Summary
Lifespan in rodents is prolonged by caloric restriction (CR) and by mutations affecting the somatotropic axis. It is not known if CR can alter the age-associated decline in GH, IGF-1 and GH secretion.
Aim
To evaluate the effect of caloric restriction on GH secretory dynamics.
Methods
Forty-three young (36.8±1.0y), overweight (BMI 27.8±0.7) men (n=20) and women (n=23) were randomized into four groups; Control=100% of energy requirements; CR=25% calorie restriction; CR+EX=12.5% CR+12.5% increase in energy expenditure by structured exercise; LCD=low calorie diet until 15% weight reduction followed by weight maintenance. At baseline and after six months, body composition (DXA), abdominal visceral fat (CT) 11-h GH secretion (blood sampling every 10 min for 11 hours; 2100h-0800h) and deconvolution analysis were measured.
Results
After six months, weight (Control:−1±1%, CR:−10±1%, CR+EX:−10±1%, LCD:−14±1%), fat mass (Control:−2±3%, CR:−24±3%, CR+EX:−25±3%, LCD:−31±2%), and visceral fat (Control: −2±4%, CR:−28±4%, CR+EX:−27±3%, LCD:−36±2%) were significantly (p<.001) reduced in the three intervention groups compared to control. Mean 11-h GH concentrations were not changed in CR or control but increased in CR+EX (p<.0001) and LCD (p<.0001) because of increased secretory burst mass (CR+EX: 34±13%, LCD: 27±22%, p<0.05) and amplitude (CR+EX: 34±14%, LCD: 30±20%, p<0.05) but not to changes in secretory burst frequency or GH half-life. Fasting ghrelin was significantly increased from baseline in all three intervention groups however total IGF-1 concentrations were increased only in CR+EX (10±7%, p<0.05) and LCD (19±4%, p<0.001).
Conclusion
A 25% CR diet for 6 months does not change GH, GH secretion or IGF-1 in non-obese men and women.
Keywords: caloric restriction, GH, IGF-1, aging
The somatotropic axis which consists of the pituitary-derived growth hormone (GH) and insulin-like growth factor 1 (IGF-1) undergoes progressive changes with age. Beginning in young adulthood, GH and IGF-1 decline exponentially (Finkelstein et al. 1972; Zadik et al. 1985) leading to GH deficiency in many older individuals (Veldhuis 2008). In epidemiological studies, declining GH concentrations correlate with weight gain, reduced insulin sensitivity, dyslipidemia and sarcopenia at a later age (Corpas et al. 1993). The strongest correlates of the age-related decline in GH are increased abdominal visceral fat and sex-steroid depletion (Weltman et al. 1994; Iranmanesh et al. 1998; Erickson et al. 2005; Veldhuis et al. 2005a; Veldhuis et al. 2005b).
Across the lifespan, studies of GH secretion indicate that reduced availability of IGF-1 is mostly explained by decreased GH secretion (Iranmanesh et al. 1994). In comparison to young adults, aged individuals have small GH pulses or reduced GH secretion per burst. Basal GH release, maximal GH secretion, the number of secretory bursts and elimination kinetics (Iranmanesh et al. 1994; Gentili et al. 2002; Veldhuis et al. 2005c) do not appear to be affected by aging. Furthermore aging does not impair GH stimulated hepatic IGF-1 production (Arvat et al. 1998; Lissett & Shalet 2003).
GH replacement therapy gained interest as an anti-aging treatment after a six month study in elderly men showed promising changes in body composition (Rudman et al. 1990). A growing database of over 220 patients (age 69±6 years) across 18 randomized controlled trials (Liu et al. 2007), now suggests that only minor improvements in body composition are observed (small decreases in fat mass and increases in lean mass) in conjunction with a high prevalence of adverse events such as edema and impaired glucose tolerance.
Caloric restriction (CR), a non-pharmacological intervention of reduced energy intake, increases health span and lifespan in most animal species and has been advocated as an anti-aging intervention in humans. An alteration in neuroendocrine function is one of the proposed explanations for delayed aging and longevity in rodents undergoing CR (Fontana 2009). Serum IGF-1 concentrations are reduced up to 4-fold in CR fed rodents in parallel to lifespan extension. Similarly manipulation of the GH-IGF-1 axis (or signaling pathway) leading to lower IGF-1 concentrations in ad libitum fed rodents extends lifespan (Flurkey et al. 2001; Ikeno et al. 2003; Bonkowski et al. 2006). Since the blunted somatotropic axis in obesity can be restored by long-term CR and/or weight loss (Williams et al. 1984; Rasmussen et al. 1995) we hypothesized that like in rodents, a decline in neuroendocrine function may also be characteristic of CR in humans. The aim of our study was to evaluate for the first time changes in GH secretory dynamics after six months of CR in non-obese humans.
