Summary
Production of IL-1β typically requires two-separate signals. The first signal, from a pathogen-associated molecular pattern, promotes intracellular production of immature cytokine. The second signal, derived from a danger signal such as extracellular ATP, results in assembly of an inflammasome, activation of caspase-1, and secretion of mature cytokine. The inflammasome component, Nalp3, plays a nonredundant role in caspase-1 activation in response to ATP binding to P2X7 in macrophages. Gingival epithelial cells (GECs) are an important component of the innate-immune response to periodontal bacteria. We had shown that GECs express a functional P2X7 receptor, but the ability of GECs to secrete IL-1β during infection remained unknown. We find that GECs express a functional Nalp3-inflammasome. Treatment of GECs with LPS or infection with the periodontal pathogen, Porphyromonas gingivalis, induced expression of the il-1β gene and intracellular accumulation of IL-1β protein. However, IL-1β was not secreted unless LPS-treated or infected cells were subsequently stimulated with ATP. Conversely, caspase-1 is activated in GECs following ATP-treatment but not P. gingivalis-infection. Furthermore, depletion of Nalp3 by siRNA abrogated the ability of ATP to induce IL-1β secretion in infected-cells. The Nalp3-inflammasome is therefore a likely to be an important mediator of the inflammatory response in gingival epithelium.
Keywords: NLR, innate immunity, purinergic receptor, inflammasome, bacteria
Introduction
Within the gingival compartment, epithelial cells are among the first host-cells encountered by periodontal pathogens such as Porphyromonas gingivalis. In addition to constituting a physical barrier to invasion by microbial pathogens, epithelial cells can sense and respond to the presence of bacteria through stimulation of pathogen recognition receptors (PRRs) such as Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (Meylan et al., 2006; Inohara et al., 2005; Janeway Jr. and Medzhitov, 2002). Consequently, gingival epithelial cells (GECs) are an important component of the innate host response to periodontal bacteria and make a significant contribution to the gingival health of the host (Eskan et al., 2008; Hasegawa et al., 2008; Yilmaz, 2008; Darveau et al., 1998)
Most studies on the innate immune response against bacterial infections have focused on immune cells of hematopoietic origin, but epithelial cells infected by P. gingivalis and other oral bacteria can also produce proinflammatory cytokines, chemokines and defensins, and upregulate cell adhesion molecules (Hasegawa et al., 2008; Eick et al., 2006; Huang et al., 2004; Kusumoto et al., 2004; Asai et al., 2001; Scannapieco et al., 2001; Krisanaprakornkit et al., 2000; Sandros et al., 2000a; Darveau et al., 1998; Darveau et al., 1997). P. gingivalis is a successful host-adapted self-limiting intracellular bacterium that is capable of intracellular replication and can maintain viability for extended periods of time. Furthermore, P. gingivalis infection promotes an anti-apoptotic phenotype in primary GECs by rendering the host cells resistant to cell death induced by potent pro-apoptotic agents ((Mao et al., 2007; Yilmaz et al., 2004; Nakhjiri et al., 2001). Although much is known about virulence factors produced by P. gingivalis (Hajishengallis et al., 2008; Hintermann et al., 2002; Michalek et al., 2002; Nisapakultorn et al., 2001; Katz et al., 2000; Curtis et al., 1999; Holt et al., 1999; Lamont and Jenkinson, 1998; Cutler et al., 1995), recognition of the bacteria by PRRs on GECs, or the pathogen’s ability to subvert an immune response, remain poorly understood.
Ligation of surface-exposed TLRs is sufficient for transcription, synthesis, and secretion of cytokines such as IL-8, IFNγ and IL-12 (Takeda et al., 2003; Janeway Jr. and Medzhitov, 2002). But given the key role played by IL-1β and IL-18 in fever and inflammatory disease (Dinarello, 2006; Ferrari et al., 2006), their production and secretion are tightly controlled, requiring typically two separate signals (Ferrari et al., 2006; Lich et al., 2006; Mariathasan et al., 2006; Meylan et al., 2006; Kahlenberg et al., 2005). The first signal, from a pathogen-associated molecular pattern (PAMP) such as flagellin or lipopolysaccharide (LPS), promotes production and intracellular accumulation of the immature cytokines. The second signal, usually derived from a “danger signal” (DS) such as ATP, results in processing and secretion of the mature cytokines. Danger signals released from infected, dying or stressed cells interact with “danger signal receptors” (DSRs) on neighboring cells to induce/alter the host response (Lich et al., 2006; Tournier and Quesnel-Hellmann, 2006; Lotze and Tracey, 2005; Sitkovsky and Ohta, 2005; Seong and Matzinger, 2004; Matzinger, 2002). Most studies on DSRs have focused on immune effector cells of hematopoietic origin (Ferrari et al., 2006; Lich et al., 2006; Meylan et al., 2006), but we find that at least one DSR, the purinergic receptor for extracellular ATP, called P2X7 (Lich et al., 2006; Ferrari et al., 1997), is also expressed and is functional on GECs (Yilmaz et al., 2008). Moreover, P. gingivalis, a successful intracellular opportunistic bacterium, can inhibit GEC apoptosis induced by ATP ligation of P2X7 (Yilmaz et al., 2008).
Recent studies show that DSs and PAMPs can synergize in promoting IL-1β secretion and inflammation. Thus, the NLR family member, Nalp3 (also known as cryopyrin or NLRP3) (Ting et al., 2008), is activated in response to stimulation of cells with extracellular ATP, some microbial products, or gout-associated uric acid crystals (Kanneganti et al., 2006; Lich et al., 2006; Martinon et al., 2006; Sutterwala et al., 2006; Mariathasan et al., 2004). Nalp3 then activates the protease caspase-1, which cleaves pro-IL-1β or pro-IL-18, resulting in their secretion. Hence, a pathogen must both express a PAMP capable of driving cytokine synthesis, and be viewed as potentially dangerous to the host organism, in order for mature IL-1β and IL-18 to be secreted efficiently.
