Abstract
Barrett's esophagus (BE) is defined by the presence of metaplastic esophageal columnar epithelium with goblet cells within endoscopically recognizable areas of the esophagus. However, some carcinomas in BE, or from the GEJ region, develop within mucosa devoid of goblet cells. However, the biological properties, pathogenesis, and risk of malignancy of metaplastic, esophageal non-goblet columnar epithelium, is, essentially, unknown. In this study, 89 patients with metaplastic esophageal columnar epithelium were evaluated immunohistochemically for markers of intestinal differentiation, such as MUC2, DAS-1, Villin, and CDX2, a marker of gastric differentiation (MUC5AC), and Ki67, a marker of cell proliferation. Of the 89 patients, 59 had columnar metaplasia with goblet cells (BE), which were further separated into low density goblet cell and high density goblet cell groups based on the percentage of crypts with goblet cells, and 30 patients had columnar metaplasia of the esophagus without goblet cells. As controls, gastric biopsies from 19 age and sex matched patients without esophageal or gastric pathology were used. The rate of positivity of the markers and the location of Ki67 staining was evaluated only in non-goblet columnar epithelium from all patient groups. Patients with metaplastic esophageal columnar epithelium without goblet cells showed positivity for MUC5AC, MUC2, DAS-1, Villin, and CDX2 in 100%, 0%, 30%, 70%, and 43% of cases, respectively. 17% of cases showed aberrant surface Ki67 positivity. These values were significantly higher than gastric controls, which showed absence of staining for all markers except MUC5AC (100%). In patients with metaplastic esophageal columnar epithelium with goblet cells (BE) a significant increased rate of staining was observed for all markers, except MUC5AC. In addition, both MUC2 and surface Ki67 staining were significantly increased in BE patients with high density goblet cells versus those with low-density goblet cells. In a separate analysis in which metaplastic esophageal non-goblet epithelium was evaluated in areas of mucosa devoid of goblet cells compared to areas of mucosa with goblet cells, from patients who had goblet cells elsewhere in the mucosa (N=59), no significant differences were observed with regard to the percentage of cases that stained with any of the markers in the non-goblet epithelium in areas devoid of goblet cells, similar to the patient group with metaplastic esophageal epithelium without goblet cells (N=30). Similar to above, in all cases, expression of intestinal markers increased in areas of mucosa adjacent to goblet cells. This study provides evidence that metaplastic esophageal columnar epithelium without goblet cells shows phenotypic evidence of intestinal differentiation and supports the theory that squamous epithelium converts initially to non-goblet columnar epithelium prior to goblet cell metaplasia. Further prospective studies are needed to evaluate the pathogenetic sequence, natural history, and risk of malignancy of metaplastic esophageal non-goblet epithelium.
Introduction
Barrett’s esophagus (BE) is a premalignant condition in which the normal squamous epithelial lining of the distal esophagus is replaced by metaplastic columnar epithelium with goblet cells.40 Barrett’s esophagus is present in approximately 10% of patients with gastroesophageal reflux disease (GERD) with an overall incidence of approximately 1.6% in the general population.34,41 Barrett’s esophagus is the most important risk factor for the development of esophageal adenocarcinoma. The incidence of adenocarcinoma in BE ranges from 1 in 52 to 1 in 441 patient-years, which represents a 30 to 125-fold increased risk.37,42
Barrett’s esophagus is believed to develop via a sequence of events that begins with chronic GERD and ends with columnar metaplasia of the esophagus with goblet cells. Metaplastic columnar epithelium with goblet cells, also referred to as “intestinal metaplasia” (IM) or “specialized IM” of the esophagus, is believed to represent the only type of columnar epithelium at significant risk of malignancy.26,36,40 Thus, the American College of Gastroenterology (ACG) has required the demonstration of IM, characterized by the presence of goblet cells, as an essential criteria for a diagnosis of BE.40 As a result, endoscopic surveillance is only recommended for patients with documented IM of the esophagus. However, the mucosa of columnar-lined esophagus is composed of several types of metaplastic epithelium.28 For instance, the glandular compartment may be composed of either pure mucous glands, pure oxyntic glands, or a mixture of both types of glands. In addition, the surface and crypt epithelium is typically composed of mucinous columnar cells, either with or without goblet cells. Unfortunately, little is known about the biological properties, pathogenesis and neoplastic potential of non-goblet columnar epithelium in patients with BE.40 In fact, recent studies suggest that metaplastic esophageal columnar epithelium without goblet cells shows chromosomal abnormalities. 5,7,21 Furthermore, two recent studies suggest that patients with esophageal columnar mucosa without goblet cells have a similar risk of neoplastic progression to patients with columnar mucosa with goblet cells.13,20 A recent study by our group has shown that non-goblet columnar epithelium demonstrates similar DNA content abnormalities as goblet-cell containing columnar epithelium.23 Finally, one recent study suggests that neoplastic changes in the esophagus occur more frequently in association with non-goblet epithelium compared to columnar epithelium with goblet cells.39
Therefore, the purpose of this study was to evaluate the biological properties of non-goblet epithelium within the tubular esophagus by examining the expression of CDX-2, a transcription factor that activates transcription of genes involved in early differentiation, and maintenance, of intestinal epithelium,12 DAS-1, a unique peptide expressed only by normal colonic goblet cells,11 Villin, a cytoskeletal protein component of microvilli in the small intestine and colon,25 MUC2, a mucin glycoprotein expressed in intestinal goblet cells and enterocytes,18 MUC5AC, a mucin glycoprotein expressed in gastric epithelium,32 and Ki67, a marker of proliferation that is expressed during all stages of the cell cycle, except G0 (resting cells).1 The data were analyzed in patients who fulfill the ACG criteria for BE, by showing metaplastic columnar epithelium with goblet cells, and also in patients with metaplastic columnar epithelium, but without goblet cells. In addition, normal gastric biopsies from patients without gastric or esophageal pathology were used as controls.
