Abstract
Objective
To analyze the structure and composition of unattached stones in idiopathic calcium oxalate stone formers (ICSF) and compare them to attached stones from the same cohort in order to investigate whether more than one pathogenic mechanism exists for stone formation in ICSF.
Patients and methods
ICSF undergoing percutaneous nephrolithotomy or ureteroscopy for treatment of nephrolithiasis were consented for this study. All accessible renal papillae were endoscopically imaged using a digital endoscope. All stones were removed and determined by the operating surgeon to be attached or unattached to the underlying papilla. Micro-computed tomography (micro-CT), which provides three-dimensional analysis of entire stones, was used to compare the structure and composition of attached versus unattached stones.
Results
Of 115 stones collected from 9 patients (12 renal units), only 25 stones were found not to be attached to renal papillae. Of these 25 stones, 4 were lost and 12 showed definite morphological evidence of having been attached to tissue, probably having been knocked off of papillae during access. For the remaining 9 stones, micro-CT analysis revealed at least one internal region of calcium phosphate within each of these unattached calcium oxalate (CaOx) stones. That is, the internal structure of the unattached stones is consistent with their having originated attached to RP, and then having become detached but retained in the kidney, with new layers of CaOx eventually covering the original attachment site.
Conclusions
Micro CT analysis supports the hypothesis that in ICSF, both attached and unattached stones occur as a result of a common pathogenic mechanism. That is, in this type of stone former, CaOx stones—even those not showing morphology that betrays attachment—all originate attached to interstitial plaque on the renal papilla.
Keywords: Randall’s plaque, nephrolithiasis, stone pathogenesis, micro computed tomography
Introduction
How stones form in kidneys remains poorly understood. Alexander Randall in the late 1930’s proposed that stones grow on the renal papilla attached to underlying interstitial apatite deposits (‘plaque’). [1, 2] Randall’s hypothesis was abandoned, primarily because it did not fit all types of stones, but has undergone a rebirth of sorts due to new data collected in calcium oxalate (CaOx) stone formers through papillary biopsy and intra-operative visualization and imaging.[3-6] Recently we have demonstrated that in the common idiopathic CaOx stone former (ICSF) most stones do indeed grow attached to the papillae, on plaque.[7] Of 115 stones visualized in 12 kidneys of 9 ICSF, 90 were attached to papillae, only 25 were found free.
The 25 stones represent an unresolved problem. If growth on plaque is indeed the main mechanism for stone formation, these 25 stones presumably formed attached to plaque and subsequently detached. If so, we should find in them apatite deposits like those in the 90 attached stones: focal, and on the surface that had faced the renal papillae during attachment. Accordingly, we compared the ultrastructure and composition of the 25 unattached stones to that of the 90 attached stones from the same group of patients using micro computed tomography (micro CT) imaging, a technique that reveals structural details of stones and can identify and localize the common stone minerals. [8]
Patients and methods
The 9 ICSF (age 34-67 years, 3 female) formed predominantly CaOx stones (stone analysis with no brushite and ≤30 percent CaP) in the absence of systemic stone-forming diseases such as primary hyperparathyroidism, sarcoidosis, distal renal tubular acidosis, bowel disease, or medullary sponge kidney [7]. All underwent percutaneous nephrolithotomy or ureteroscopy for stone removal. During surgery all accessible renal papillae were endoscopically imaged with a digital endoscope, and a continuous digital recording was made of the entire surgical procedure. All stones were recorded by the operating surgeon as being attached or unattached. Micro CT analysis was performed on both attached and unattached stones. Stones were scanned using a mCT20 system (SCANCO Medical AG, Bruttisellen, Switzerland) with voxel sizes of 20-24 μm, or with a SkyScan 1172 system (SkyScan, Kontich, Belgium) with voxel sizes of 3.5-15 μm. Three-dimensional reconstructions of stones were examined using Image J (http://rsbweb.nih.gov/ij/) and Voxx2 (http://www.nephrology.iupui.edu/imaging/voxx/) to verify the morphological arrangements of CaP versus CaOx in each stone.
Results
Of the 25 stones that appeared to be loose during surgery, 12 showed definite signs of having been attached to tissue (Figure 1). Each had a mucus-covered, cupped region on one surface that by micro CT analysis contained apatite; that surface was concave as expected for growth on a papillum. Another surface of each stones was convex and smooth, as expected for the portion that had faced outward into the urinary space. These 12 stones resemble the attached stones from our prior report [7] and probably had been detached during the placement of guide and safety wires.