We have observed that GECs express Nalp3, and that cells infected with P. gingivalis transcribe the gene encoding IL-1β. However, infected GECs do not secrete IL-1β protein. We therefore explored the possibility that P2X7 ligation by ATP, through its ability to activate a Nalp3 inflammasome in GECs, may stimulate processing and secretion of IL-1β during P. gingivalis infection.
Results
GECs express components of an inflammasome
We first verified that P. gingivalis infection of GECs leads to transcription of the il-1β gene by real-time PCR, using primers specific for human il-1β. In fact, the expression of the il-1β gene increased by a factor of 1.95 ± 0.04 (P < 0.001) after a 6 hr infection of primary GECs with P. gingivalis 33277 at a multiplicity of infection (MOI) of 100 (Fig. 1A), compared to basal levels. These results are consistent with a previous report using oligonucleotide arrays of host-cell genes, which showed a two-fold upregulation of the il-1β gene after a 30 min of P. gingivalis infection, and significant (P < 0.001) upregulation of il-1β transcription after a 2 hrs of infection (Handfield et al., 2005; Sandros et al., 2000b). These results imply that P. gingivalis produces at least the PAMPs necessary for inducing il-1β gene transcription in GECs.
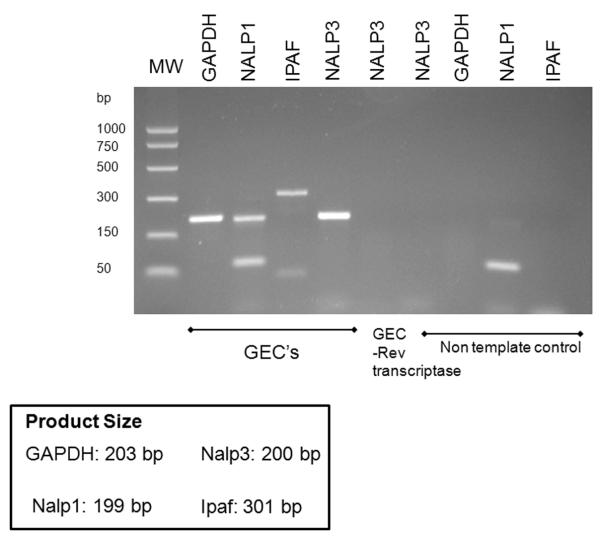
Figure 1.

Primary GECs express mRNA for NLR family members and P. gingivalis infection stimulates the il-1β gene transcription.
(A) il-1β gene expression was measured by quantitative, real time PCR (qPCR) from GECs infected with P. gingivalis at an MOI = 100 for 6 hrs, and uninfected (control). GAPDH was included as an internal control. Fold change was calculated by the comparative cycle threshold method. P < 0.001 for cells uninfected, control (*), compared with cells infected with P. gingivalis (**). Data are representative of two independent experiments performed in duplicates.
(B) PCR amplification was carried out with primers specific for Nalp3, Nalp1 and Ipaf, as described in Experimental Procedures. An amplicon of the expected size (200 bp) was found for Nalp3. The control, without primers (NTC), did not produce a detectable band.
In addition, by RT-PCR, we find that primary GECs express the Nalp3 gene at high levels (Fig. 1B). PCR amplification was carried out with primers specific for human Nalp3 (amplicon expected size, 200 bp). The RT-PCR control, without reverse transcriptase, did not produce a detectable band. Primary GECs also express the NLR family members Nalp1 and Ipaf (Fig. 1B). In humans, Nalp1 is a PRR for cytosolic peptidoglycan, while Ipaf is mainly a PRR for cytosolic flagellin. Thus primary GECs could potentially stimulate any of these three inflammasomes.
We had previously shown that a functional purinergic receptor, P2X7, is expressed on primary GECs and P. gingivalis can inhibit the GEC apoptosis induced by ATP ligation of P2X7 (Yilmaz et al., 2008). Furthermore, only ligation of P2X7 by ATP is known to stimulate activation of the Nalp3 inflammasome and caspase-1, resulting in processing and secretion of the IL-1β protein, as demonstrated by studies with macrophages deficient in P2X7 (Ferrari et al., 2006; Mariathasan et al., 2006). We therefore evaluated whether GECs may be capable of secreting IL-1β following stimulation of either LPS-primed or P. gingivalis-infected GECs with ATP.
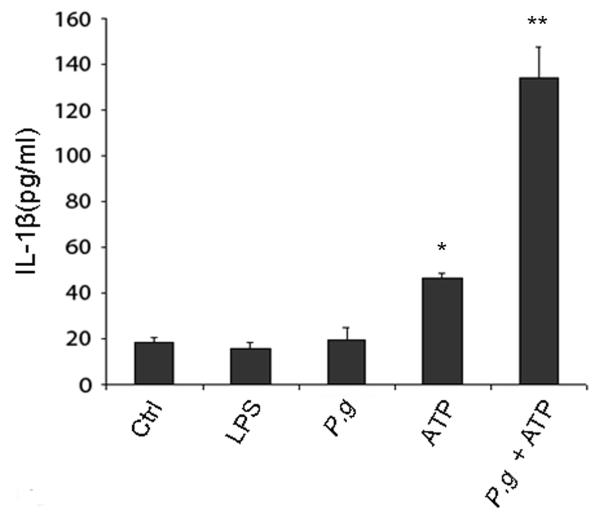
Unless P. gingivalis can provide its own DS in addition to PAMPs, there should be no IL-1β secretion following infection alone. Consistent with this possibility, we find that primary GECs do not secrete any IL-1β (above basal levels) following either treatment with E. coli LPS for 6 hrs or P. gingivalis infection for 6 hrs (Fig. 2). There was a small, reproducible increase in IL-1β secretion when uninfected GECs were treated with 5 mM ATP for 3 hrs. However, a high level of IL-1β secretion was observed only in GECs that were infected with P. gingivalis for 6 hrs, followed by incubation with ATP for 3 hrs (Fig. 2).
Figure 2.