Results
There was an equal distribution of males and females within the four groups and no group differences were observed at baseline for anthropometric or body composition characteristics.
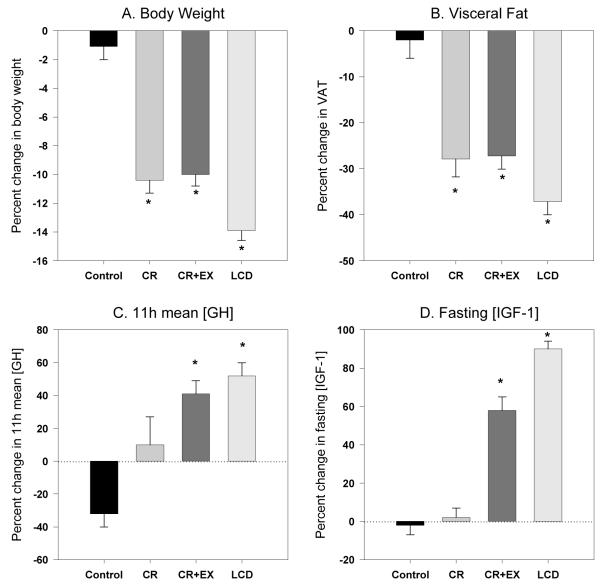
Body weight and composition changes (Figure 1)
Figure 1.
The effect of 6 month of caloric restriction on body weight (panel A), visceral fat (panel B), 11-h mean growth hormone (panel C) and total IGF-1 (panel D) concentrations. * Significant changes from baseline (p=0.05).
As previously reported (Redman et al. 2007), body weight, FM, FFM and visceral adipose mass were reduced from baseline in all three intervention groups and remained stable in the control group. By design, body weight and composition were stabilized in the LCD group between months 3 and 6, while both continued to decrease in CR and CR+EX. After six months body weight was significantly reduced in CR (−10.4±0.9%), CR+EX (−10.0±0.8%) and LCD (−13.9±0.7%) compared to the control group (−1.1±0.9%) and each intervention group had significant losses of fat mass (CR:−24±3%, CR+EX:−25±3%, LCD:−32±3%) and fat-free mass (CR:−5±1%, CR+EX: −3±1%, LCD −6±1%). Abdominal visceral fat was significantly reduced in the three intervention groups compared to control (Control:−2±4%, CR:−28±4%, CR+EX:−27±3%, LCD:−36±2%).
GH Secretion
At baseline we did not observe any differences in 11-h mean GH concentrations, GH secretion or fasting IGF-1 and ghrelin concentrations between groups (Table 1). Table 2 summarizes the changes in GH secretion dynamics after 6 months of intervention in the four treatment groups. The control group had no significant changes from baseline for any of the measured variables. For the CR group, there were no changes in mean 11-h GH concentrations or GH secretion after 6 months of calorie restriction. There were however significant increases in ApEn (21±14%, p=0.04) and in the apparent half life (11±5%, p=0.02) of GH. In the CR+EX group we did observe a significant increase in 11-h mean GH concentration by 41±8% (p<.0001) mostly due to increases in both pulse amplitude (34±14%, p=0.02) and pulse mass (34±14%, p=0.04). 11-h mean GH concentrations were increased by 53±8% (p<.0001) in the LCD group after 6 months of the intervention due to an increase in secretory burst mass (28±22%, p<0.05) and amplitude (30±20%, p<0.01) without changes in the frequency of secretory bursts, interburst interval or GH half-life.
Table 1.