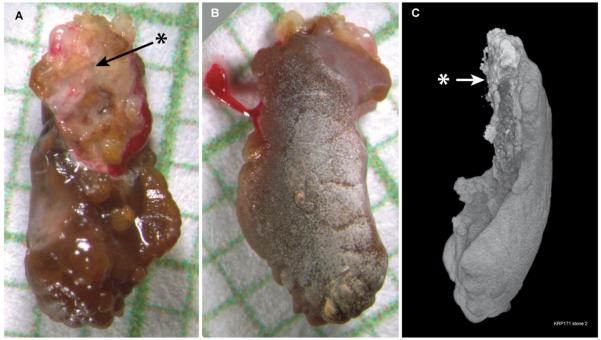
Figure 1.
Example of a stone found loose in the collecting system of a patient, but which shows morphological evidence of having been recently attached to tissue. A: View of stone immediately after removal, showing region (*) that appeared to be covered with a mixture of tissue and mucus. Background grid shows 1 mm squares. B: Opposite surface of stone, presumably the surface exposed to the urine; clot of blood on the left is that seen in panel A clinging to right side of stone. C: Surface image of micro CT reconstruction of stone; closest to the viewer is the right edge of view A (left edge of view B). Arrow indicates surface corresponding to marked region in panel A. This region also showed material at the surface that was x-ray bright, indicating the presence of calcium phosphate (CaP), most likely in the form of apatite.
Of the 13 remaining stones 4 were lost and could not be studied. The remaining 9 did not possess mucus-covered, apatite-containing regions on a concave surface. Instead the surfaces were dark brown and uniform (Figure 2). The uniform outer layer of all 9 stones was also uniform by micro CT with an X-ray attenuation value consistent with CaOx monohydrate [8]. All 9 stones also contained internal regions with X-ray attenuation values consistent with apatite. Using the fact that micro CT permits rotation of the stone image, we could establish that all apatite regions in these stones were internal (Figure 3), unlike the surface apatite on attached stones [7] and the 12 stones that seemingly were recently attached (Figure 1).
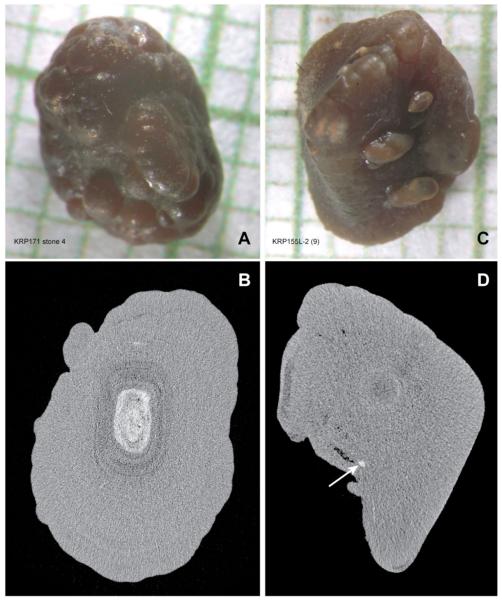
Figure 2.
Examples of two stones that showed no evidence of having been recently attached to tissue. A and C: Micrographs of stones on mm background grid. Images of other sides of these stones looked very similar to those shown. B and D: Micro CT section through each stone, shown below its corresponding micrograph. Stone in A shows significant region of interior CaP (white region in panel B), surrounded by calcium oxalate monohydrate. Stone in C displayed smaller regions of interior CaP, one of which is indicated by arrow in D.
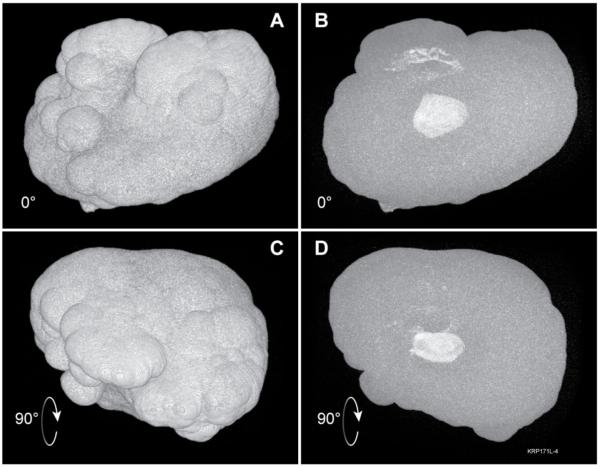
Figure 3.
Demonstration of interior position of internal CaP regions. A: Surface rendering of same stone as in Figure 2A/C. B: Maximum intensity projection with same orientation as in panel A; white regions within the stone indicate CaP. C and D: Similar surface and maximum-intensity views as A and B, but image stack was rotated 90° about the horizontal axis; note that regions of CaP are completely interior, without any portions that extend to the stone surface.
Discussion
Our results support the hypothesis that in ICSF stones grow mainly, if not exclusively, on papillae over regions of interstitial apatite plaque. We already have proven a great surplus vs. chance for such growth [7], and here deal with the stones found at surgery in the urinary system and not attached to the kidney at all. How did they grow? Possibly they did not grow on papillae over plaque, but in some other way.