Primary GECs express a functional inflammasome. IL-1β secretion was measured in supernatants from GECs incubated with control buffer (Ctrl), incubated with 2 μg/ml of E. coli LPS for 6 hrs, infected with P. gingivalis (P.g.) at an MOI = 100 for 6 hrs, incubated with 5 mM ATP for 3 hrs, or infected with P. gingivalis for 6 hrs and then incubated for an additional 3 hrs with 5 mM ATP. IL-1β concentrations were quantified with an IL-1β ELISA kit. The values show averages and S.D. from 3 samples of a representative experiment, and represent results obtained from at least three experiments. P < 0.05 for cells infected with P. gingivalis and cells treated with ATP (*), compared with cells infected with P. gingivalis and then incubated with ATP (**).
Among several inflammasomes that have been characterized, only the Nalp3 inflammasome can be activated by extracellular ATP through P2X7 ligation (Pétrilli et al., 2007). These results therefore suggest that GECs express a functional Nalp3 inflammasome. Furthermore, since P. gingivalis infection by itself does not induce IL-1β secretion, the results imply that P. gingivalis does not activate to a significant level either the Nalp1 or Ipaf inflammasome.
GEC infection by P. gingivalis leads to production of intracellular cytokine
IL-1β is first synthesized as pro-IL-1β, which is cleaved by caspase-1 into mature IL-1β. In order to rule out the possibility that ATP may be stimulating synthesis of pro-IL-1β, instead of simply promoting secretion of mature IL-1β protein, we also measured intracellular accumulation of IL-1β by immunofluorescence microscopy, using antibodies against IL-1β protein. This antibody can not distinguish between pro-IL-1β or processed IL-1β, but nonetheless allowed us to quantify the total amount of cytokine (pro-IL-1β and IL-1β) that had accumulated within the cell.
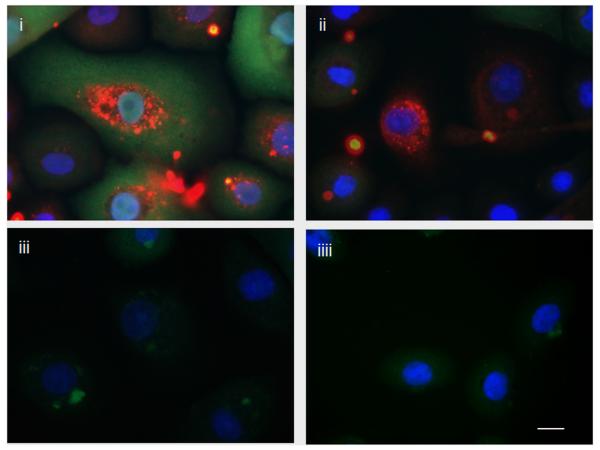
GECs were mock-infected or infected with P. gingivalis for 6 hrs, and ATP or control buffer was added for an additional 3 hrs. As shown in Fig. 3, there is no measurable IL-1β in uninfected cells treated with control buffer (Fig. 3A-iiii) or uninfected cells treated with ATP for 3 hrs (Fig.3A-iii). However, there is a large increase in IL-1β staining in GECs infected with P. gingivalis (Fig. 3A-i). The IL-1β staining is visibly lower in infected GECs that were subsequently incubated with ATP, indicating the release of accumulated IL-1β from infected GECs into the extracellular medium (Fig. 3A-ii).
Figure 3.
Cytokine is produced intracellularly during P. gingivalis infection but is released from cells following ATP treatment.
(A) Intracellular cytokine (pro-IL-1β and IL-1β) was detected by immunofluorescence using antibodies against IL-1β (green). The samples were also stained with P. gingivalis antibody (red) and DAPI (blue) to visualize the nuclei. (i) There is a large increase in IL-1β staining in GECs infected with P. gingivalis at an MOI = 100 for 6 hrs. (ii) Most cytokine is released from cells that had been infected with P. gingivalis for 6 hrs and then incubated with 5 mM ATP for an additional 3 hrs. (iii) There is very little cytokine in GECs incubated with 5 mM ATP (iiii) or control buffer for 3 hrs. The images are representative of 150 cells studied per sample from at least two separate experiments performed in duplicate. Bar 10 μm.
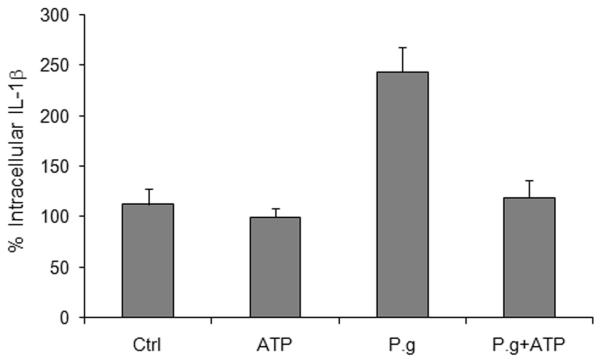
(B) Images of cells prepared as in Fig. 3A were captured with a fluorescence microscope equipped with a cooled CCD. The levels of intracellular cytokine (pro-IL-1β and IL-1β) in cells incubated with control buffer (Ctrl), treated with 5 mM ATP for 3 hrs, infected with P. gingivalis at an MOI = 100 for 6 hrs (P.g.), or infected with P. gingivalis for 6 hrs and then treated with ATP for 3 hrs, were quantified by measuring relative fluorescence with NIH ImageJ analysis software, as described in Experimental Procedures. Data were expressed as a percentage of the fluorescence intensity of the control samples. Results are representative images of 150 cells studied per sample from at least two individual experiments performed in duplicate. The values show averages and S.D. from a representative experiment. P = 0.05 for cells infected with P. gingivalis, compared with cells infected with P. gingivalis and then incubated with ATP.
We quantified the extent of ATP-induced release of IL-1β from infected cells by measuring relative fluorescence staining with NIH ImageJ analysis software, as we had previously done for quantifying P. gingivalis infection (Yilmaz et al., 2006). Thus, ATP alone, which is not thought to induce cytokine production on its own (Petrilli et al., 2007; Ferrari et al., 2006), has no effect on intracellular IL-1β staining in GECs, compared to untreated controls (Fig. 3B). However, a 6 hr infection with P. gingivalis resulted in a large increase in intracellular IL-1β staining. The intracellular IL-1β levels decreased substantially in infected cells that were then treated with ATP for 3 hrs (Fig. 3B).