Subject characteristics and G H secretory dynamics at baseline. Data are summarized as mean ± sem
| Control N=11 |
CR N=11 |
CR+EX N=12 |
LCD N=9 |
Treatment Effect, p-value |
|
|---|---|---|---|---|---|
| Gender (F/M) | 6/5 | 5/6 | 7/5 | 5/4 | |
| Age, years | 37.7±2.2 | 38.4±1.6 | 35.5±1.6 | 38.1±2.6 | 0.62 |
| Weight, kg | 81.0±2.7 | 82.6±3.1 | 81.7±3.2 | 81.4±3.6 | 0.99 |
| 11-h mean concentration, ug/L | 1.02±0.66 | 0.93±0.63 | 1.06±0.66 | 1.09±0.79 | 0.95 |
| Total production rate, ug/L·11-h | 33.0±22.8 | 37.4±32.2 | 37.6±26.1 | 45.4±36.1 | 0.81 |
| ApEn | 0.49±0.19 | 0.43±0.14 | 0.41±0.17 | 0.45±0.13 | 0.67 |
| GH Half life, min | 19.13±2.15 | 17.79±3.07 | 19.09±1.48 | 20.31±2.58 | 0.12 |
| Number of bursts per 11-h | 10.6±4.5 | 10.0±2. 6 | 11.9±3.6 | 10.8±2.1 | 0.56 |
| Burst Amplitude, ug/L·min | 0.17±0.10 | 0.18±0.14 | 0.17±0.12 | 0.23±0.22 | 0.74 |
| Burst Mass, ug/L | 3.0±1.6 | 3.6±2.9 | 3.4±2.5 | 4.3±3.6 | 0.75 |
| Interburst interval, min | 52.5±16.0 | 60.4±19.1 | 58.1±15.8 | 61.7±11.5 | 0.17 |
| Fasting total IGF-1, ug/L | 424.3±37.9 | 418.0±28.4 | 380.1±34.8 | 408.4±62 .2 | 0.74 |
| Fasting total Ghrelin, pmol/L | 2095±105 | 2357±137 | 2277±230 | 2723±198 | 0.18 |
Control=healthy weight maintenance diet, CR=25% caloric restriction from baseline energy requirements, CR+EX=12.5% caloric restriction and 12.5% increase in energy expenditur through structured aerobic exercise and LCD=low calorie diet (890kcal/day) to achieve a 15% weight loss followed by weight maintenance.
Table 2.
Changes in GH concentration and GH secretion dynamics after 6 months of calorie restriction (6 month - baseline)
| CR | CR+EX | LCD | Control | |
|---|---|---|---|---|
| 11-h mean concentration, ug/L | 0.15±0.16 | 0.83±0.21* | 1.47±0.41* | −0.33±0.11 |
| Total production rate, ug/L·11-h | 1.3±10.8 | 31.2±11.9* | 32.8±12.6* | −12.9±4.5 |
| Approximate Entropy (ApEn) | 0.20±0.09* | −0.03±0.05 | 0.02±0.08 | 0.04±0.06 |
| GH Half life, min | 2.49±0.95* | 0.43±0.79 | 0.61±0.51 | 0.65±0.88 |
| Number of bursts per 11-h | 3.2±1.9 | 0.0±1.1 | 1.1±0.9 | 0.9±0.4 |
| Burst Amplitude, ug/L·min | 0.00±0.03 | 0.16±0.06* | 0.19±0.09* | −0.07±0.02 |
| Burst Mass, ug/L | −0.18±0.71 | 3.00±1.16* | 3.70±1.89* | −1.17±0.49 |
| Interburst interval, min | −3.1±17.0 | 6.4±8.5 | −1.8±6.7 | 3.1±7.6 |
| Fasting total IGF-1, ug/L | 2.0±20.9 | 57.7±30.2* | 89.8±19.5* | −1.9±20.4 |
| Fasting total Ghrelin, pg/mL | 187.5±26.8* | 250.0±31.6* | 91.0±52.6* | 20.6±26.9 |
Significant changes from baseline (p=0.05).
Ghrelin and IGF-1
In response to 6 months of caloric restriction, fasting ghrelin concentrations were significantly increased (p<0.03) from baseline in all three intervention groups but not in the control group: 7±1% in CR; 7±3% in CR+EX; and 8±3% in LCD. Caloric restriction significantly increased total IGF-1 concentrations in CR+EX (10±7%, p<0.05) and LCD (19±4%, p<0.001) only (Table 2).