Materials and Methods
1. Study Group
The study consisted of 89 patients retrieved by a retrospective search through the pathology files of the Brigham and Women’s Hospital in Boston, Massachusetts, the Fred Hutchinson Cancer Research Center in Seattle, Washington, and the Veterans Administration Medical Center in Dallas, Texas between the years 2001 to 2007. All patients had endoscopically confirmed columnar metaplasia of the distal esophagus and had four-quadrant biopsies obtained from every 1-2 cm of metaplastic mucosa. Overall, 331 biopsies from 89 patients with columnar metaplasia of the esophagus were included. Of these 89 patients, 59 had BE, all long-segment type (≥ 3cm), defined by the presence of visible tongues of columnar mucosa in the esophagus and goblet cells in mucosal biopsies of this region. The remaining 30 patients had short (1-3 cm) (N=15) and ultra-short (0-1 cm) (N=15) segments of columnar metaplasia of the distal esophagus, but without goblet cells. This group was chosen for a comparison to the BE patients with goblet cells. Of the 59 patients with long-segment BE, 25 were considered to have low density goblet cells (LDGC) based on the fact that goblet cells were present in less than 50% of the crypts, and 34 had high density goblet cells (HDGC) based on the presence of goblet cells in 50% or more of the metaplastic crypts. As controls, 19 biopsies of normal gastric corpus mucosa, from 19 age and sex matched patients without esophageal or gastric pathology, were used. In all patient groups, only non-goblet columnar epithelium was evaluated in this study, even in patients with BE who had goblet cells in other biopsies from the same individual. A comparison of the data was performed between the patients with columnar metaplasia with goblet cells (both LDGC and HDGC) and the patients with columnar metaplasia without goblet cells, and controls. Separate analyses were also performed in the patients with columnar metaplasia with goblet cells (BE) by comparing non-goblet epithelium in patients with LDGC to non-goblet epithelium in patients with HDGC. Finally, in patients with goblet cells (BE), non-goblet columnar epithelium in specific biopsies without goblet cells was compared to non-goblet columnar epithelium in other biopsies, from the same individual, that had goblet cells in the same tissue sample.
The clinical and endoscopic findings of all patients were obtained by a review of the patients’ electronic medical records. Clinical information included gender, age, and length of esophageal columnar epithelium at endoscopy. The length of esophageal columnar mucosa was calculated as the distance between the gastroesophageal junction (GEJ), defined as the most proximal aspect of the gastric folds, and the most proximal extent of columnar epithelium in the esophagus. Tissue samples were obtained during routine endoscopic surveillance for patients with BE, or during endoscopy for patients with symptoms of GERD or dysphagia. A mean of 3.7 biopsies per patient (range 1-16) were obtained. The research protocol was approved by the institutional review boards of the participating hospitals.
2. Histologic evaluation
All biopsy specimens were fixed in 10% buffered formalin and processed routinely. Hematoxylin and eosin (H and E) stained slides were reviewed by one of the authors (HPH) in order to confirm the diagnosis and for quantitation of the number (density) of goblet cells, as indicated above. None of the patients had dysplasia, acute inflammation, ulceration, or H. pylori gastritis. For patients with esophageal columnar epithelium without goblet cells (N=30), in order to ensure that the biopsies were obtained from the anatomic esophagus and not from the gastric cardia, histologic evidence of origin from the tubular esophagus was confirmed by the presence of esophageal glands, esophageal ducts, or multilayered epithelium within the columnar mucosa, based on previously published criteria.38
3. Immunohistochemistry
Immunohistochemical studies were performed on 4-μm-thick formalin-fixed, paraffin-embedded tissue sections. The panel of antibodies, and their functions, utilized in this study are listed in Table 1. The tissue slides were baked at 60°C for 1 hour, then deparaffinized and dehydrated through a series of xylene and alcohol solutions (4 washes in 100% xylene for 3 minutes each and 4 washes in 100% ethanol for 3 minutes each) followed by a wash under running water for 5 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in ethanol for 15 minutes, followed by a wash under running water for 5 minutes. Slides stained with CDX-2, Das-1, Ki67, and Villin underwent microwave antigen retrieval (800 watts, General Electric, Louisville, KY) at 199F for 30 in preheated Buffer. Tissue sections to be stained with MUC2 and MUC5AC were treated using a digital decloaking chamber (Pacific Southwest Lab Equipment Inc., Vista, CA). Sections to be stained with CDX-2, Das-1, MUC2 and Ki67 were treated in 10mM citrate buffer, pH 6.0. All slides were cooled for 15 minutes, and then transferred to phosphate buffered saline (PBS). All sections were blocked with the appropriate 1.5% serum for 15 minutes. The blocking serum was decanted and the sections were incubated with the primary antibody for 1 hour at room temperature. Primary antibodies were detected using the Vectastain Elite ABC kit (Vector laboratories, Inc., Burlington, CA). Slides were washed with PBS prior to incubation with the secondary antibody at 1:200 dilution in a 2% serum for 30 minutes. The Avidin-biotin complex was incubated for 30-40 minutes, then rinsed with PBS. Staining was developed using 3,3’-diaminobenzidine (DAB) (Sigma Chemical Company) as a substrate and Gill’s Hematoxylin (Fisher Scientific, Pittsburgh, PA) as a counter stain.
Table 1.