Given that the concentration of IL-1β in the supernatant of P. gingivalis-infected GECs increased significantly after ATP treatment (Fig. 2), these results strongly suggest that P. gingivalis infection stimulates production of pro-IL-1β and its intracellular accumulation, but ATP induces release of IL-1β from the infected cells. This interpretation is also supported by the observation that addition of ATP to GECs infected with P. gingivalis does not further augment the two-fold increase in il-1β gene transcription due to infection alone (not shown).
Nalp3 depletion inhibits ATP-dependent IL-1β secretion from P. gingivalis infected GECs
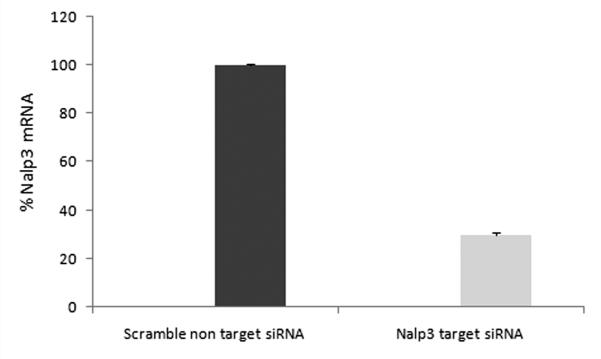
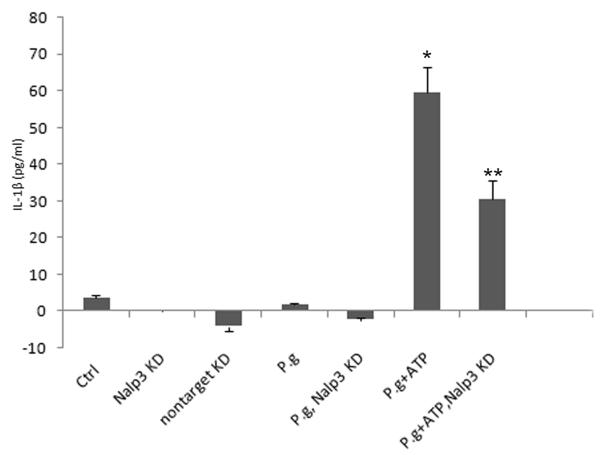
Our results suggest that P. gingivalis infection of GECs leads to intracellular accumulation of pro- IL-1β, but the cytokine is not secreted unless the infected cells are subsequently treated with extracellular ATP, presumably via P2X7 ligation. P2X7-mediated inflammasome activation in macrophages requires participation of a Nalp3 inflammasome. Therefore, we investigated the potential involvement of Nalp3 in IL-1β secretion by depleting Nalp3 in GECs through RNA interference. As shown in Fig. 4A, Nalp3 expression in GECs was decreased by more than half in GECs treated with Nalp3-specific siRNA, while transfection with nontarget siRNA had no effect. Depletion of Nalp3 had no effect on IL-1β secretion from primary GECs treated with LPS (not shown) or infected with P. gingivalis. However, there was a significant effect of Nalp3 depletion on IL-1β secretion from cells that were infected with P. gingivalis and subsequently stimulated with ATP (Fig. 4B). The results demonstrate that activation of a Nalp3 inflammasome is required for secretion of most of the IL-1β in P. gingivalis-infected cells treated with ATP.
Figure 4.
Depletion of Nalp3 abrogates the ability of ATP to induce IL-1β secretion from P. gingivalis-infected GECs.
(A) Nalp3 gene expression was transiently silenced in primary by siRNA transfection, as described in Experimental Procedures. The expression levels for each condition were analyzed by qRT-PCR using the Ct method, and were represented as percentages of the non-target siRNA sample normalized to 100. The knockdown experiments were repeated at least three separate times demonstrating consistently ~ 70% decrease in the Nalp3 gene transcript in each time.
(B) Primary GECs were treated with Nalp3 or non-target siRNA reagents, and IL-1β secretion from Nalp3 knockdowns (KD) and controls was measured by ELISA. The cells infected with P. gingivalis (P.g.) with or without transfection with siRNA (Nalp3 KD) did not produce any noticeable IL-1β secretion. On the other hand, depletion of Nalp3 in cells infected with P. gingivalis and then incubated with ATP (Nalp3 KD + P.g. + ATP) resulted in significant inhibition in secretion of IL-1β, compared with GECs that were not treated with siRNA (P.g. + ATP). The values show averages and S.D. from duplicate samples of a representative experiment, and show results obtained from at least 2 separate experiments. P = 0.05 for cells infected with P. gingivalis and then incubated with ATP (P.g. + ATP *), compared with cells that were transfected with Nalp3 siRNA, infected with P. gingivalis, and then incubated with ATP (P.g. + ATP, Nalp3 KD **).
ATP treatment stimulates activation of caspase-1 in GECs
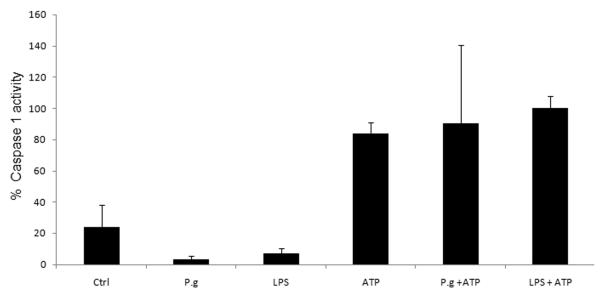
We further examined a role for an inflammasome by measuring caspase-1 activation in LPS-primed or P. gingivalis-infected GECs. Caspase-1 activation was measured by incubating LPS-treated or infected cells with the caspase-1 substrate, FAM-YVAD-FMK, whose relative fluorescence increases inside the cells that have activated caspase-1 (Thornberry et al., 1997). Neither 6 hr treatment with LPS nor 6 hr infection with P. gingivalis had a measurable effect on caspase-1 activation in primary GECs (Fig. 5). However, subsequent treatment with ATP for 3 hrs induced a large increase in caspase-1 activation. Incubation with ATP by itself, in the absence of LPS treatment or P. gingivalis infection, also induced caspase-1 activation (Fig. 5). Since extracellular ATP does not activate caspase-1 in macrophages that are deficient in Nalp3 (Pétrilli et al., 2007; Mariathasan et al., 2006) — and extracellular ATP has never been shown to activate inflammasomes containing NLRs other than Nalp3 — these results are consistent with ATP-mediated assembly of a Nalp3 inflammasome in GECs, which leads to caspase-1 activation. This interpretation is supported by our observation that ATP does not stimulate IL-1β secretion from infected GECs in Nalp3-deficient GECs (Fig. 4B). The results also suggest that caspase-1 is not activated in the cells in which Nalp3 had been depleted by siRNA.
