heijnen CJ. Predictors of persistent and new-onset fatigue in adolescent girls. Pediatrics. 2008;121:449–457. doi: 10.1542/peds.2007-1093. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: An Overview. Am Fam Physician. 2008;78:1173–1179. [PubMed] [Google Scholar]
- 48.Varni JW, Limbers CA, Bryant WP, Wilson DP. The PedsQL™ Multidimensional Fatigue Scale in type 1 diabetes: feasibility, reliability, and validity. Pediatr Diabetes. 2009;10:321–328. doi: 10.1111/j.1399-5448.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 49.Viner R, Christie D. Fatigue and somatic symptoms. BMJ. 2005;330:1012–1015. doi: 10.1136/bmj.330.7498.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]