Discussion
A progressive decline in GH often leading to somatopause, or relative GH deficiency, is a characteristic of normal aging. CR is an intervention postulated to delay the onset of age-related disease and increase mean and maximal lifespan. One of the proposed theories by which CR promotes longevity in rodents, is via an inhibition of the somatotropic axis and GH-IGF-1 signaling pathways. In this randomized controlled trial of CR in overweight humans we characterized changes in GH secretory dynamics before and after 6 months of CR. On its own, a 25% CR diet did not modulate GH concentrations, GH secretion or IGF-1 concentrations. Interestingly CR in conjunction with exercise (CR+EX) and a low calorie diet followed by weight maintenance (LCD) increased GH concentrations by more than 40%, altered GH secretion and increased serum IGF-1.
In rodents, CR is one of the most robust experimental strategies known to delay aging and extend lifespan. Concentrations of IGF-1 are reduced in proportion to the level of CR (Hursting et al. 1993; Dunn et al. 1997; Berrigan et al. 2002) and lower levels of IGF-1 have been associated with the anti-carcinogenic benefits of CR in rodents (Anisimov 2001). Furthermore, genetic mutants of GH and GH-resistance display a delayed aging phenotype and an extended lifespan (Bartke 2005). The lifespan of growth hormone-receptor knockout mice resembles that of normal mice subjected to a 30% CR diet (Bonkowski et al. 2006). Similarly the Laron dwarf mouse, produced by a targeted disruption of the growth hormone receptor, is GH resistant and long lived (Bartke & Brown-Borg 2004). Dwarf mice (Ames and Snell), which lack growth hormone live longer than normal siblings and display many signs associated with delayed aging (Bartke et al. 2001; Flurkey et al. 2001; Bartke & Brown-Borg 2004). These genetically modified mice that suffer defects in the production of GH or IGF-1 or in responsiveness to GH that is, they express significantly lower levels of circulating IGF-1, have decreased onset of age-associated cancers (Hursting et al. 2003).
Most of the metabolic benefits of CR associated with longevity in rodents however are also observed in humans (Heilbronn et al. 2006). In both humans and rodents, CR leads to increased insulin sensitivity (Larson-Meyer et al. 2006), a lowering of the metabolic rate and core body temperature (Heilbronn et al. 2006), decreased DNA damage (Heilbronn et al. 2006) and inflammation (Lefevre et al. 2009) and reduced concentrations of insulin and triiodothyronine (Heilbronn et al. 2006). Our finding in this randomized trial of CR supports the recent observation that IGF-1 levels are not decreased in individuals self imposing nutritious CR diets for 6 years (Fontana et al. 2008), suggesting that long-term CR diets also may not modulate the GH:IGF-1 axis in humans. Collectively these observations indicate that CR in humans, at least over the short term, does not involve changes GH or IGF-1 and that the metabolic improvements of CR observed in humans occur independent of the somatotropic axis. Chronic CR such as anorexia nervosa however, does increase GH secretion and reduce IGF-1 concentrations (Argente et al. 1997; Scacchi et al. 1997) however this is likely due to severe under-nutrition.
The results from our study cannot fully explain why GH secretion was increased with the CR+EX and LCD interventions. Declining levels of growth hormone mostly attributed to impairment in pulsatile GH secretion are negatively associated with body fatness. Centrally, a blunted response of GH secretion is observed with pharmacological stimulation at both the hypothalamus (Topper et al. 1984; Kopelman et al. 1985) and pituitary levels (Williams et al. 1984; Kopelman & Noonan 1986). Diet-induced weight loss (Williams et al. 1984; Tanaka et al. 1990; Rasmussen et al. 1995) and even fasting in obese subjects have been shown to normalize GH secretion and GH response to growth hormone stimulating hormone (Kelijman & Frohman 1988). Weight loss alone in our 6 month study does not fully support the previously reported changes in GH. Body weight and body fatness were reduced in the three intervention groups and to the same degree in the CR and CR+EX groups. The larger reduction in body weight and body fatness in the LCD group may be a potential explanation for the greatest improvement in GH. However, body weight and composition changes alone cannot explain why an increase in GH was not observed in the CR group. It should be noted that by design, our participants were overweight and not obese. Visceral adiposity is a strong negative predictor of GH secretion (Clasey et al. 2001). The change in visceral fat may be involved in the increased GH response but is unlikely to be the sole factor. Visceral fat was reduced by ~30% in the CR+EX group and also in the CR group where GH secretion was unchanged. The LCD group however had the most marked changes in GH secretion and the greatest loss of visceral fat.