Figure 5.
Stimulation with ATP activates caspase-1 in GECs. Primary GECs were infected with P. gingivalis at an MOI = 100 for 6 hrs (P.g.), treated with 2 μg/ml of E. coli LPS for 6 hrs, incubated with 5 mM ATP for 3 hrs, or incubated with 5 mM ATP for 3 hrs after 6 hrs of infection or LPS-treatment. Caspase-1 activation was measured by incubating treated or infected cells with a fluorescent caspase-1 substrate, as described in Experimental Procedures. LPS treatment or P. gingivalis infection have no effect on caspase-1 activity, but subsequent treatment with ATP induces a large increase in caspase-1 activation. The values show percent averages and S.D. from 3 samples of a representative experiment, and represent results obtained from at least three experiments. P = 0.02 for cells infected with P. gingivalis, compared with cells infected with P. gingivalis and then incubated with ATP.
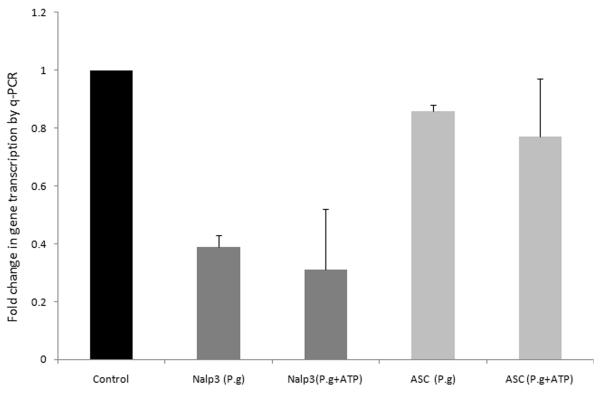
Since a previous study of P. gingivalis infection in a monocytic cell line showed upregulation of Nalp3 during infection (Bostanci et al., 2009), we examined whether infection of primary GECs may have sensitized the cells to ATP-induced caspase-1 activation. Unexpectedly, expression of the Nalp3 gene decreases partially after P. gingivalis infection (Fig. 6). Infection by P. gingivalis had no effect on expression of the inflammasome adaptor protein, ASC, and ATP had no additional effect on Nalp3 or ASC expression in the infected GECs (Fig. 6). The results suggest that Nalp3 downregulation by P. gingivalis in GECs, by itself, does not have measurable consequences for Nalp3 inflammasome activity, since ATP could activate caspase-1 as efficiently in uninfected cells as in infected cells (Fig. 5). Further depletion by siRNA is required for a decrease in inflammasome activity (Fig. 4).
Figure 6.
The gene expression levels of Nalp3 but not ASC decreased partially after P. gingivalis infection. GECs were uninfected and untreated (control), infected with P. gingivalis at an MOI of 100 for 6 hrs, or infected and treated for an additional 3 hrs with 5 mM ATP. The expression levels of Nalp3 and ASC were compared with GAPDH and normalized to uninfected GEC responses, as described in Experimental Procedures. Data are representative of two independent experiments performed in duplicates.
Discussion
Ligation of a PRR by a PAMP is sufficient for transcription and synthesis of pro-IL-1β in macrophages. However, a second signal is needed in most cases for processing and secretion of the mature cytokine, IL-1β (Ferrari et al., 2006; Lich et al., 2006; Mariathasan et al., 2006; Meylan et al., 2006; Kahlenberg et al., 2005). Processing of pro-IL-1β requires activation of caspase-1, which cleaves pro-IL-1β into IL-1β.
Recently, the molecular machinery behind caspase-1 activation has been revealed. Two caspase-1 molecules are recruited to a macromolecular complex containing an NLR family member and the adaptor protein, ASC, to form the “inflammasome”. This leads to the activation of caspase-1 and subsequent IL-1β and IL-18 maturation. To date, four different inflammasomes have been characterized, containing the NLRs: Nalp1, Nalp2, Nalp3, and Ipaf (Lamkanfi et al., 2007; Pétrilli et al., 2007).
The human Nalp1 inflammasome is activated by cytosolic muramyl-dipeptide, a degradation product of peptidoglycan (Martinon et al., 2002). The Nalp2 inflammasome has been assembled in vitro, but the activators are still unknown (Bruey et al., 2004).
The Nalp3 inflammasome is ASC dependent and, as noted above, requires two signals. First a PAMP binds to its PRR (mainly TLRs); and second, a danger signal, for example ATP ligation to its purinergic receptor P2X7, stimulates formation of the Nalp3 inflammasome and activation of caspase-1 (Lich et al., 2006; Martinon et al., 2006).
The Ipaf inflammasome is activated during S. typhimurium and L. pneumophila infections. Studies have shown that flagellin from these bacteria are recognized by Ipaf, which can lead to activation of caspase-1 independently of Nalp3 (Creagh and óNeill, 2006; Meylan et al., 2006). Furthermore, Ipaf-dependent activation of caspase-1 can take place in the absence of surface TLRs such as TLR5, which recognizes extracellular flagellin (Franchi et al., 2006; Miao et al., 2006). Thus, ligation of only TLR5 initiates synthesis of pro-IL-1β but not its secretion, whereas Ipaf ligation leads to both cytokine synthesis and its processing by caspase-1 (Franchi et al., 2006; Miao et al., 2006). Clearly, the presence of flagellin in the cytosol is sufficient for the immune system to recognize this PAMP as being dangerous.