Summary of antibodies used in this study
| Antigen | Antibody Clone | Function | Dilution | Antigen Retrieval | Company |
|---|---|---|---|---|---|
| MUC 2 | Ccp58 | Glycoprotein expressed in intestinal goblet cells and enterocytes | 1:800 | Digital Decloaking | Novocastra Laboratories |
| MUC 5AC | 45M1/HGM | Glycoprotein expressed in gastric epithelium | 1:200 | Digital Decloaking | Novocastra Laboratories |
| Das-1 | Das-1 | Unique peptide expressed in colonic goblet cells | 1:20 | Microwave | (Gift of Kiron M. Das) |
| Villin | ID2C3 | Cytoskeletal protein component of microvilli in small intestine and colon | 1:250 | Microwave | Immunotech |
| CDX-2 | CDX2-88 | Transcription factor involved in differentiation and maintenance of intestinal phenotype | 1:500 | Microwave | BioGenex |
| Ki67 | MIB-1 | Marker of proliferation | 1:200 | Microwave | DakCytomation |
The immunostained slides were evaluated, in a blinded fashion, by one of the authors (HPH) without knowledge of the patient group from which the samples were obtained. Staining was evaluated only in non-goblet columnar epithelium from each of the patient groups. Immunostains for MUC2, MUC5AC, CDX-2, Das-1 and Villin antigens were scored as positive if staining was observed in the cytoplasm (MUC2, MUC5, DAS-1, Villin) or nucleus (CDX-2) within crypt and/or surface non-goblet columnar epithelium. Staining for Ki67 was scored as positive if there was aberrant nuclear staining of columnar epithelium in surface epithelium, and negative if staining was limited to the (normal) basal half of the crypt epithelium.
4. Statistical analysis
Fisher’s exact test was used for statistical analysis and for comparison between patient groups. A p value < 0.05 was considered statistically significant.
Results
1. Clinical features
The clinical features of the patient groups are summarized in table 2. The 59 patients with columnar metaplasia with goblet cells (BE) had an overall mean age of 61 years (range: 41-85 years) with a mean length of endoscopically identified metaplastic epithelium of 4.0 cm (range: 1-16 cm). BE patients with LDGC consisted of 18 males and 7 females with a mean age of 61 years (range: 43-85 years). The mean length of endoscopically identified BE was 4.0 cm (range: 1-11 cm). BE patients with HDGC consisted of 28 males and 6 females with a mean age of 61 years (range: 41-82 years). The mean length of endoscopically identified metaplastic epithelium in these patients was also 4.0 cm (range: 1-16 cm). Finally, patients with columnar metaplasia of the esophagus, but without goblet cells, consisted of 17 males and 13 females with a mean age of 60 years (range: 48-90 years). The mean length of esophageal columnar epithelium in these patients was less than 1 cm (range: 0.1-3.0 cm). None of the clinical features were significantly different between the patient groups, except for length of esophageal columnar mucosa, which was significantly shorter in patients with non-goblet columnar metaplasia compared to those with BE (p<0.05).
Table 2.
Clinical features of patient groups with columnar metaplasia of the distal esophagus
| Feature | Patients with columnar metaplasia with goblet cells (Barrett’s Esophagus) | Patients with columnar metaplasia without goblet cells | Gastric Controls | ||
|---|---|---|---|---|---|
| LDGC N=25 | HDGC N=34 | Total N=59 | N=30 | N=19 | |
| Male/Female ratio | 18/7 | 28/6 | 46/13 | 17/13 | 10/9 |
| Mean age (years) | 61 | 61 | 61 | 59 | 60 |
| Mean length of columnar metaplasia (cm; range) | 4.0 (1-11) | 4.0 (1-16) | 4.0 (1-16) | 1.0 (0.1-3.0) | NA |
NA = Not applicable
2. Immunohistochemistry results
A. Evaluation of All Study Groups and Controls
Table 3 summarizes the immunohistochemical data in gastric controls, patients with columnar metaplasia without goblet cells and in patients with columnar metaplasia with goblet cells. As expected, all 19 gastric controls (100%) showed MUC5AC staining in surface and crypt mucinous columnar epithelium. However, none of the gastric controls (0%) stained for any of the intestinal epithelium specific markers (MUC-2, DAS-1, Villin, CDX-2), and none showed surface epithelial staining with Ki67. Patients with columnar metaplasia without goblet cells also showed MUC5AC positivity in 100% of cases. However, 0 (0%), 9 (30%), 5 (17%) and 13 (43%) of these patients showed staining for MUC2, DAS-1, Villin, and CDX-2, respectively. In addition, 5 (17%) of the cases showed surface epithelial staining with Ki67. The percentage of cases with DAS-1 and CDX-2 staining was significantly higher (p<0.05) than the gastric controls.
Table 3.
Phenotypic markers in non-goblet columnar epithelium from patients with columnar metaplasia of the distal esophagus either with or without goblet cells
| Marker | Patients with columnar metaplasia with goblet cells (Barrett’s Esophagus) | Patients with columnar metaplasia without goblet cells | Gastric Controls | ||
|---|---|---|---|---|---|
| LDGC N=25 | HDGC N=34 | Total N=59 | N=30 | N= 19 | |
| MUC5AC | 25 (100%) | 34 (100%) | 59 (100%) | 30 (100%) | 19 (100%) |
| MUC2 | 18 (72%)c | 32 (94%)c | 50 (85%) | 0 (0%) | 0 |
| Das-1 | 21 (84%) | 32 (94%) | 53 (90%) | 9 (30%) | 0 |
| Villin | 23 (92%) | 33 (97%) | 56 (95%) | 5 (17%) | 0 |
| CDX-2 | 24 (96%) | 34 (100%) | 58 (98%) | 13 (43%) | 0 |
| Ki67 | 12 (48%)a | 25 (74%)b | 37 (63%) | 5 (17%)a,b | 0 |
LDGC = low density goblet cells, HDGC = high density goblet cells, N = number of patients
p<0.02
p<0.0007
p<0.03
In the total group of patients with columnar metaplasia with goblet cells (N=59), including both the LDGC and HDGC subgroups, the background non-goblet columnar epithelium stained with MUC5AC in all cases (100%), similar to the patients with columnar metaplasia without goblet cells and the gastric controls. However, a significant increase in the percentage of cases that stained for MUC2 (85%), DAS-1 (90%), Villin (95%), and CDX-2 (98%) was observed in this BE group of patients compared to the patients with columnar metaplasia without goblet cells, and the gastric controls. The percentage of cases with Ki67 surface epithelial staining was also significantly more common in the BE patients with goblet cells compared to the other patient groups (P<0.05).
