Abstract
Background
Despite advances in cross-sectional imaging and the use of molecular markers, distinguishing between benign and malignant cysts remains a clinical challenge.
Aims
The aim of the study was to identify both preoperative clinical and cyst characteristics at time of EUS that predict malignancy.
Methods
A retrospective analysis was performed on consecutive patients with pancreatic cysts at a tertiary center that underwent endoscopic ultrasound (EUS) and surgical resection from May 1996 to December 2007. Clinical history, EUS characteristics, cytology, tumor markers, and surgical histology were collected. Predictors of malignancy were determined by univariate and multivariate analysis using logistic regression.
Results
153 patients underwent an EUS and subsequent surgical intervention. 57 of 153 (37%) had a histologic diagnosis of malignancy. On univariate analysis, older age (p <0.001), male gender (p = 0.010), jaundice (p = 0.039), history of other malignancy (p = 0.036), associated mass in cyst (p = 0.004), and malignant cytology (p <0.001) were associated with malignancy. History of pancreatitis (p = 0.008) and endoscopist impression of pseudocyst (p = 0.001) were associated with benign cysts. Multivariate analysis found that only older age (Odds ratio [OR], 1.04; 95% confidence interval [CI], 1.01-1.08), male gender (OR, 2.26; 95% CI, 1.08-4.73), and malignant cytology (OR, 6.60; 95% CI, 2.02-21.58) were independent predictors of cancer.
Conclusions
Older age, male gender and malignant cytology from EUS predict cancer at surgical resection. These characteristics may be used to estimate the probability of malignancy in a cyst and aid in management.
Keywords: Pancreas, Pancreatic cancer, Oncology, Outcomes research
Introduction
Pancreatic cysts are increasingly being detected due to improving imaging technology and are identified in about 1% of patients who undergo abdominal computed tomography (CT).1 Depending on the histologic type, the risk of malignancy is variable. Differentiation between benign and malignant cysts remains a diagnostic challenge.2
Preoperative evaluation of pancreatic cysts has relied mainly on cross sectional imaging (e.g. magnetic resonance imaging (MRI) and CT) and the use of endoscopic ultrasound (EUS) with fine needle aspiration (FNA). Imaging modalities can be helpful in distinguishing certain cystic lesions such as intraductal papillary mucinous neoplasm (IPMN).3 However, these characteristic features are variable and thus, lack sensitivity.4 The accuracy of CT in providing a definitive diagnosis ranges from 26% to 92%, while MRI has been reported to have relatively similar accuracy as CT.4-8 Another study found that CT has an accuracy of 61% in predicting malignant potential of pancreatic cysts.9 Similarly, EUS-FNA has limitations. Although many studies have investigated the role of EUS-FNA in predicting the malignant potential of cysts, EUS has poor interobserver agreement for diagnosis of neoplastic verus non-neoplastic lesions.10-14 The sensitivity of cytology for detecting malignancy is variable, ranging from 27%-64%, with an accuracy of 59%.10, 12, 15, 16 Furthermore, difficulty in obtaining sufficient cells often reduces the utility of cytologic examination, and tumor markers such as carcinoembryonic antigen (CEA) has limited accuracy.10
Numerous studies have attempted to evaluate both imaging studies and patient characteristics in order to improve the diagnostic yield of detecting malignancy. But many have used post-hoc surgical histology of cysts to determine predictors of malignancy. For example, studies have collected cases with surgical histology on specific types of cysts such as IPMN and then determine the predictors for malignancy.14, 17-19 However, this excludes the cases misdiagnosed preoperatively as IPMN. As a result, the application of these predictors is based on confirmed diagnosis and not presumptive diagnosis. Due to limitations of EUS-FNA and radiological imaging in diagnosis, the use of these results may be difficult in the preoperative setting. Selecting the study subjects using preoperative diagnosis of pancreatic cystic lesion, which is less prone to misdiagnosis, can eliminate this potential diagnostic limitation.
The aim of this study was to identify both preoperative clinical and cyst characteristics at the time of EUS that predict malignancy.