The role played by the Nalp1, Nalp2, Nalp3 or Ipaf inflammasomes in cytokine secretion by epithelial cells infected with oral pathogens has never been investigated. The function of NLRs has also been characterized mainly in macrophages. Given that epithelial cells represent the preferred target host-cell for many pathogens and that P. gingivalis infects GECs, we have turned our attention to the possibility that primary GECs may express a functional inflammasome.
We have recently shown that primary GECs express a functional P2X7 receptor (Yilmaz et al., 2008), which binds extracellular ATP. The source of the extracellular ATP remains to be shown, but is thought to be released from stressed or infected cells in oral tissue. P. gingivalis is found to modulate the P2X7 activity and protect the host cells against cell death maximally around 60 hrs of infection, which in turn may also aid the organism’s intracellular survival in the gingival epithelial tissues (Yilmaz et al., 2008). In this report, we show that infection of GECs with P. gingivalis stimulates transcription of the il-1β gene and production of intracellular pro-IL-1β, but the cytokine is not secreted unless the infected cells are treated subsequently with ATP. Moreover, Nalp3 depletion by RNA interference abrogated partially the ability of ATP to induce IL-1β secretion in P. gingivalis infected cells. Taken together, these results suggest that primary GECs indeed express a functional Nalp3 inflammasome. Although inflammasomes containing other NLR members have been characterized, none of them is sensitive to extracellular ATP (Pétrilli et al., 2007). Since infection by itself did not stimulate IL-1β secretion, the results therefore imply that P. gingivalis can not activate the Ipaf, Nalp1 or Nalp2 inflammasomes. Consistent with our results, the first histological characterization of the distribution of the Nalp3 protein in different tissues shows that Nalp3, unlike Nalp1, is expressed in epithelial cells of the oral cavity (Kummer et al., 2007).
Mutations in the Nalp3/cryopyrin inflammasome cause hereditary inflammatory diseases in humans. The most common of these disorders are autoimmune and are considered gain-of-function mutations. The diseases are marked by skin rashes and prolonged episodes of fever, including Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and neonatal-onset multisystem inflammatory disease, and are collectively known as the Cryopyrin-Associated Periodic Syndromes (CAPS) (Kanneganti et al., 2007; Martinon and Tschopp, 2004). Studies have shown that mutations involved in CAPS result in enhanced caspase-1 activation and spontaneous IL-1β and IL-18 secretion (Kanneganti et al., 2007; Agostini et al., 2004). Confirmation of the mechanism of disease is revealed by the effectiveness of the treatment of CAPS using an IL-1 receptor antagonist (Kanneganti et al., 2007).
While the role of Nalp3 mutations in periodontal disease have not been investigated until now, a correlation has been observed between IL-1β single-nucleotide polymorphisms and periodontal disease (Akman et al., 2008). Significantly, higher levels of IL-1β have also been detected in saliva from patients with periodontitis, compared to healthy controls (Tobón-Arroyave et al., 2008), suggesting a role for this cytokine in development of disease. Future studies are thus likely to also uncover a role for Nalp3 and IL-1β polymorphisms in periodontal disease. As mutations of Nalp3 and P2X7 are associated with autoinflammatory disease or cancer, they should allow us to identify genetic markers for host susceptibility to periodontitis.
Experimental Procedures
Bacteria and cell culture
P. gingivalis ATCC 33277 was cultured anaerobically for 24 hrs at 37°C in trypticase soy broth supplemented with yeast extract (1 mg ml−1), haemin (5 mg ml−1) and menadione (1 mg ml−1). Bacteria were grown for 24 hrs, harvested by centrifugation at 6000 g and 4°C for 10 min, washed twice, and resuspended in Dulbecco’s Phosphate-buffered saline (PBS, from Sigma), pH 7.3, before incubation with host cells (Yilmaz et al., 2008). Bacteria were quantified using a Klett-Summerson photometer.
Primary GECs were obtained after oral surgery from healthy gingival tissue as previously described (Yilmaz et al., 2004). Cells were cultured as monolayers in serum-free keratinocyte growth medium (KGM) (Lonza) at 37°C in 5% CO2. GECs were used for experimentation at 75–80% confluence and cultured for 48 hrs before infection with bacterial cells or exposure to other test reagents in KGM.
Analysis of mRNA expression for Nalp3 and the other NLRs
In order to determine whether GECs express the Nalp3 (Nlrp3), Nalp1 (Nlrp1) and Ipaf (Nlrc4) genes, PCR amplification was carried out with primers specific for the human genes. The sequences of the primers used were as follows. Nalp3: forward primer, 5′ CTTCTCTGATGAGGCCCAAG 3′; reverse primer, 5′ GCAGCAAACTGGAAAGGAAG 3′ (amplicon expected size, 200 bp). Nalp1: forward primer, 5′-ACCTGATCCCAAGTGACTGC-3′, reverse primer, 5′-TCTTCTCCAGGGCTTCGATA-3′. And Ipaf: forward primer 5′-CTCTCATGGTGGAAGCCAGTCC-3′, reverse primer 5′-GACAGAGACTTGACTATGTAATCC-3′.
The PCR cycling protocol for all primers was 94°C at 5 sec, 55°C at 5 sec, and 68°C at 15 sec. The protocol was repeated for 45 cycles and included an initial 5-min enzyme activation step at 94°C and a final 10-min extension step at 72°C. PCR products were separated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining.