In addition to body composition changes, the different response of GH secretion to our 3 CR diets draws attention to the possible roles of ghrelin, IGF-1 and exercise. In the current study, ghrelin, a GH secretagogue, is probably not mediating the increase in GH secretion since ghrelin concentrations were increased in all the intervention groups but not to a greater extent in CR+EX and LCD where GH and IGF-1 increase.
It is well known that a single bout of exercise increases GH secretion from pre-exercise levels (Sutton & Lazarus 1976; Friedmann & Kindermann 1989) and exercise training has been shown to increase 24h GH secretion in young obese women (Weltman et al. 1992). In our study, the inconsistent findings between CR and CR+EX despite similar weight losses and similar body composition changes could reflect an increased GH secretion due to exercise training. In an earlier study, exercise training over one year however was not a stimulus for GH secretion in older adults (Hartman et al. 2000) which was attributed to a lack of change in adiposity (Wideman et al. 2002).
The IGF-1 data are less clear. In obesity, the role of IGF-1 in the blunted GH axis is debated. Generally obese individuals have normal total IGF-1 levels, and caloric restriction in normal weight (Fontana et al. 2008) or overweight subjects (Walford et al. 2002; Fontana et al. 2008) does change IGF-1 concentrations. IGF-1 concentrations are reduced in proportion to decreased dietary protein (Fontana et al. 2008). In our study, total IGF-1 concentration was not altered by CR alone, but was increased in the LCD and CR+EX groups. We did not have sufficient serum available to measure concentrations of IGF-1 binding proteins, which are important for the estimation of free IGF-1 and further interpretation of these findings.
In summary, 25% CR in overweight but non-obese young humans did not increase GH secretion or change IGF-1 concentrations over 6 months. This finding questions the involvement of the somatotropic axis in nutritionally sound CR diets in humans. Calorie restriction in combination exercise and a low calorie diet with weight loss greater than 10% however, increased GH secretion. We were unable to explain the non-uniform findings between the three CR interventions (CR, CR+EX, LCD) by changes in body fatness, visceral adiposity, ghrelin or IGF-1. Further studies are needed to determine whether longer interventions of CR alone can reverse the age-associated decline in the somatotropic axis or if the addition of exercise is necessary to retain youthful GH secretion.
Experimental Procedures
Subjects
The study was approved by the Pennington Biomedical Research Center IRB and the Data Safety Monitoring Board of CALERIE and subjects provided written informed consent prior to participating. Forty-six healthy, overweight (25 ≥ BMI < 30) men (25 – 50 y) and pre-menopausal women (25 – 45 y) completed the study. Participants were excluded if they smoked, exercised more than twice per week, were pregnant, lactating or post-menopausal, had a history of obesity (BMI>32), diabetes, cardiovascular disease, eating disorders, psychological disorders, substance abuse or regularly used medications except for birth control. Details of the screening process and study population have been extensively described (Heilbronn et al. 2006). The physical characteristics of the participants during weight maintenance at baseline are summarized in Table 1.
Study design
In brief, participants were randomized into one of four groups for 24 weeks: control = healthy weight maintenance based on AHA Step 1 diet, CR = 25% caloric restriction from baseline energy requirements, CR+EX = 12.5% caloric restriction and 12.5% increase in energy expenditure through structured aerobic exercise and LCD = low calorie diet (890kcal/d) to achieve a 15% reduction in body mass followed by weight maintenance. The group assignment was stratified to ensure the equal distributions of sex and BMI (Table 1).
Energy Requirements
As detailed previously (Redman et al. 2009), the energy intake required for weight maintenance during the baseline testing and the subsequent energy deficit prescribed during the intervention were calculated from 4-week data including two 14-day periods by doubly labeled water (DLW). During the first DLW study participants followed their usual diet at home (B1) whereas they were provided with a weight maintenance diet adjusted to maintain weight within 0.25 kg during the second DLW study (B2). Individual energy requirements were calculated as the average of total daily energy expenditure (TDEE) during B1 and energy intake for weight maintenance during B2.
Diet and behavioral intervention
During weeks 1–12 and 22–24 of the intervention, participants were provided with all meals which were prepared by the metabolic kitchen at the Center and based on individual energy intake targets. During weeks 13–22 participants self-selected a diet based on their individual targets. The diet composition was based on the American Heart Association guidelines, 30% calories from fat, 15% from protein and 55% from carbohydrate. During self-selected feeding, compliance to the dietary intervention was monitored from weekly self-reported food records and changes in body weight. Participants attended weekly behavioral group or individual sessions and cognitive-behavioral techniques were used to not only teach subjects how to adhere to their meal and exercise plans but to boost motivation to the demanding study interventions.