Methods
Eligibility
This retrospective study was carried out at a tertiary care, academic medical center. Study patients were identified using a prospectively maintained pancreatic cyst database which comprised of all patients referred for endoscopic evaluation of pancreatic cystic lesions from May 1996 to December 2007.10 All patients in our institution undergoing EUS for cystic lesions have FNA unless contraindicated. A comprehensive medical record review was performed. 156 consecutive patients who underwent subsequent surgical pancreatic cyst resection at our institution were eligible for analysis. Patients were excluded based on the following: FNA was not performed, pathology results were not available, or there was a separate pancreatic mass not associated with the cyst prior to or at the time of EUS. Patients who had solid lesions with cystic components were not excluded from the analysis. In patients with multiple EUS studies, only the index EUS was included. The study was approved by the Institutional Review Board of the Massachusetts General Hospital.
Data collection
Electronic and paper medical records as well as endoscopy, cytology, and pathology reports were used for all data extraction without any direct interview of patients. Two independent reviewers (E.S.H., B.G.T.) performed all data extraction without prior knowledge of surgical histology. A trial extraction was performed to ensure inter-reviewer consistency. Patient characteristics were determined using all available records prior to the time of the surgical resection. The following clinical information was collected: age, gender, body mass index (BMI), presence or absence of symptoms (jaundice, abdominal pain, weight loss), family history of pancreatic cancer, history of diabetes, history of pancreatitis, history of other malignancies (defined as any non-pancreatic cancer), smoking status, alcohol use and regular aspirin use. Age and BMI were classified as continuous variables, while all other variables were binary. Patients were considered to be aspirin users if aspirin use was listed on any pre-operative visit note. Family history was positive if there were any relatives with pancreatic cancer. Smoking and alcohol status were considered positive if patients were either current or former users.
Endoscopic Ultrasound
Endoscopy reports were used to determine cyst characteristics. Cystic lesions were aspirated using EUS guidance (linear video EUS scope, Pentax Medical, Montvale, NJ) with a 19- or 22-guage needle (Cook Medical, Winston-Salem, North Carolina, or Mediglobe, Tempe, AZ) occluded with a stylet. The following morphological findings were included: cyst size, location (head, body and tail), multicystic (defined as clusters of cysts), multifocality, pancreatic main duct dilation (defined as ≥3 mm), associated mass, and endoscopist impression. Cyst size was used as a continuous variable in the model, while all others were used as dichotomous variables.
Cytology and CEA
All cyst aspirates were reviewed by a cytopathologist. Samples were first classified as diagnostic (n=87) or non-diagnostic (n=66) based on the adequacy of cellular material. Diagnostic samples were then classified as malignant or non-malignant. Malignant samples included cellular material interpreted as frank malignancy (n=17) or highly suspicious for malignancy (n=2). All other samples were considered non-malignant for analytical purposes [normal (n= 44) and atypical (n=24)]. CEA was measured using an Abbott Diagnostics IMX-MEIA immunodiagnostics analyzer and recorded as a continuous variable.
Surgery and Surgical Histology
All patients underwent abdominal exploration either by laparoscopy or laparotomy. All surgical specimens were reviewed by a staff gastrointestinal pathologist. Histology consisting of atypia, borderline, or dysplasia was categorized as benign lesions. Specimens containing invasive carcinoma or carcinoma-in-situ were classified as malignant lesions. Cystic endocrine tumors and solid pseudopapillary tumors were considered malignant because of their metastatic potential and also aggressive surgical management in most cases.20-22
Statistical Analysis
Statistical analysis was performed using SAS version 9.1. (Cary, NC). Student t-test or Wilcoxon rank sum test was used for continuous variables in the univariate analysis. For categorical variables, chi-square test or Fisher exact test was used. Odd ratios with 95% CI were calculated using logistic regression analysis.
A multivariate logistic regression analysis using forward selection was performed using the five most significant variables in the univariate analysis. All p-values were two-sided and considered to be statistically significant when less than 0.05. Area under the curve was calculated to determine the goodness-of-fit of the model. The Hosmer-Lemeshow test was performed to assess if the model is significantly different from a perfect prediction model.