Measurement of IL-1β, Nalp3, and ASC gene transcription
Transcription of the il-1β, nalp3, and asc genes was measured by quantitative, real-time PCR (qPCR), using primers specific for human il-1β, nalp3, and asc and an Mx3000P (Stratagene) instrument. Briefly, GECs were infected with P. gingivalis 33277 at an MOI of 100 for 6 hrs. RNA was prepared and 2 μg per sample were reverse-transcribed from uninfected, infected, and infected and 5 mM ATP treated GECs, as described (Darville et al., 2003). Quantitative PCR was performed with 1/50 of the cDNA preparation in the Mx3000P (Stratagene) in 25 μl final volumes with the Brilliant QPCR Master Mix (Stratagene). cDNA was amplified using 200 nM of each specific sense and antisense primers. Quantitative PCR was conducted at 95°C for 10 min, followed by 40 cycles at 95°C for 30 sec, 55°C for 1 min and 72°C for 30 sec. The expression levels of cytokine cDNA and Nalp3, ASC were compared with GAPDH and normalized to uninfected GEC responses by the comparative cycle threshold method, as described by the manufacturer (Stratagene). The primers for the genes coding IL-1β, Nalp3, ASC, and GAPDH were as follows. For human IL-1β: 5′ CAGCCAATCTTCATTGCTCA 3′ (forward), 5′ TCGGAGATTCGTAGCTGGAT 3′ (reverse). For human Nalp3: 5′ CTTCCTTTCCAGTTTGCTGC 3′ (forward), 5′ TCTCGCAGTCCACTTCCTTT 3′ (reverse). For human ASC: 5′ AGTTTCACACCAGCCTGGAA 3′ (forward), 5′ TTTTCAAGCTGGCTTTTCGT 3′ (reverse). For human GAPDH: 5′ AACGGATTTGGTCGTATTGGGC 3′ (forward), 5′ CTTGACGGTGCCATGGAATTTG 3′ (reverse).
Depletion of Nalp3 by RNA interference
Primary cultures of GECs at 50% confluence were transfected in GEC growth media using 125 nM multiple short interfering RNA (siRNA) duplexes in 3 μl siRNA DharmaFECT1 agent (Dharmacon). Briefly, 3 μl transfection agent was added drop-wise into 100 μl of GEC growth media. After gentle mixing, the incubation was performed for 10 min at room temperature. Then 125 nM siRNA Nalp3 sequences were added to diluted transfection agent, mixed gently, and incubated for 10 min at room temperature. Finally, 195 μl of this mixture was added to each well, the plate was rocked gently, and further incubated for 48 hrs at 37 °C 5% CO2. The sequences of the siRNA for Nalp3 consisted of the following mixture: 5′-GGAUCAAACUACUCUGUGAUU-3′ UUCCUAGUUUGAUGAGACACU 5′-UGCAAGAUCUCUCAGCAAAUU-3′ UUACGUUCUAGAGAGUCGUUU 5′-GAAGUGGGGUUCAGAUAAUUU-3′ UUCTTCACCCCAAGUCUAUUA 5′-GCAAGACCAAGACGUGUGAUU-3′ UUCGUUCUGGUUCUGCACACU Non-target pool siRNA (Dharmacon) and transfection agent alone were used as negative controls. The qRT-PCR analysis was performed up to 48 hrs post-transfection to confirm the Nalp3 gene knockdown. The expression level for the each condition was normalized to housekeeping gene GAPDH using the comparative cycle threshold (Ct) method. The fold changes were calculated as 2−ΔΔCt, by comparing target siRNA with non-target siRNA (normalized to 1) from the ΔΔCt value. Results were then expressed as percentages.
Measurement of cytokine secretion by ELISA
GECs were either treated with 2 μg/ml of Escherichia coli LPS (InvivoGen) for 6 hrs or infected with P. gingivalis (MOI = 100) for 6 hrs. Some cells were subsequently treated with 5 mM ATP in cell culture medium for an additional 3 hrs. Cell culture supernatants from LPS-treated, P. gingivalis-infected or control GECs were collected and assayed for cytokine activity by ELISA using a commercial cytokine ELISA kit for IL-1β (BD Biosciences Pharmingen).
Analysis of intracellular cytokine production by fluorescence microscopy
Intracellular accumulation of IL-1β protein (pro-IL-1β and IL-1β) was visualized by immunofluorescence microscopy, using antibodies against IL-1β protein (R&D Systems). GECs growing on 4-well chambered glass slides (Nalge-Nunc International) were infected with P. gingivalis at an MOI of 100 for 6 hrs, and 5 mM ATP or control buffer was added for an additional 3 hrs. Infected cells were fixed in 10% neutral buffered formalin for 20 min. The cells were permeabilized for 10 min with 0.1% Triton X-100, and the slides were incubated with fluorescein-conjugated anti-IL-1β monoclonal antibody at a 1:100 dilution for 1 hr at room temperature. The samples were incubated with anti-P. gingivalis 33277 antibody and reacted simultaneously with Alexa Fluor 633 secondary antibody (Invitrogen) (Yilmaz et al., 2008). Samples with no primary antibody incubation were included as control. The samples were treated with 4,6-diamidino-2-phenylindole (DAPI) 1 mg ml−1 (Sigma) to visualize the nuclei. After washing, the slides were mounted in Vectashield mounting medium and examined with an epifluorescence microscope. The images were captured with a Zeiss Axio imager A1 fluorescence microscope equipped with a cooled CCD camera (Qimaging) controlled by QCAPTURE software v.1394. The levels of intracellular IL-1β in infected and uninfected cells were quantified by measuring the intensity of fluorescence emitted from the acquired images with NIH ImageJ analysis software, as previously done for quantifying P. gingivalis infection (Yilmaz et al., 2006). Briefly, the threshold of relative fluorescence intensity was determined with samples that were uninfected and untreated based on pixel intensity in each field of interest. The results were then expressed as a percentage of the fluorescence intensity, compared to the control samples. The same microscopy settings were employed throughout all samples.