Exercise Prescription and Compliance
Participants in CR, LCD and Control were required to continue their usual pattern of physical activity while participants in CR+EX increased their energy expenditure by 12.5% above baseline through structured aerobic exercise, five days per week. At least three of the five weekly exercise sessions were performed under supervision at the center. The exercise program was implemented gradually and by week six all participants were expending the required 12.5% of baseline energy expenditure. Exercise energy expenditure was initially determined by indirect calorimetry and thereafter heart rate was used to assess compliance during all exercise sessions. On average, the target energy cost was maintained at 403±63 kcal per session for women and 569±118 kcal per session for men resulting in an average exercise duration of 53±11 min and 45±14 min per session, respectively.
Physiological Testing
All physiological testing (Heilbronn et al. 2006) was conducted during a 5-day inpatient stay in the inpatient unit at baseline and during weeks 12 and 24 of intervention. Following a 12h fast and morning void, percent body fat was measured using DXA (Hologics QDR 4500A, Bedford, MA) and fat mass (FM) and fat-free mass calculated. Abdominal fat distribution was measured by multi-slice computed tomography (GE Light Speed, General Electric, Milwaukee, WI) as previously described (Larson-Meyer et al. 2006).
Sampling Protocol and hormone assays
On the 4th inpatient day, frequent blood samples were obtained from an intravenous catheter placed in an anticubital vein for 24 hours for the determination of leptin circadian rhythm (not reported) and GH pulsatility. From 0800h until 2100h samples were collected at 30 minute intervals after which the frequency was increased to every 10 minutes until 0800h the following morning. Participants were not permitted to sleep during the day. Standard meals calculated on the basis of calorie levels were consumed at 0900h (breakfast), 1130h (lunch) and 1700h (dinner). Water was consumed ad libitum. Lights were turned off from 2030h to 0700h. Samples were kept on ice and processed every 30 minutes with aliquots of serum frozen at −80°C until measured in a single batch. Serum GH concentrations were determined by chemiluminescent immunoassay using the Immulite 2000 instrument (Siemens Healthcare Diagnostics, Deerfield, IL); intra- and inter- assay coefficient of variation (CV) were 3.7% and 5.7%, respectively. Serum IGF-1 concentrations were measured by ELISA (Diagnostic System Laboratories, Inc. Webster, TX); intra- and inter-assay CVs were 6.0% and 6.7%, respectively. Total ghrelin concentrations were determined in plasma by radioimmunoassay (Linco Research Inc., St Charles, MO); intra- and interassay CVs were 6.4% and 16.3%, respectively.
Deconvolution analysis
Biexponential deconvolution analysis was used to quantitate basal and pulsatile secretion from the hormone concentration profiles (Veldhuis et al. 1987). In view of parameter interdependence, estimation was conditioned statistically on mean rapid-phase kinetics and a priori pulse times (Veldhuis et al. 1995). The directly measured rapid-phase half for GH was 3.5 min with a fractional amplitude of 0.37 (Faria et al. 1989). Putative GH pulse times were determined by Cluster (discrete peak-detection) analysis at a nominal P < 0.05 false-positive rate, as validated from simulations and empirical data (Veldhuis & Johnson 1986; Urban et al. 1989). Rapid-phase kinetics and peak positions were held constant, allowing valid simultaneous estimation of the amplitude and half-duration (and thereby mass or integral) of secretory bursts, slow-phase half-life and underlying basal secretion (initially estimated as corresponding to the lowest 5% concentration). The outcomes are basal (time-invariant), pulsatile (sum of secretory-burst mass) and total (sum of basal and pulsatile) secretion expressed in hormone concentration units per time.
Approximate entropy (ApEn) analysis
ApEn is a regularity statistic (Pincus 1991; Pincus & Goldberger 1994), which measures relative orderliness. Elevated ApEn denotes greater irregularity of subpattern evolution over time, which, in turn, signifies loss of feedback and/or feedforward control (Hartman et al. 1994; Pincus et al. 1999; Veldhuis et al. 2001). Studies have demonstrated the validity, reliability and utility of ApEn to discriminate regularity of hormone patterns with high sensitivity [> 90%] and specificity [> 90%] (Pincus et al. 1999; Veldhuis et al. 2001).