Results
Baseline Characteristics
A total of 156 patients with pancreatic cysts who underwent EUS with FNA and subsequent pancreatic surgery were identified in the database and comprised the study population. 153 patients were included in the final analysis. Three patients were excluded due to a separate concurrent pancreatic mass not associated with the cyst. One was a 56 year old male with mass in the head and cysts in the body/tail. Another was a 66 year old male with mass in the uncinate and a cyst in the tail. Third was a 62 year old female with mass adjacent to pancreatic body and cyst in the head of pancreas. Mean age of patients was 60.4 years (±14.4 years) at time of EUS and 39% were male. BMI was available in 88 patients with a mean of 25.7 kg/m2 (±5.6 kg/m2).
Thirty-eight (26%) were asymptomatic. Among those with symptoms, abdominal pain (62%) was the most common followed by weight loss (21%) and jaundice (10%). Eleven (7%) patients had family history of pancreatic cancer. Diabetes mellitus was present in 33 patients (22%). Forty-one (27%) had a history of pancreatitis prior to EUS procedure. History of other malignancy was present in 31 (21%) of patients. Sixty (45%) patients were current or former smokers and 72 (54%) used alcohol currently or in the past. Regular aspirin use was listed in the medication lists of 37 (25%) patients (Table 1).
Table 1. Patient Characteristics (n = 153)a.
| Clinical variable | Benign (n=96) | Malignant (n=57) | P valueb |
|---|---|---|---|
| Median Age, years (range) | 59.5 (24-83) | 68 (34-83) | <0.001 |
| Unknown | 0 | 0 | |
| Sex | |||
| Male (n, %) | 29 (30) | 30 (53) | 0.010 |
| Female (n, %) | 67 (70) | 27 (47) | |
| Unknown | 0 | 0 | |
| Median BMI, kg/m2 (range) | 26.4 (15.7-45.1) | 24.5 (17.3-38.5) | 0.263 |
| Unknown | 43 | 22 | |
| Symptoms | |||
| None (n, %) | 21 (23) | 17 (31) | 0.247 |
| Abdominal pain (n, %) | 63 (68) | 28 (52) | 0.078 |
| Jaundice (n, %) | 5 (5) | 9 (17) | 0.039 |
| Weight Loss (n, %) | 18 (19) | 13 (24) | 0.533 |
| Unknown | 3 | 3 | |
| Family history of pancreatic cancer | |||
| Yes (n, %) | 4 (6) | 7 (18) | 0.105 |
| No (n, %) | 58 (94) | 33 (83) | |
| Unknown | 34 | 17 | |
| Diabetes Mellitus | |||
| Yes (n, %) | 20 (21) | 13 (23) | 0.840 |
| No (n, %) | 76 (79) | 43 (77) | |
| Unknown | 1 | 1 | |
| Smoking | |||
| Yes (n, %) | 38 (45) | 22 (44) | >0.99 |
| No (n, %) | 46 (55) | 28 (56) | |
| Unknown | 12 | 7 | |
| Alcohol Use | |||
| Yes (n, %) | 46 (55) | 26 (52) | 0.858 |
| No (n, %) | 38 (45) | 24 (48) | |
| Unknown | 12 | 7 | |
| History of Pancreatitis | |||
| Yes (n, %) | 33 (35) | 8 (14) | 0.008 |
| No (n, %) | 62 (65) | 48 (86) | |
| Unknown | 1 | 1 | |
| History of Other Malignancies | |||
| Yes (n, %) | 14 (15) | 17 (30) | 0.036 |
| No (n, %) | 81 (85) | 39 (70) | |
| Unknown | 1 | 1 | |
| Regular Aspirin Use | |||
| Yes (n, %) | 22 (24) | 15 (27) | 0.697 |
| No (n, %) | 70 (76) | 40 (73) | |
| Unknown | 4 | 2 |
Smoking and alcohol use was defined to be positive for current or former users. History of other malignancy is defined any previous diagnosis of cancer other than pancreatic origin.
P-values were calculated using chi-square or Fisher exact test for categorical variables and t-test or Wilcoxon for continuous variables.