What we found is that a majority display a kind of morphological ‘signature’ of attachment: cupped apatite regions on their concave surface that look just like what one finds on attached stones when they are removed from the kidney surface. The others have no surface apatite but contain internal apatite where attachment might have occurred. We accept that the inner apatite is not as convincing or specific as the external apatite, and therefore that some leeway still exists; but for the most part, this work, taken with its prior counterpart [7], strongly supports the idea of CaOx stones as papillary overgrowths on plaque. We propose that each unattached stone had originated attached to plaque, but became detached and was then retained in the collecting system. Exposure of the area of plaque on the stone to urine supersaturated with CaOx allowed subsequent layering of CaOx over the plaque site, internalizing it, and creating the images we found in our micro CT analysis.
Detailed analyses of spontaneously passed calculi—which thus must have become unattached—have supported an origin of CaOx stones on plaque. Using scanning electron microscopy and x-ray dispersive elemental analysis, Cifuentes and colleagues [9] examined 87 passed stones that had a morphology suggestive of having been previously attached. Sixty-three of these stones had evidence of plaque at the surface and 13 of these 63 showed calcified renal tubules suggesting a papillary tip origin. Thus, there is ample evidence that stones that had been attached can spontaneously lose their attachment and be passed. If such a stone were to lose its attachment and be retained in the kidney for sufficient time, it is possible that signs of its previous attachment could be covered over by new layers of mineral. Such a case is described by Fernandez et al., who reported a stone that showed no evidence of plaque at its surface but, upon cleavage, the interior of the stone showed a region composed of CaP with calcified renal tubules; they proposed that the stone must have originated on plaque, was dislodged, and then grew layers of CaOx that covered over the residue of plaque [10].
Micro CT has been shown to be an excellent tool for non-destructively studying the internal and surface microstructure of urinary calculi.[8, 11, 12] Here, we were able to carry out a study similar to that of Fernandez et al., but we did not have to rely on fortuitous cleavage of the unattached stones to reveal internal regions of apatite. Instead, micro CT allowed non-destructive analysis of the entire volume of each CaOx stone, and revealed, in all 9 unattached stones, at least one internal region of apatite. In other words, we have confirmed the Fernandez conjecture, and the Cifuentes observations using a new technology.
In summary, we have previously shown evidence that all attached stones appear to be attached to Randall’s plaques, and we show here that even stones that were found loose within the collecting system of these patients all show evidence that they once were attached to plaque. Altogether, the plaque hypothesis is supported by this work, and has not as yet been contradicted by studies designed to falsify it; therefore it can be accepted as a useful contemporary paradigm for planning future stone research.
Acknowledgements
This work was supported by NIH grants P01DK56788, R01DK59933 and S10RR023710. We thank Molly Jackson and Christian Beuschel for the work of scanning the stones by micro CT.
References
- 1.Randall A. The etiology of primary renal calculus. International Abstract of Surgery. 1940;71:209–40. [Google Scholar]
- 2.Randall A. The origin and growth of renal calculi. Annals of Surgery. 1937;105:1009–27. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–16. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evan AP, Lingeman J, Coe FL, Worcester E. Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313–8. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 5.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, et al. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec. 2007;290:1315–23. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 6.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall’s plaques in the pathogenesis of calcium stones. J Urol. 2007;177:31–8. doi: 10.1016/j.juro.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 7.Miller NL, Gillen DL, Williams JC, Jr., Evan AP, Bledsoe SB, Coe FL, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU International. 2009;103:966–71. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarse CA, McAteer JA, Sommer AJ, Kim SC, Hatt EK, Lingeman JE, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urology. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifuentes Delatte L, Miñón-Cifuentes JL, Medina JA. Papillary stones: calcified renal tubules in Randall’s plaques. J Urol. 1985;133:490–4. doi: 10.1016/s0022-5347(17)49039-2. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez Conde M, Cifuentes Delatte L, Medina JA, Rodriguez Minon JL, Caralps A. Núcleo con tubos renales calcificados en el interior de un pequeño cálculo de Whewellita. Arch Esp Urol. 1986;39:71–3. [PubMed] [Google Scholar]
- 11.Zarse CA, McAteer JA, Tann M, Sommer AJ, Kim SC, Paterson RF, et al. Helical CT accurately reports urinary stone composition using attenuation values: In vitro verification using high resolution micro CT calibrated to FT-IR microspectroscopy. Urology. 2004;63:828–33. doi: 10.1016/j.urology.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Williams JC, Jr., Matlaga BR, Kim SC, Jackson ME, Sommer AJ, McAteer JA, et al. Calcium oxalate calculi found attached to the renal papilla: Preliminary evidence for early mechanisms in stone formation. J Endourol. 2006;20:885–90. doi: 10.1089/end.2006.20.885. [DOI] [PubMed] [Google Scholar]