Statistical Analysis
Data in text and tables are provided as means ± SEM. SAS Version 9.12 (SAS Institute, Cary, NC) was used for analysis. The changes in all variables from baseline to month 6 were computed and data were analyzed by ANCOVA with treatment and time interactions and baseline values as covariates. P<0.05 was considered statistically significant.
Acknowledgements
The authors thank the remaining members of Pennington CALERIE Research Team including: Enette Larson-Meyer, Corby Martin, Hie Xie, Steve Anton, Julia Volaufova, Marlene Most, Lilian de Jonge, Tuong Nguyen, Frank Greenway, Emily York-Crow, Catherine Champagne, Brenda Dahmer, Andy Deutsch, Paula Geiselman, Jennifer Howard, Jana Ihrig, Michael Lefevre, Darlene Marquis, Connie Murla, Sabrina Yang, Robbie Durand, Sean Owens, Aimee Stewart and Vanessa Tarver. Our gratitude is extended to the excellent staffs of the Inpatient Clinic and Metabolic Kitchen. Finally, our profound gratitude goes to all the volunteers who spent so much time in participating in this very demanding research study. This work was supported by U01 AG20478 (ER) and in part by 1P30 DK072476 (ER), and AG196595 and AG29362 (JDV). LMR was supported by a Neil Hamilton-Fairley Training Fellowship awarded by the NHMRC of Australia (ID 349553).
Grant Support: This work was supported by U01 AG20478 (ER) and in part by 1P30 DK072476 to ER and AG196595 and AG29362 to JDV. LMR was supported by a Neil Hamilton-Fairley Training Fellowship awarded by the NHMRC of Australia (ID 349553).
Footnotes
Disclosure summary: The authors have nothing to disclose
Clinical trial registration number: CALERIE, NCT00099151 (clinicaltrials.gov)
References
- Anisimov VN. Mutant and genetically modified mice as models for studying the relationship between aging and carcinogenesis. Mech Ageing Dev. 2001;122:1221–1255. doi: 10.1016/s0047-6374(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, Morande G, Hernandez M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82:2084–2092. doi: 10.1210/jcem.82.7.4090. [DOI] [PubMed] [Google Scholar]
- Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Broglio F, Deghenghi R, Ghigo E. Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRPs in human aging. Pituitary. 1998;1:51–58. doi: 10.1023/a:1009970909015. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasey JL, Weltman A, Patrie J, Weltman JY, Pezzoli S, Bouchard C, Thorner MO, Hartman ML. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab. 2001;86:3845–3852. doi: 10.1210/jcem.86.8.7731. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Farhy L, Mielke K, Bowers CY, Veldhuis JD. Determinants of dual secretagogue drive of burst-like growth hormone secretion in premenopausal women studied under a selective estradiol clamp. J Clin Endocrinol Metab. 2005;90:1741–1751. doi: 10.1210/jc.2004-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AC, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab. 1989;68:535–541. doi: 10.1210/jcem-68-3-535. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab. 1972;35:665–670. doi: 10.1210/jcem-35-5-665. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L. The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. 2009;25:144–150. doi: 10.1097/MOG.0b013e32831ef1ba. [DOI] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann B, Kindermann W. Energy metabolism and regulatory hormones in women and men during endurance exercise. Eur J Appl Physiol Occup Physiol. 1989;59:1–9. doi: 10.1007/BF02396572. [DOI] [PubMed] [Google Scholar]
- Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ML, Weltman A, Patrie J. Exercise training for one year does not increase 24-h GH secretion in older adults; 82nd Annual Meeting of the Endocrine Society; Toronto, Canada. 2000; p. 396. al. e. [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Switzer BR, French JE, Kari FW. The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res. 1993;53:2750–2757. [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Grisso B, Veldhuis JD. Low basal and persistent pulsatile growth hormone secretion are revealed in normal and hyposomatotropic men studied with a new ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1994;78:526–535. doi: 10.1210/jcem.78.3.8126122. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, South S, Liem AY, Clemmons D, Thorner MO, Weltman A, Veldhuis JD. Unequal impact of age, percentage body fat, and serum testosterone concentrations on the somatotrophic, IGF-I, and IGF-binding protein responses to a three-day intravenous growth hormone-releasing hormone pulsatile infusion in men. Eur J Endocrinol. 1998;139:59–71. doi: 10.1530/eje.0.1390059. [DOI] [PubMed] [Google Scholar]