EUS characteristics
Mean cyst size based on EUS findings was 3.2 cm (±2.2cm). Eighty-one (53%) of cysts were located in the head, 36 (24%) in the body, and 34 (22%) in the tail. A dilated main pancreatic duct was found in 35 (23%) patients. Twenty-two (14%) of the cysts were multicystic in nature while 9 patients (6%) had multifocal cysts. An associated mass seen on EUS was present in 41 (27%) of the patients (Table 2).
Table 2. Cyst Characteristics (n=153).
| Clinical variable | Benign (n=96) | Malignant (n=57) | P valuea |
|---|---|---|---|
| Median Size, mm (range) | 28 (2.5-140) | 30 (12-120) | 0.140 |
| Unknown | 10 | 7 | |
| Location | |||
| Head (n, %) | 49 (52) | 32 (56) | 0.618 |
| Body (n, %) | 22 (23) | 14 (25) | 0.846 |
| Tail (n, %) | 23 (24) | 11 (19) | 0.550 |
| Unknown | 1 | 0 | |
| Multifocal | |||
| Yes (n, %) | 6 (6) | 3 (5) | >0.99 |
| No (n, %) | 90 (94) | 54 (95) | |
| Unknown | 0 | 0 | |
| Multicystic | |||
| Yes (n, %) | 12 (13) | 10 (18) | 0.476 |
| No (n, %) | 84 (88) | 47 (82) | |
| Unknown | 0 | 0 | |
| Dilated main pancreatic duct | |||
| Yes (n, %) | 21 (22) | 14 (25) | 0.696 |
| No (n, %) | 75 (78) | 43 (75) | |
| Unknown | 0 | 0 | |
| Associated Mass or Solid component | |||
| Yes (n, %) | 17 (18) | 24 (42) | 0.004 |
| No (n, %) | 79 (82) | 33 (58) | |
| Unknown | 0 | 0 | |
| Endoscopist Impression | |||
| Pseudocyst (n, %) | 13 (14) | 1 (2) | 0.001 |
| Cyst (n, %) | 80 (86) | 55 (98) | |
| Unknown | 3 | 1 | |
| Median CEA, ng/ml (range) | 159 (0-87,780) | 270 (0.3-28,534) | 0.930 |
| Unknown | 29 | 18 | |
| Cytology | |||
| Malignant cells | 4 (4) | 15 (26) | <0.001b |
| Non-malignant cells | 48 (50) | 20 (35) | 0.093 |
| Non-diagnostic | 44 (46) | 22 (39) | 0.404 |
| Unknown | 0 | 0 |
P-values were calculated using chi-square or Fisher exact test for categorical variables and t-test or Wilcoxon for continuous variables.
P-value <0.05 vs non-malignant cells and non-diagnostic samples
Pathology
Surgical pathology revealed 57 (37%) malignant and 96 (63%) benign cystic lesions. Table 3 shows the histologic types for benign and malignant cysts. Forty-seven (31%) and 34 (22%) were found to be IPMN and mucinous cystic neoplasm (MCN) on surgical histology, respectively. Pseudocysts were diagnosed in 27 (18%) cysts.
Table 3a. Histology of Benign Cystic Lesions.