When a separate comparison was made between the BE patients with LDGC versus those with HDGC, a significant increase in the proportion of cases with MUC2 staining (72% versus 94%, p=0.03) and surface Ki67 staining (48% versus 74%) was observed in the latter group compared to the former. Although DAS-1, Villin, and CDX-2 expression were also more prevalent in HDGC versus LDGC BE patients, the differences did not reach statistical significance. In addition, except for MUC5AC expression, all markers were significantly higher than the patients with columnar metaplasia without goblet cells, and the gastric controls, for each of the two BE subgroups independently.
B. Sub Analysis of the Barrett’s Esophagus Group
Table 4 summarizes the immunohistochemical results in the LDGC and HDGC subgroup of patients with columnar metaplasia with goblet cells (BE). For each goblet cell density subgroup, non-goblet columnar epithelium was evaluated in areas of mucosa without goblet cells and compared to areas of mucosa with goblet cells. Apart from MUC5AC, which, as indicated above, showed staining in mucinous columnar epithelium from all patient groups in 100% of cases, the percentage of cases that stained with MUC2, DAS-1, Villin, CDX-2, and Ki67 (surface epithelium staining) was increased in mucinous columnar epithelium in areas with goblet cells compared to mucinous columnar epithelium in areas without goblet cells, for both BE subgroups. In fact, MUC2 staining was observed in non-goblet epithelium in areas devoid of goblet cells in only 2 of 59 (3.3%) patients with metaplastic esophageal columnar epithelium with goblet cells in other areas of the mucosa. No significant differences were observed with regard to the percentage of cases that stained with any of the markers in non-goblet epithelium in cases of LDGC versus HDGC versus patients with columnar epithelium without goblet cells. In addition, non-goblet epithelium in areas of goblet cells, from the LDGC and HDGC groups, were similar for all of the markers evaluated, except for DAS-1, which was significantly higher in non-goblet epithelium in areas of HDGC compared to areas of LDGC (p<0.05).
Table 4.
Phenotypic markers in non-goblet columnar epithelium in areas of mucosa either with or without goblet cells in patients with Barrett’s esophagus
| Marker | Patients with columnar metaplasia with goblet cells (Barrett’s Esophagus) | |||
|---|---|---|---|---|
| LDGC N = 25 | HDGC N = 34 | |||
| CM-NG | CM-WG | CM-NG | CM-WG | |
| MUC 5AC | 25 (100%) | 25 (100%) | 34 (100%) | 34 (100%) |
| MUC 2 | 2 (8%)a | 18 (72%) a | 0 (0%) b | 32 (94%) b |
| Das-1 | 8 (32%) c | 17 (68%) c | 13 (38%) d | 32 (94%) d |
| Villin | 7 (28%) e | 23 (92%) e | 12 (35%) f | 33 (97%) f |
| CDX-2 | 10 (40%) g | 24 (96%) g | 9 (26%) h | 34 (100%) h |
| Ki67 | 2 (8%) i, k | 11 (44%) i | 10 (29%) j, k | 19 (56%) j |
LDGC = low density goblet cells, HDGC = high density goblet cells, CM-NG = columnar mucosa -no goblet cells, CM-WG = columnar mucosa -with goblet cells
p<0.0005
p<0.0005
p<0.02
p<0.0005
p<0.0005
p<0.0005
p<0.000
p<0.0005
p<0.009
p<0.04
p=0.051
Finally, the prevalence of staining for all of the markers evaluated in this study did not correlate with the length of columnar epithelium, or with any of the clinical features, such as patient age and gender.
Discussion
The purpose of this study was to evaluate the expression of markers of intestinal and gastric differentiation in metaplastic, esophageal non-goblet columnar epithelium, since the biological properties, risk of malignancy, and pathogenetic sequence of events that lead to fully differentiated goblet cell metaplasia in BE are not well characterized. The study was designed to evaluate metaplastic esophageal non-goblet columnar epithelium in patients who express either no, or different degrees of, goblet cell metaplasia. One patient group had columnar metaplasia of the esophagus, but without goblet cells. The other patient group had metaplastic esophageal columnar epithelium with goblet cells (which fulfills the ACG criteria for BE), and this group was further subdivided into those with LDGC or HDGC for comparison.
Overall, our results show that metaplastic esophageal columnar epithelium without goblet cells reveals phenotypic evidence of intestinal differentiation. In fact, expression of intestinal markers in metaplastic non-goblet columnar epithelium was similar regardless of the presence or absence, or degree, of goblet cell metaplasia elsewhere in the esophagus. Furthermore, an increase in the rate of expression of intestinal markers in metaplastic non-goblet columnar epithelium was noted in areas of mucosa that contained goblet cells, but the degree of intestinal differentiation in non-goblet columnar epithelium was not related to the density of goblet cells in the mucosa. This data provides evidence that metaplastic non-goblet columnar epithelium of the esophagus shows phenotypic evidence of intestinal differentiation, and provides support for the theory that squamous epithelium converts initially to non-goblet columnar epithelium prior to goblet cell metaplasia.