- Kelijman M, Frohman LA. Enhanced growth hormone (GH) responsiveness to GH-releasing hormone after dietary manipulation in obese and nonobese subjects. J Clin Endocrinol Metab. 1988;66:489–494. doi: 10.1210/jcem-66-3-489. [DOI] [PubMed] [Google Scholar]
- Kopelman PG, Noonan K. Growth hormone response to low dose intravenous injections of growth hormone releasing factor in obese and normal weight women. Clin Endocrinol (Oxf) 1986;24:157–164. doi: 10.1111/j.1365-2265.1986.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Kopelman PG, Noonan K, Goulton R, Forrest AJ. Impaired growth hormone response to growth hormone releasing factor and insulin-hypoglycaemia in obesity. Clin Endocrinol (Oxf) 1985;23:87–94. doi: 10.1111/j.1365-2265.1985.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM. The insulin-like growth factor-I generation test: peripheral responsiveness to growth hormone is not decreased with ageing. Clin Endocrinol (Oxf) 2003;58:238–245. doi: 10.1046/j.1365-2265.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266:H1643–1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol. 1999;277:E948–957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Hilsted J, Skakkebae NE. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab. 1995;80:1407–1415. doi: 10.1210/jcem.80.4.7536210. [DOI] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Scacchi M, Pincelli AI, Caumo A, Tomasi P, Delitala G, Baldi G, Cavagnini F. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab. 1997;82:3225–3229. doi: 10.1210/jcem.82.10.4275. [DOI] [PubMed] [Google Scholar]
- Sutton J, Lazarus L. Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J Appl Physiol. 1976;41:523–527. doi: 10.1152/jappl.1976.41.4.523. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Inoue S, Numata K, Okazaki H, Nakamura S, Takamura Y. Very-low-calorie diet-induced weight reduction reverses impaired growth hormone secretion response to growth hormone-releasing hormone, arginine, and L-dopa in obesity. Metabolism. 1990;39:892–896. doi: 10.1016/0026-0495(90)90296-o. [DOI] [PubMed] [Google Scholar]
- Topper E, Gil-Ad I, Bauman B, Josefsberg Z, Laron Z. Plasma growth hormone response to oral clonidine as compared to insulin hypoglycemia in obese children and adolescents. Horm Metab Res. 1984;16(Suppl 1):127–130. doi: 10.1055/s-2007-1014915. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Johnson ML, Veldhuis JD. Biophysical modeling of sensitivity and positive accuracy of detecting episodic endocrine signals. Am J Physiol. 1989;257:E88–94. doi: 10.1152/ajpendo.1989.257.1.E88. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD. Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev. 2008;7:189–208. doi: 10.1016/j.arr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci U S A. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Iranmanesh A, Miles JM, Bowers CY. Sex-steroid control of the aging somatotropic axis. Endocrinol Metab Clin North Am. 2005a;34:877–893. viii. doi: 10.1016/j.ecl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on growth hormone secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab. 2005b;90:6006–6013. doi: 10.1210/jc.2005-0854. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Johnson ML. Complicating effects of highly correlated model variables on nonlinear least-squares estimates of unique parameter values and their statistical confidence intervals: estimating basal secretion and neurohormone half-life by deconvolution analysis. Meth Neurosci. 1995;28:130–138. [Google Scholar]
- Veldhuis JD, Iranmanesh A, Bowers CY. Joint mechanisms of impaired growth-hormone pulse renewal in aging men. J Clin Endocrinol Metab. 2005c;90:4177–4183. doi: 10.1210/jc.2005-0336. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe C, Barkan A, Johnson ML, Pincus S. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R721–729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab. 1994;78:543–548. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Schurrer R, Evans WS, Veldhuis JD, Rogol AD. Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. J Appl Physiol. 1992;72:2188–2196. doi: 10.1152/jappl.1992.72.6.2188. [DOI] [PubMed] [Google Scholar]
- Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A. Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med. 2002;32:987–1004. doi: 10.2165/00007256-200232150-00003. [DOI] [PubMed] [Google Scholar]
- Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA. Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med. 1984;311:1403–1407. doi: 10.1056/NEJM198411293112203. [DOI] [PubMed] [Google Scholar]
- Zadik Z, Chalew SA, McCarter RJ, Jr., Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]