| Types of Benign Lesion | Subjects |
|---|---|
| IPMN | |
| Adenoma | 9 |
| Borderline | 15 |
| Mucinous Cystic Neoplasm | |
| Adenoma | 24 |
| Borderline | 5 |
| Indeterminate Mucinous Cyst | 1 |
| Inflammatory | |
| Pseudocyst only | 27 |
| Retention cyst only | 6 |
| Serous cystadenoma | 6 |
| Other | |
| Lymphangioma | 1 |
| Peritoneal cyst | 1 |
| Simple cyst | 1 |
| Total Lesions | 96 |
| Table 3b. Histology of Malignant Cystic Lesions | |
| Types of Maligant Lesion | Subjects |
| IPMN | |
| Carcinoma in-situ | 13 |
| Invasive Cancer | 10 |
| Mucinous Lesions | |
| Carcinoma in-situ | 3 |
| Invasive Cancer | 2 |
| Ductal Adenocarcinoma | 10 |
| Adenocarcinoma | 6 |
| Endocrine Tumor | 7 |
| Solid Pseudopapillary Tumor | 1 |
| Other | |
| Acinar cell | 2 |
| Squamous CA | 1 |
| Papillary AdenoCA | 1 |
| Cholangiocarcinoma | 1 |
| Total Lesions | 57 |
Predictors
In the univariate analysis, patient characteristics significantly associated with malignancy included age (65.5 ± 11.3 years in patients with malignant cyst vs 57.3 ± 15.2 years in patients with benign cyst, p< 0.001), sex (53% males with malignant cyst vs 30% male with benign cyst, p = 0.010), symptoms of jaundice (17% in malignant cyst vs 5% in benign cyst, p = 0.039), and history of other malignancy (30% of patients with malignant cyst had prior history of other malignancy vs 15% in benign cyst, p = 0.036). Benign cysts were significantly associated with a history of pancreatitis (35% of patients with benign cyst had history of pancreatitis vs 14% in malignant cyst, p = 0.008) (Table 1). There was no significant association between malignant cysts and other patient characteristics including BMI, abdominal pain, weight loss, lack of symptoms, family history of pancreatic cancer, diabetes mellitus, smoking status, alcohol use, and regular aspirin use.
In terms of cyst characteristics, associated mass or a solid component in a cyst (42% of malignant cysts had associated mass vs 18% of benign cysts, p = 0.004) and malignant cytology (26% of malignant cysts vs 4% of benign cysts, p <0.001) were associated with malignancy. The endoscopists' impression of pseudocyst was found to be associated with benign cysts (14% of benign cysts vs 2% of malignant cysts, p = 0.001) (Table 2). No significant association was found with other cyst characteristics including cyst size, location, multifocality of cysts, multicystic nature of cysts, dilation of main pancreatic duct, and CEA of the cyst. We also performed a similar analysis comparing patients with CIS versus those with benign cystic lesions. Cystic lesions in the head of the pancreas were significantly associated with CIS lesions compared with benign cystic lesions (p = 0.031).
In the forward logistic regression analysis (Table 4), only age (OR, 1.04; 95% CI, 1.01-1.08), male sex (OR, 2.26; 95% CI, 1.15-4.73), and malignant cytology (OR, 6.60; 95% CI, 2.02-21.58) were independent predictors of malignancy. Area under receiver operating curve (ROC) was 0.751 (95% CI 0.673-0.830). Hosmer-Lemeshow test had p-value of 0.901, supporting model validity. Figure 1 illustrates the probability of malignancy based on the age, sex and presence or absence of malignant cytology of a patient.
Table 4. Univariate and Multivariate Analysis.
| Clinical variable | Univariatea (95% CI) | Multivariateb (95% CI) | P value |
|---|---|---|---|
| Age | 1.05 (1.02-1.08) | 1.04 (1.01-1.08) | 0.005 |
| Male Sex | 2.57 (1.30-5.06) | 2.26 (1.08-4.73) | 0.031 |
| Malignant cytology c | 8.21 (2.57-26.25) | 6.60 (2.02-21.58) | 0.002 |
Logistic regression was used to calculate the odd ratios.
Multivariate logistic regression used forward selection of five most significant covariates in univariate analysis. Area under ROC curve was 0.751 (95% CI; 0.673-0.830).
Odds ratio and P-value vs non-malignant cells and non-diagnostic samples.
Figure 1.