There are several other studies that have evaluated markers of intestinal differentiation, such as MUC2, MUC6, Sucrase Isomaltase, Dipeptidyl Peptidase, CDX2, DAS1, Hepatocyte antigen, and CD10, in patients with BE.4,7,9,15-17,29,35 However, ours is the first to systematically evaluate markers of intestinal differentiation in non-goblet columnar epithelium in patients either with, or without, goblet cell metaplasia elsewhere in the esophagus, and to evaluate the latter group based on goblet cell density. For instance, Chaves et al evaluated MUC2, MUC5AC, and MUC6 in 46 patients with columnar-lined esophagus, including 9 cases without IM, 22 cases with IM and 15 BE cases with adenocarcinoma.7 In that study, MUC5AC and MUC6 were detected in non-goblet columnar epithelium in 100% and 100% of cases without IM, respectively, and in 100% and 86.3% of cases with IM. However, in that study, MUC2 was present in 72.7% of cases with IM, but not in any of the cases of columnar-lined esophagus without IM. These results are similar to ours in which MUC2 was expressed in non-goblet epithelium in 85% of patients with goblet cells (and in these positive cases, almost exclusively in areas of mucosa with goblet cells compared to areas of mucosa without goblet cells) but in none (0%) of the cases of esophageal columnar metaplasia without goblet cells. These data suggest that, in contrast to MUC5AC (and MUC6), MUC2 expression in metaplastic esophageal columnar epithelium represents a late intestinal alteration, and corresponds specifically to the onset of goblet cell metaplasia in BE. Thus, further studies should be performed to determine if MUC2 expression in metaplastic non-goblet columnar epithelium in biopsies from the esophagus represents a specific marker of goblet cell metaplasia elsewhere in the patient, and whether expression of this marker coincides with an increased risk of neoplastic progression. In contrast, data from both the Chaves and our current study suggest that MUC5AC and MUC6 are expressed early in the pathogenesis of BE, prior to the onset of goblet cell metaplasia.7
Several studies have evaluated other markers of intestinal differentiation, such as CDX2, Sucrase Isomaltase, DAS-1, and CD10 in metaplastic esophageal non-goblet columnar epithelium and also showed various degrees of positivity.4,9,16,17,29,35 The human CDX2 gene is a member of the caudal-related type homeobox gene family, whose gene product has been shown to be important in early differentiation and maintenance of intestinal epithelium by regulation of intestinal specific gene transcription.12 In fact, CDX2 expression has been shown to regulate transcription of genes, such as Villin, Mucins (MUC2), Sucrase Isomaltase, human defensin, alkaline phosphatase, galactin, and trefoil factors.19,22 In one study by Phillips et al, expression of CDX2 was evaluated in 134 biopsy or resection specimens of patients with metaplastic esophageal columnar epithelium, including 62 cases without goblet cells.29 They showed that CDX2 was present in 100% of biopsies with goblet cell metaplasia (BE) and in 30% of cases of metaplastic esophageal columnar epithelium without goblet cells. In another study by Groisman et al, CDX2 immunoexpression was evaluated in 90 patients with short-segment BE, including 45 with, and 45 without, goblet cells.17 In that study, CDX2 was present in all (100%) cases with goblet cells, in both goblet and non-goblet epithelium, but was also focally present in 38% of patients without goblet cells in their esophagus. In our current study, our finding of CDX2 expression in 43% of patients with metaplastic esophageal columnar epithelium without goblet cells, and in 98% of patients with goblet cells, are consistent with the studies by Phillips and Grossman, and suggests that CDX2 expression is an early event in intestinal differentiation in the esophagus and may help promote goblet cell metaplasia. In fact, in our sub-analysis of BE patients with LDGC and HDGC (table 4), CDX2 was expressed significantly more often in areas of mucosa with goblet cells (96% and 100%, respectively) compared to areas of mucosa without goblet cells (40% and 26%, respectively), which supports this latter theory.
Our findings regarding DAS-1, an antibody that recognizes a colonic goblet cell protein, and Villin, an intestinal brush border specific peptide, in 30% and 17% of patients with metaplastic esophageal columnar metaplasia without goblet cells, respectively, in this study, and in 90% and 95% of patients with metaplastic esophageal columnar epithelium with goblet cells, respectively, further supports the “intestinalized” nature of the background non-goblet columnar epithelium in patients with BE.16,31 Similar to CDX-2, expression of DAS-1 and Villin probably represent early differentiation events prior to goblet cell metaplasia, and are increased in areas of mucosa with goblet cells. These data are also supported by other studies that have shown 1. DAS-1 in 88% and 91% of patients with short and long-segment BE, respectively, by Glickman et al,16 and 2. CD10 expression (which labels the intestinal brush border similar to Villin) in 25-30% of patients with BE, by Sarbia et al.35 Unfortunately, neither of these two previous studies evaluated expression in metaplastic esophageal columnar epithelium according to the density of goblet cells or evaluated patients who had columnar metaplasia of the esophagus, but without goblet cells elsewhere in the esophagus.
