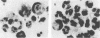Abstract
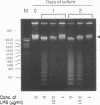
In previous studies, neutrophil-ingesting macrophages were clearly and easily observed in the peritoneal cavity of guinea pigs after intraperitoneal injection of thioglycolate medium, and phagocytosis of neutrophils by macrophages could be detected in in vitro cultures of peritoneal exudate cells. Using an in vitro system, we examined the effect of bacterial lipopolysaccharide and recombinant human granulocyte colony-stimulating factor on the apoptosis (programmed cell death) of neutrophils and their subsequent ingestion by macrophages. Lipopolysaccharide delayed karyopyknosis and apoptosis of neutrophils, as shown by endogenous endonuclease activity and a high proportion of trypan blue-excluding cells, and subsequent ingestion by autologous macrophages. Granulocyte colony-stimulating factor also delayed neutrophil karyopyknosis and ingestion by macrophages. When a thioglycolate medium was coinjected intraperitoneally with lipopolysaccharide into guinea pigs in the in vivo system, delays in neutrophil disappearance and ingestion by macrophages in the peritoneal cavity were also observed. We suggest that bacterial products and cytokines regulate neutrophil apoptosis and subsequent ingestion by macrophages at inflamed sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Neutrophil responses to lipopolysaccharide. Effect of adherence on triggering and priming of the respiratory burst. J Immunol. 1991 Feb 15;146(4):1271–1276. [PubMed] [Google Scholar]
- BREWER D. B. ELECTRON-MICROSCOPE OBSERVATIONS ON THE PHAGOCYTOSIS OF NEUTROPHIL POLYMORPHONUCLEAR LEUCOCYTES BY MACROPHAGES. J Pathol Bacteriol. 1964 Jul;88:307–309. doi: 10.1002/path.1700880139. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Lopez A. F., Nicola N. A., Warren D. J., Vadas M. A., Sanderson C. J., Metcalf D. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood. 1986 Jul;68(1):162–166. [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Granulocyte activation by endotoxin. II. Role of granulocyte adherence, aggregation, and effect of cytochalasin B, and comparison with formylated chemotactic peptide-induced stimulation. J Immunol. 1983 Feb;130(2):863–868. [PubMed] [Google Scholar]
- Dresch C., Flandrin G., Breton-Gorius J. Phagocytosis of neutrophil polymorphonuclears by macrophages in human bone marrow: importance in granulopoiesis. J Clin Pathol. 1980 Nov;33(11):1110–1113. doi: 10.1136/jcp.33.11.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall E., Wyllie A. H., Morris R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985 Oct;56(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Fittschen C., Sandhaus R. A., Worthen G. S., Henson P. M. Bacterial lipopolysaccharide enhances chemoattractant-induced elastase secretion by human neutrophils. J Leukoc Biol. 1988 Jun;43(6):547–556. doi: 10.1002/jlb.43.6.547. [DOI] [PubMed] [Google Scholar]
- Grigg J. M., Savill J. S., Sarraf C., Haslett C., Silverman M. Neutrophil apoptosis and clearance from neonatal lungs. Lancet. 1991 Sep 21;338(8769):720–722. doi: 10.1016/0140-6736(91)91443-x. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Lee A., Savill J. S., Meagher L., Whyte M. K. Apoptosis (programmed cell death) and functional changes in aging neutrophils. Modulation by inflammatory mediators. Chest. 1991 Mar;99(3 Suppl):6S–6S. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. C., Penfold P. L., Holden B. A., Billson F. A. Ultrastructural features of contact lens-induced deep corneal neovascularization and associated stromal leukocytes. Cornea. 1990 Apr;9(2):144–151. [PubMed] [Google Scholar]
- Mangan D. F., Wahl S. M., Sultzer B. M., Mergenhagen S. E. Stimulation of human monocytes by endotoxin-associated protein: inhibition of programmed cell death (apoptosis) and potential significance in adjuvanticity. Infect Immun. 1992 Apr;60(4):1684–1686. doi: 10.1128/iai.60.4.1684-1686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Synthesis by mouse peritoneal cells of G-CSF, the differentiation inducer for myeloid leukemia cells: stimulation by endotoxin, M-CSF and multi-CSF. Leuk Res. 1985;9(1):35–50. doi: 10.1016/0145-2126(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K., Nagayama A., Nagata S. Constitutive production of granulocyte colony-stimulating factor by hybrids of a SV40-transformed mouse macrophage and a renal adenocarcinoma cell line. Growth Factors. 1991;5(3):183–189. doi: 10.3109/08977199109000282. [DOI] [PubMed] [Google Scholar]
- Pekin T. J., Jr, Malinin T. I., Zvaifler N. J. Unusual synovial fluid findings in Reiter's syndrome. Ann Intern Med. 1967 Apr;66(4):677–684. doi: 10.7326/0003-4819-66-4-677. [DOI] [PubMed] [Google Scholar]
- Sanui H., Yoshida S., Nomoto K., Ohhara R., Adachi Y. Peritoneal macrophages which phagocytose autologous polymorphonuclear leucocytes in guinea-pigs. I: induction by irritants and microorgansisms and inhibition by colchicine. Br J Exp Pathol. 1982 Jun;63(3):278–284. [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Henson P. M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel "charge-sensitive" recognition mechanism. J Clin Invest. 1989 Nov;84(5):1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Simpson D. M., Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972 Aug;51(8):2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs A. I., Boddington M. M., Mowat A. G. Joint fluid cytology in Reiter's disease. Ann Rheum Dis. 1978 Dec;37(6):557–560. doi: 10.1136/ard.37.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M., Meagher L., Savill J., Haslett C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J Immunol. 1992 Jun 1;148(11):3543–3549. [PubMed] [Google Scholar]
- Vellenga E., Rambaldi A., Ernst T. J., Ostapovicz D., Griffin J. D. Independent regulation of M-CSF and G-CSF gene expression in human monocytes. Blood. 1988 Jun;71(6):1529–1532. [PubMed] [Google Scholar]
- Verghese M. W., Snyderman R. Endotoxin (LPS) stimulates in vitro migration of macrophages from LPS-resistant mice but not from LPS-sensitive mice. J Immunol. 1982 Feb;128(2):608–613. [PubMed] [Google Scholar]
- Watari K., Asano S., Shirafuji N., Kodo H., Ozawa K., Takaku F., Kamachi S. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989 Jan;73(1):117–122. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Yoshida S., Mizuguchi Y. Phagocytosis of polymorphonuclear leukocytes by guinea pig peritoneal macrophages: effects of serum and temperature in vitro. FEMS Microbiol Immunol. 1992 Oct;5(4):211–218. doi: 10.1111/j.1574-6968.1992.tb05903.x. [DOI] [PubMed] [Google Scholar]