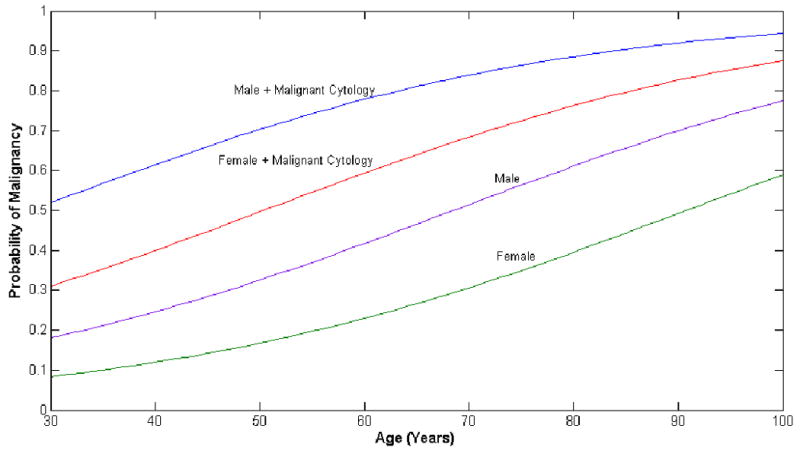
Probability of Malignancy. Using the odds ratio from multivariate analysis (Table 4), the probability of malignancy is plotted against age of the patient. Each curve represents a different probability curve based on the gender and the presence of malignant cytology from EUS.
Subgroup analysis
Mucinous lesions (IPMN and MCN) are considered premalignant lesions and warrant closer surveillance. We performed a subgroup analysis in patients (n=81) with a histologic diagnosis of mucinous lesion (IPMN and MCN) to identify predictors of malignancy. Using the same covariates, we found that older age (69.4±7.7 years in benign cysts vs 58.4 ±16.2 years in malignant cysts, p = 0.002), male gender (52% male in malignant cysts vs 26% male in benign cysts, p = 0.027), and jaundice (15% in malignant cysts vs 0% in benign cysts, p = 0.01) were significantly associated with malignancy. The presence of non-malignant cells on cytology was significantly associated with benign cysts (22% malignant cysts had non-malignant cells on cytology vs 48% benign cysts, p = 0.031). All other clinical variables were not significantly associated with malignancy.
Discussion
In patients with cystic lesions of the pancreas, the ability to detect malignancy can can be challenging. Many studies have used patient and cyst characteristics as the primary predictors to evaluate the malignant potential of the cysts. 14, 18, 19, 23-25 However, these analyses are based on post-hoc surgical histology from patients with documented IPMN and does not consider cases with preoperative misdiagnosis of IPMN. Since the preoperative classification of cyst types is often inaccurate, the use of predictors from these studies may be incorrectly applied to individuals with a preoperative diagnosis of cystic lesions such as IPMN, but who do not truly have IPMN.9, 10 Our study attempts to eliminate this bias by using preoperative diagnosis of pancreatic cystic lesions, which is less prone to misdiagnosis. In doing so, the results of our study will be more useful and applicable in the preoperative setting to aid in the differentiation of benign and malignant cysts.
Patient characteristics can be helpful in determining the risk of malignancy in cysts. In our study, we found that older age and male gender were significant predictors of malignant cysts in the multivariate analysis consistent with prior studies.1, 17-19, 23, 24, 26-28 Spinelli et al found age greater than 70 is a risk factor for having malignant cysts.1 Similarly, studies have also found that malignant IPMN and MCN occurred more frequently in older patients.17, 19, 27 However, unlike previous studies, we used age as a continuous variable in order to capture the changing probability of malignancy over time (Figure 1). The continuous age variable gives a more precise estimation of risk of malignancy for clinical decision making. A recent study by Lee et al reported a higher incidence of malignancy in males compared to females (28% vs12%) incidence in their univariate analysis.26 Our analysis showed that malignancy was also associated with male gender even after adjusting for age and malignant cytology. We found that 79.4% of MCN are women which was significantly different (p =0.019) than IPMN which consists of only 53.2% women. Since 5 of 34 (14.7%) MCN and 23 of 47 (48.9%) IPMN are malignant, this may explain why male gender is a predictor of malignancy.