The pathogenesis of conversion of squamous to columnar epithelium in the esophagus is, essentially, unknown.14,33 However, many studies suggest that this is a multistep process that probably involves activation of multiple genes involved in intestinal differentiation, even at the embryonic level.22 In fact, some studies do support the concept that the initial step in the conversion of squamous to columnar epithelium in the esophagus is the development of metaplastic columnar epithelium without goblet cells.2,8,10,14,27,28 For instance, in one case series by Chaves et al, four patients developed recurrence of metaplastic esophageal columnar epithelium, without goblet cells, after a distal esophageal resection for adenocarcinoma.8 In all patients, the metaplastic columnar epithelium was uniformly positive for Sucrase Isomaltase, which is an intestinal cell specific enzyme. Interestingly, only one of the four cases developed goblet cells, and this occurred after 10 years of surveillance. Other studies in children with BE, who have a low prevalence of goblet cells, and in adults with short and ultra-short segments of BE, also support this hypothesis.2,10,14,27,28
Recent data also suggests that metaplastic esophageal non-goblet columnar epithelium in patients either with, or without, goblet cells in other portions of the mucosa, shows a variety of molecular and DNA content abnormalities in addition to intestinal differentiation, and, as a result, may be at risk for neoplastic progression.5,23,24,33 For instance, in one recent study by our group, DNA content abnormalities, such as elevated DNA index, elevated DNA heterogeneity index, aneuploidy and increased percent of cells with DNA content greater than 5N, occurred with equal frequency, and extent, in patients with metaplastic esophageal columnar epithelium without goblet cells compared to patients with metaplastic columnar epithelium with goblet cells, by analysis of high fidelity DNA histograms.23 Similarly, in a study by Chaves et al, a similar degree of chromosomal instability was observed in metaplastic esophageal columnar epithelium without goblet cells, compared to metaplastic columnar epithelium with goblet cells.5 Romagnoli et al also showed molecular alterations, such as LOH and allelic imbalances, in patients with metaplastic esophageal columnar epithelium without goblet cells.33 The potential risk of neoplastic progression in metaplastic esophageal non-goblet columnar epithelium is also supported by the fact that previous studies showed a significant risk of dysplasia and/or adenocarcinoma in patients with esophageal non-goblet columnar epithelium upon long term follow up.6,13,20 Takubo et al also documented that most neoplasms develop in association with esophageal columnar epithelium devoid of goblet cells.39 Our current results showing aberrant surface Ki67 staining in 17% of patients with metaplastic esophageal columnar metaplasia without goblet cells, which was similar to the value obtained in areas of mucosa with metaplastic columnar epithelium in patients with goblet cells, supports the proliferative potential of this type of epithelium.3
In summary, our study provides evidence that metaplastic esophageal columnar epithelium without goblet cells shows phenotypic evidence of intestinal differentiation, and also supports the theory that squamous epithelium converts initially to non-goblet columnar epithelium prior to goblet cell metaplasia. MUC5AC, DAS-1, Villin, and particularly CDX2, probably represent early changes of intestinal differentiation, whereas MUC2 expression likely represents a late “intestinal” alteration that corresponds specifically to the onset of goblet cell metaplasia. These data, in conjunction with other studies that have shown molecular alterations in non-goblet epithelium, support the controversial concept that metaplastic esophageal columnar epithelium without goblet cells has neoplastic potential. These findings have important clinical implications with regard to the current ACG definition of BE,40 which requires goblet cells to be documented in mucosal biopsies in order to establish the diagnosis, and provides evidence in favor of the British Society of Gastroenterology guidelines which do not require demonstration of goblet cells in order to diagnose BE.30 Further prospective studies are needed to evaluate the pathogenetic sequence, natural history, and risk of malignancy of metaplastic non-goblet epithelium in the esophagus.
Figure 1.
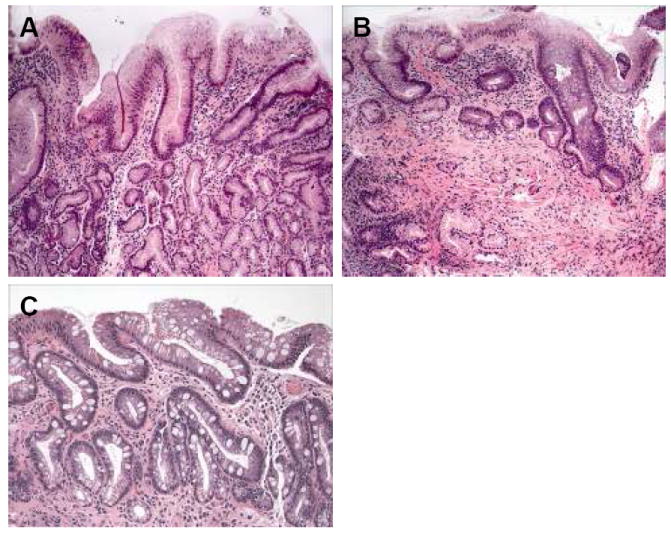
(A-C): Representative H&E-stained tissue sections from patients with columnar metaplasia of the esophagus without goblet cells (A), columnar metaplasia with low-density goblet cells (B), and columnar metaplasia with high-density goblet cells (C).
Figure 2.
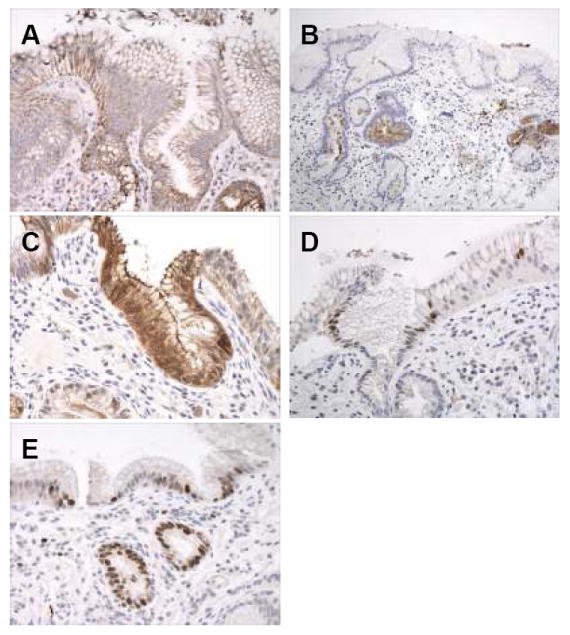
(A-E): Immunohistochemical results of intestinal markers and Ki67 in a patient with columnar metaplasia of the esophagus, but without goblet cells. A. MUC-5AC showing diffuse cytoplasmic reactivity in surface and crypt epithelium. B. DAS-1 reactivity in columnar mucous cells within the deep portions of the crypt epithelium and within mucosal glands. C. Strong reactivity for villin in mucinous columnar cells in the surface and crypt epithelium. D. Nuclear staining for CDX2 in scattered cells in the surface and crypt epithelium. E. Nuclear Ki67 staining in the surface and crypt epithelium in an area of mucosa without active inflammation or ulceration.