Measurement of caspase-1 activity
GECs were treated with 2 μg/ml of E. coli LPS or control buffer, or infected with P. gingivalis (MOI = 100) for 6 hrs. LPS-treated, infected, or control cells were then incubated for an additional 3 hrs with 5 mM ATP or control buffer. Caspase-1 activation was measured by incubating cells with the caspase-1 substrate, FAM-YVAD-FMK at a 1:30 dilution (Immunochemistry Technologies), whose relative fluorescence increases inside whole living cells that have activated caspase-1 (Thornberry et al., 1997). Specificity of substrate binding to caspase-1 was confirmed by pretreating the cells with excess of nonfluorescent z-YVAD,FMK, before addition of FAM-YVAD-FMK. The fluorescence increase in cells with activated caspase-1 was measured with a 96-well fluorescence plate reader (excitation at 488 nm, emission at 520 nm with black microtiter plates). Caspase-1 activation was quantified as the amount of green fluorescence emitted from the FLICA probes bound to the enzyme. The relative fluorescence intensities were obtained by subtracting the value of the sample with the smallest fluorescence intensity from each sample. The results were then expressed as percentages of the sample with highest intensity normalized to 100.
Statistical analysis
Student’s t-test was used to calculate the statistical significance of the experimental results between two conditions (significance at P < 0.05). The uninfected cell populations were rejected from the analysis.
Acknowledgments
This study was supported by NIDCR grants R01DE016593 and R01DE019444. We also thank Dr. Chulhee Choi, University of Florida, and Department of Periodontology for his kind assistance.
References
- Agostini L, Martinon F, Burns K, McDermott MF, P.N. H, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Akman A, Ekinci NC, Kacaroglu H, Yavuzer U, Alpsoy E, Yegin O. Relationship between periodontal findings and specific polymorphisms of interleukin1alpha and -1beta in Turkish patients with Behçet’s disease. Arch. Dermatol. Res. 2008;300:19–26. doi: 10.1007/s00403-007-0794-1. [DOI] [PubMed] [Google Scholar]
- Asai Y, Ohyama Y, Gen K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect. Immun. 2001;69:7387–7395. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, Belibasakis GN. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157:415–422. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J. Biol. Chem. 2004;279:51897–51907. doi: 10.1074/jbc.M406741200. [DOI] [PubMed] [Google Scholar]
- Creagh EM, O’Neill LAJ. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kuramitsu HK, Lantz M, Macrina FL, Nakayama K, Potempta J, et al. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J. Periodontal Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Kalmar JR, Genco CA. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- Darveau R, Belton CM, Reife R, Lamont RJ. Local chemokine paralysis: a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Darville T, O’Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 2003;171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- Eick S, Reissmann A, Rodel J, Schmidt KH, Pfister W. Porphyromonas gingivalis survives within KB cells and modulates inflammatory response. Oral Microbiol. Immunol. 2006;21:231–237. doi: 10.1111/j.1399-302X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Benakanakere MR, Rose BG, Zhang P, Zhao J, Stathopoulou P, et al. Interleukin-1β modulates proinflammatory cytokine production in human epithelial cells. Infect. Immun. 2008;76:2080–2089. doi: 10.1128/IAI.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MKA, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65 (RelA) J. Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nature Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Shakhatreh MA, James D, Nishiyama S, et al. Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Adv Exp Med Biol. 2008;632:203–219. doi: 10.1007/978-0-387-78952-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, et al. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell. Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Tribble GD, Baker HV, Mans JJ, Handfield M, Lamont RJ. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76:2420–2427. doi: 10.1128/IAI.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann E, Haake SK, Christen U, Sharabi A, Quaranta V. Discrete proteolysis of focal contact and adherens junction components in Porphyromonas gingivalis-infected oral keratinocytes: a strategy for cell adhesion and migration disabling. Infect. Immun. 2002;70:5846–5856. doi: 10.1128/IAI.70.10.5846-5856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Huang GT, Zhang HB, Dang HN, Haake SK. Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb. Pathog. 2004;37:303–312. doi: 10.1016/j.micpath.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Ann. Rev. Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-κB-driven protein synthesis. J. Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- Kanneganti T-D, Ozoren N, Body-Malapel M, Amer A, Park J-H, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect. Immun. 2000;68:1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- Kusumoto Y, Hirano H, Saitoh K, Yamada S, Takedachi M, Nozaki T, et al. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J. Periodontol. 2004;75:370–379. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Franchi L, Nńũez G. Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the fum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lich JD, Arthur JC, Ting JP-Y. Cryopyrin: in from the cold. Immunity. 2006;24:241–243. doi: 10.1016/j.immuni.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Reviews Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Michalek SM, Katz J, Childers NK, Martin M, Ballovetz DF. Microbial/host interactions: mechanisms involved in host responses to microbial antigens. Immunol. Res. 2002;26:223–234. doi: 10.1385/IR:26:1-3:223. [DOI] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect. Immun. 2001;69:4242–4247. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Sandros J, Karlsson C, Lappin DF, Madianos PN, Kinane DF, Papapanou PN. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 2000a;79:1808–1814. doi: 10.1177/00220345000790101301. [DOI] [PubMed] [Google Scholar]
- Sandros J, Karlsson C, Lappin DF, Madianos PN, Kinane DF, Papapanou PN. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J Dent Res. 2000b;79:1808–1814. doi: 10.1177/00220345000790101301. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Wang B, Shiau HJ. Oral bacteria and respiratory infection: effects on respiratory pathogen adhesion and epithelial cell proinflammatory cytokine production. Ann. Periodontol. 2001;6:78–86. doi: 10.1902/annals.2001.6.1.78. [DOI] [PubMed] [Google Scholar]
- Seong S-Y, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nature Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Ohta A. The “danger” sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobón-Arroyave SI, Jaramillo-González PE, Isaza-Guzmá DM. Correlation between salivary IL-1beta levels and periodontal clinical status. Arch. Oral Biol. 2008 doi: 10.1016/j.archoralbio.2007.11.005. in press. [DOI] [PubMed] [Google Scholar]
- Tournier J-N, Quesnel-Hellmann A. Host-pathogen interactions: a biological rendez-vous of the infectious nonself and danger models? PLoS Pathogens. 2006;2:e44. doi: 10.1371/journal.ppat.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infec. Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect. Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X(7)-mediated host-cell apoptosis. Cell. Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]