EUS continues to play an increasing role in the evaluation of pancreatic cysts. In particular, FNA for cytological and tumor marker examination has been useful in characterization of the pancreatic cyst. CEA has been helpful in differentiating between mucinous and non-mucinous lesions, depending on the threshold value.10, 29, 30 However, the role of CEA is not clear in distinguishing between malignant and benign cysts. Pais et al reported no significant difference in CEA between malignant and benign IPMN.14 Similarly, our study did not show CEA level to distinguish between malignant and benign lesions, which may be due to inadequate power. However, CEA levels in malignant and benign cysts overlap considerably, which means that any cutoff threshold value used would suffer from a large numbers of false positives or false negatives, limiting its utility. Cytology has proven to be highly specific, but sensitivity ranges from 27-64% for diagnosing malignancy.12, 15, 16, 31 Similarly, our study showed that diagnostic cytology has a sensitivity and specificity of 43% and 92%, respectively. Our analysis found that in comparison to non-diagnostic or benign cells, malignant cells found on cytology was significantly associated with malignancy even after adjusting for age and sex. This suggests that the results of EUS with FNA are helpful when the cytology is malignant, but less helpful when cytology is benign or non-diagnostic because it cannot exclude malignancy due to its poor negative predictive value.
Interestingly, other clinical characteristics found to be associated with malignancy in previous studies did not appear to be significant in this study after controlling for potential confounders. In our study, jaundice and associated mass on EUS were significant predictors of malignancy in the univariate analysis which is similar to prior studies.18, 19, 25, 32 However, many studies have not examined these characteristics adjusting for potential confounders, while others did not use EUS cytology as a potential confounders.18, 19, 32 In our multivariate analysis, jaundice and associated mass on EUS were no longer significant predictors. Wiesenauer et al also found similar results in their multivariate analysis.25 We believe that malignant cytology (p <0.001 in univariate analysis) is a better predictor of malignancy than either jaundice (p = 0.039) or associated mass (p = 0.004) because patients can have jaundice or associated mass for other reasons (ie chronic pancreatitis, cyst wall thickening), rendering them less specific symptoms. Consequently, both jaundice and associated mass did not add to the predictability of malignancy in the multivariate analysis once malignant cytology was introduced into the model. Furthermore, other studies have found that dilated main pancreatic duct and increased cyst size in IPMN were associated with malignancy, which we did not find in our study.14, 33-35 However, it is unclear if these characteristics are also risk factors for other cyst types. One study did not find dilated pancreatic duct to be significantly associated with malignancy in mucinous cystadenoma.36 Another large study did not find pancreatic cyst size to be a significant predictor of malignancy for all cystic neoplasm of pancreas.1 Since our study included all types of pancreatic cysts, this may explain the difference in findings. In addition, we defined pancreatic duct dilation as size greater than 3 mm, but Sugiyama et al found that only dilated ducts ≥7 mm were a predictor of malignancy. This suggests that perhaps only those with largest dilation have higher risk of malignancy, which may explain why our study did not find a significant association.33
There are limitations to this study. First, our analysis only evaluated patients who underwent surgical resections for their pancreatic cysts. The study population might be biased by indication. Patients with preoperative suspicion of malignancy are more likely to undergo surgery, which could have inflated our malignancy rates. Second, our study was based on patients from a tertiary care center which limits generalizability outside of such a setting. Third, certain clinical variables such as family history of pancreatic cancer, and CEA were missing in some patients which could have limited the power to detect a significant difference. We attempted to minimize this effect by comprehensively and exhaustively reviewing all available medical records.
A major strength of the study is that it is one of the largest analyses to evaluate the predictors of malignancy in pancreatic cysts. Furthermore, unlike other analyses that defined study participants by post-operative histology, our study selected subjects based on presumed pancreatic cysts from a prospective cohort.14, 17 Retrospective analyses based on histological diagnosis have conclusions that are difficult to use in the preoperative setting because the analyses excludes the cases which are misdiagnosed. Due to the limitations of diagnostic modalities, preoperative diagnosis of the cyst type may be different from the postoperative diagnosis. The current study addresses this issue and has more direct applicability at the time of EUS to determine malignancy of pancreatic cysts and its management.
In conclusion, this study found that in the evaluation of pancreatic cysts, older age, male gender and the presence of malignant cytology from EUS-FNA are the best predictors in estimating the probability of malignancy in patients. Our findings may aid in the risk stratification of patients with pancreatic cysts considering surgical resection.