Figure 3.
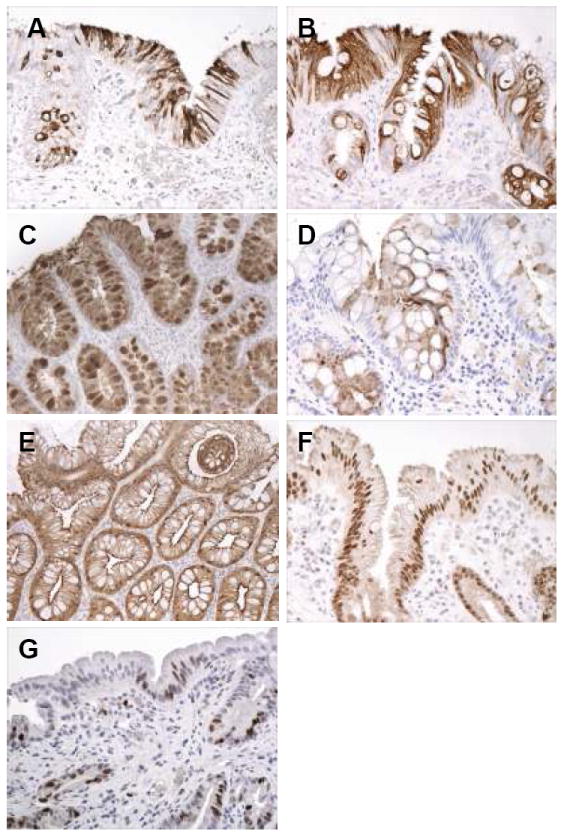
(A-G): Intestinal phenotypic markers and Ki67 staining in patients with Barrett’s esophagus, either low-density or high-density goblet cell subgroups. A. Strong cytoplasmic MUC2 staining in surface and crypt mucinous columnar cells, and in goblet cells, in this patient with columnar metaplasia of the esophagus with low-density goblet cells. B. Strong diffuse cytoplasmic staining in surface and crypt columnar cells, and goblet cells, in a patient with columnar metaplasia of the esophagus with high-density goblet cells. C. Strong MUC-5AC staining in columnar cells and goblet cells in this patient with high-density goblet cells. D. Cytoplasmic staining for DAS-1 in columnar cells and scattered goblet cells in this patient with columnar metaplasia of the esophagus with high-density goblet cells. E. Strong diffuse cytoplasmic staining in the surface and crypt epithelium for villin in this patient with high-density goblet cells. F. Nuclear staining for CDX2 in most cell nuclei in the surface and crypt epithelium in this patient with columnar metaplasia of the esophagus with low-density goblet cells. G. Nuclear staining for Ki67 in scattered surface columnar cells and crypt cells in this patient with columnar metaplasia of the esophagus and low-density goblet cells.
Footnotes
Presented in part at the 96th annual meeting of the United States and Canadian Academy of Pathology in San Diego, CA, March 24-30, 2007.
References
- 1.Cattoretti G, Becker MHG, Key G, et al. Monoclonal and antibodies against recombinant parts of the Ki67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave processed formalin-fixed paraffin sections. J Pathol. 1992;168:357. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasoma PT, Deer R, Dalton P, et al. Distribution and significance of epithelial types in columnar-lined esophagus. Am J Surg Pathol. 2001;25:1188–1193. doi: 10.1097/00000478-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Chao D, Sanchez C, Galipeau P, et al. Cell proliferation, cell cycle abnormalities, and cancer outcome in patietns with Barrett’s esophagus: A long-term prospecitve study. Clin Cancer Research. doi: 10.1158/1078-0432.CCR-07-5063. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaves P, Cardoso P, Crespo Mendes De Almeida J, et al. Non-goblet cell population of Barrett’s esophagus: An immunohistochemical demonstration of intestinal differentiation. Hum Pathol. 1999 Nov;30(11):1291–1295. doi: 10.1016/s0046-8177(99)90058-8. [DOI] [PubMed] [Google Scholar]
- 5.Chaves P, Crespo M, Ribeiro C, et al. Chromosomal analysis of Barrett’s cells: demonstration of instability and detection of the metaplastic lineage involved. Mod Pathol. 2007 Jul;20(7):788–796. doi: 10.1038/modpathol.3800787. Epub 2007 May 25. [DOI] [PubMed] [Google Scholar]
- 6.Chaves P, Cruz C, Cardoso P, et al. Enterocytic columnar non-goblet cells of Barrett’s esophagus -an immunohistochemical demonstration of association with malignant evolution. J Exp Clin Cancer Res. 2003;22(2):273–278. [PubMed] [Google Scholar]
- 7.Chaves P, Cruz C, Pereira D, et al. Gastric and intestinal differentiation in Barrett’s metaplasia and associated adenocarcinoma. Dis Esoph. 2005;18:383–387. doi: 10.1111/j.1442-2050.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaves P, Pereira AD, Cruz C. Recurrent columnar-lined esophageal segments –study of the phenotypic characteristics using intestinal markers. Dis Esoph. 2002;15:282–286. doi: 10.1046/j.1442-2050.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 9.Chu PG, Jiang Z, Weiss L. Hepatocyte antigen as a marker of intestinal metaplasia. Am J Surg Pathol. 2003;27(7):952–959. doi: 10.1097/00000478-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Dahms BB, Rothstein FC. Barrett’s esophagus in children: a consequence of chronic gastroesophageal reflux. Gastroenterology. 1984;86:318–323. [PubMed] [Google Scholar]
- 11.Das KM, Prasad I, Garla S, et al. Detection of a shared colon epithelial epitope on Barrett epithelium by a novel monoclonal antibody. Ann Intern Med. 1994;5:753–756. doi: 10.7326/0003-4819-120-9-199405010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Freund JN, Domon-Dell C, Kedinger M, et al. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957–969. doi: 10.1139/o99-001. [DOI] [PubMed] [Google Scholar]
- 13.Gatenby PA, Ramus JR, Caygill CP, et al. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43(5):524–530. doi: 10.1080/00365520701879831. [DOI] [PubMed] [Google Scholar]
- 14.Glickman JN, Chen YY, Wang H, et al. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett’s esophagus. Am J Surg Pathol. 2001;25(5):569–578. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Glickman JN, Shahsafaei A, Odze RD. Mucin core peptide expression can help differentiate Barrett’s esophagus from intestinal metaplasia of the stomach. Am J Surg Pathol. 22(10):1357–1365. doi: 10.1097/00000478-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Glickman JN, Wang H, Das KM, et al. Phenotype of Barrett’s esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction. Am J Surg Pathol. 2001;25(1):87–94. doi: 10.1097/00000478-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Groisman GM, Amar M, Meir A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett’s) metaplasia. Mod Pathol. 2004;17:1282–1288. doi: 10.1038/modpathol.3800182. [DOI] [PubMed] [Google Scholar]
- 18.Guillem P, Billeret V, Buisine MP, et al. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int J Cancer. 2000;88:856–861. doi: 10.1002/1097-0215(20001215)88:6<856::aid-ijc3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 20.Kelty CJ, Gough MD, Van Wyk Q, et al. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007 Nov;42(11):1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 21.Kerkhof M, Bax DA, Moons LM, et al. For the Cypar Study Group. Does CDX2 expression predict Barrett’s metaplasia in oesophageal columnar epithelium without goblet cells? Aliment Pharmacol Ther. 2006 Dec;24(1112):1613–1621. doi: 10.1111/j.1365-2036.2006.03163.x. [DOI] [PubMed] [Google Scholar]
- 22.Krishnadath K. Novel findings in the pathogenesis of esophageal columnar metaplasia or Barrett’s esophagus. Curr Opin Gastroenterol. 2007;23:440–445. doi: 10.1097/MOG.0b013e32814e6b4f. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Hahn H, Odze R, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell containing epithelium. Am J Gastroenterol. 2008 doi: 10.1038/ajg.2009.85. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord RV, Wickramasinghe K, Johansson JJ, et al. Cardiac mucosa in the remnant esophagus after esophagectomy is an acquired epithelium with Barrett’s-like features. Surgery. 2004 Sep;136(6):633–640. doi: 10.1016/j.surg.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Maroux S, Coudrier E, Feracci H. Molecular organization of intestinal brush border. Biochimie. 1988 Sep;70(9):1297–1306. doi: 10.1016/0300-9084(88)90198-8. [DOI] [PubMed] [Google Scholar]
- 26.Murray L, Watson P, Johnston B, et al. Risk of adenocarcinoma in Barrett’s oesophagus: population based study. BMJ. 2003;327:534–535. doi: 10.1136/bmj.327.7414.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odze RD. Unraveling the mystery of the gastroesophageal junction: A pathologist’s perspective. Am J Gastroenterol. 2005;100:1853–1867. doi: 10.1111/j.1572-0241.2005.50096.x. [DOI] [PubMed] [Google Scholar]
- 28.Paull A, Trier JS, Dalson MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976 Aug 26;295(9):476–480. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 29.Phillips RW, Frierson HF, Moskaluk CA. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27(11):1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006 Apr;55(4):442. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regalado SP, Nambu Y, Iannettoni MD, et al. Abundant expression of the intestinal protein villin in Barrett’s metaplasia and esophageal adenocarcinomas. Mol Carcinog. 1998 Jul;22(3):182–189. [PubMed] [Google Scholar]
- 32.Reis CA, David L, Nielson PA, et al. Immunohistochemical study of MUC5AC expression in human gastric carcinoma using a novel monoclonal antibody. Int J Canc. 1997;74:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Romagnoli S, Roncalli M, Graziani D, et al. Molecular alterations of Barrett’s esophagus on microdissected endoscopic biopsies. Lab Invest. 2001;81:241–247. doi: 10.1038/labinvest.3780232. [DOI] [PubMed] [Google Scholar]
- 34.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Sarbia M, Donner A, Frank C, et al. Distinction between intestinal metaplasia in the cardia and in Barrett’s esophagus: The role of histology and immunohistochemsitry. Hum Pathol. 2004;35(3):371–376. doi: 10.1016/j.humpath.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago workshop. Gastroenterology. 2004;127:310–330. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J Clin. 2005;55(6):334–351. doi: 10.3322/canjclin.55.6.334. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava A, Odze RD, Lauwers G, et al. Morphologic features are useful in distinguishing Barrett’s esophagus from carditis with intestinal metaplasia. Am J Surg Pathol. 2007 Nov;31(11):1733–1741. doi: 10.1097/PAS.0b013e318078ce91. [DOI] [PubMed] [Google Scholar]
- 39.Takubo K, Aida J, Namoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol. 2008 doi: 10.1016/j.humpath.2008.06.008. In Press. [DOI] [PubMed] [Google Scholar]
- 40.Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008 Mar;108(3):788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 41.Winters C, Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92(1):118–124. [PubMed] [Google Scholar]
- 42.Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: A systematic review and meta-analysis. Am J Epidemiol. 2008 Aug;168:237–249. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]