Acknowledgments
Grant Support: Edward S. Huang and Brian G. Turner are supported by NIH T32DK007191. Chin Hur is supported by NIH K07CA107060.
Abbreviations
- IPMN
intraductal papillary mucinous neoplasm
- EUS-FNA
endoscopic ultrasound and fine needle aspiration
- MRI
magnetic resonance imaging
- CT
computed tomography
- OR
odds ratio
- CI
confidence interval
- CEA
carcinoembryonic antigen
Footnotes
Specific author contributions:
Conception and design: ESH, CH
Acquisition of data: ESH, BGT
Analysis and interpretation of data: ESH, BGT, CFC, WRB, CH
Drafting of manuscript: ESH
Critical revision: ESH, BGT, CFC, WRB, CH
Final approval: ESH, BGT, CFC, WRB, CH
Disclosure: none
Writing Assistance: none
References
- 1.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–7. doi: 10.1097/01.sla.0000124299.57430.ce. discussion 657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 3.Megibow AJ. Update in imaging of cystic pancreatic masses for gastroenterologists. Clin Gastroenterol Hepatol. 2008;6:1194–7. doi: 10.1016/j.cgh.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari VV, Raman SS, Vuong NL, et al. Pancreatic cystic lesions: discrimination accuracy based on clinical data and high resolution CT features. J Comput Assist Tomogr. 2007;31:860–7. doi: 10.1097/RCT.0b013e318039b277. [DOI] [PubMed] [Google Scholar]
- 6.Gerke H, Jaffe TA, Mitchell RM, et al. Endoscopic ultrasound and computer tomography are inaccurate methods of classifying cystic pancreatic lesions. Dig Liver Dis. 2006;38:39–44. doi: 10.1016/j.dld.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Procacci C, Biasiutti C, Carbognin G, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906–12. doi: 10.1097/00004728-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol. 2007;189:648–56. doi: 10.2214/AJR.07.2365. [DOI] [PubMed] [Google Scholar]
- 9.Fisher WE, Hodges SE, Yagnik V, et al. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford) 2008;10:483–90. doi: 10.1080/13651820802291225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 12.Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–7. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 13.Koito K, Namieno T, Nagakawa T, et al. Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc. 1997;45:268–76. doi: 10.1016/s0016-5107(97)70269-4. [DOI] [PubMed] [Google Scholar]
- 14.Pais SA, Attasaranya S, Leblanc JK, et al. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489–95. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mallery SQD, Lewandrowski K, Centeno B, Warshaw A, Brugge WR. EUS-guided FNA with cyst fluid analysis in pancreatic cystic lesions. Gastrointest Endosc. 1998;47:149A. [Google Scholar]
- 16.Pinto MM, Meriano FV. Diagnosis of cystic pancreatic lesions by cytologic examination and carcinoembryonic antigen and amylase assays of cyst contents. Acta Cytol. 1991;35:456–63. [PubMed] [Google Scholar]
- 17.Bernard P, Scoazec JY, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–8. doi: 10.1001/archsurg.137.11.1274. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–9. doi: 10.1053/j.gastro.2007.05.010. quiz 309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54 4:iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberg K, Jelic S. Neuroendocrine gastroenteropancreatic tumors: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19 2:ii104–5. doi: 10.1093/annonc/mdn117. [DOI] [PubMed] [Google Scholar]
- 22.Mortenson MM, Katz MH, Tamm EP, et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100–13. doi: 10.1016/j.amjsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–51. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651-4. [DOI] [PubMed] [Google Scholar]
- 24.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiesenauer CA, Schmidt CM, Cummings OW, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610–7. doi: 10.1001/archsurg.138.6.610. discussion 617-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–42. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 27.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–12. doi: 10.1097/00000658-200002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 29.Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516–24. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 30.Hammel P, Levy P, Voitot H, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230–5. doi: 10.1016/0016-5085(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 31.Maire F, Couvelard A, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701–6. doi: 10.1016/s0016-5107(03)02032-7. [DOI] [PubMed] [Google Scholar]
- 32.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–82. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–9. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 34.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–7. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–5. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–6. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]


