Abstract
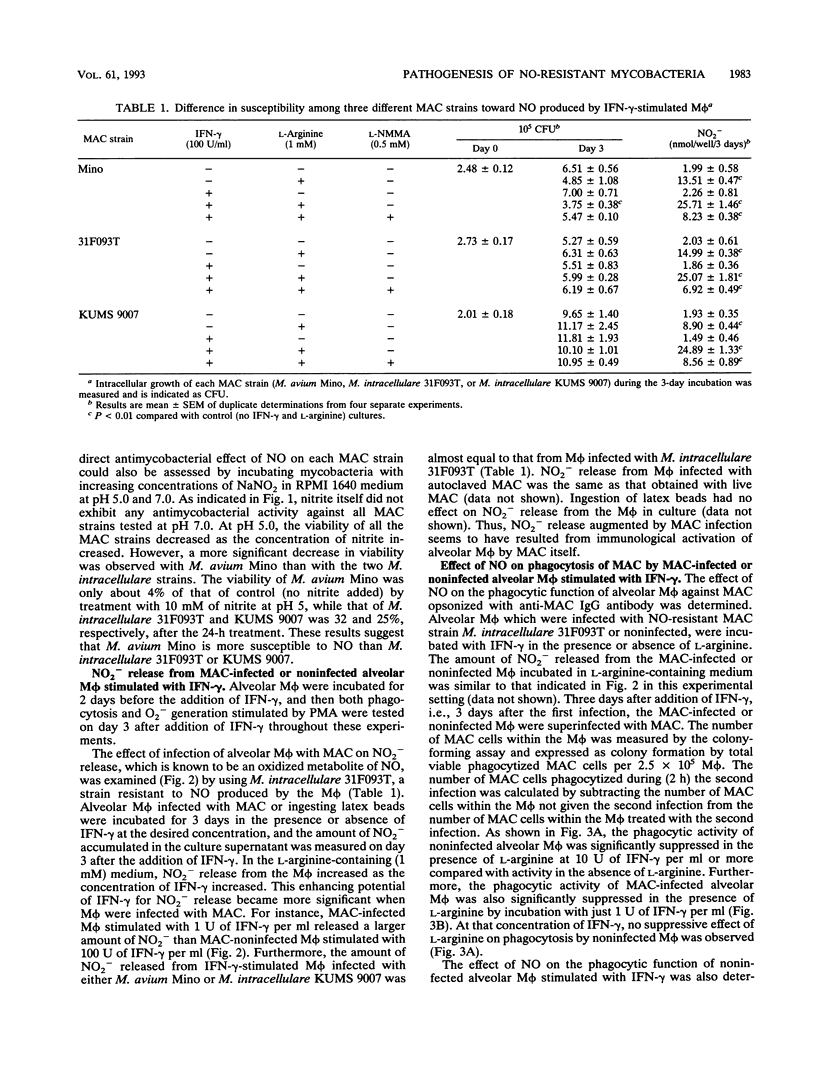
Susceptibility of three different strains of Mycobacterium avium complex (MAC), i.e., one strain of M. avium (Mino) and two strains of M. intracellulare (31F093T and KUMS 9007), to nitric oxide (NO) generated by rat alveolar macrophages (M phi) or NO generated chemically by acidification of NO2- was examined in vitro. We also investigated the effects of NO on phagocytosis and superoxide anion (O2-) generation by M phi. The intracellular growth of M. avium Mino was significantly suppressed by NO generated by gamma interferon (IFN-gamma)-stimulated M phi, whereas that of two strains of M. intracellulare (31F093T and KUMS 9007) was not. M. avium Mino was also more susceptible to NO generated chemically by acidification of NO2- than the two M. intracellulare strains. In L-arginine (1 mM)-containing medium, NO release from the M phi assessed by measuring NO2- increased as the concentration of IFN-gamma increased. The enhancing potential of IFN-gamma for NO release became more pronounced when M phi were infected with 31F093T, an NO-resistant strain. A large amount of NO generated by IFN-gamma-stimulated M phi suppressed both phagocytosis and O2- generation by the M phi, especially after infection of the M phi with strain 31F093T. These results indicate that the intracellular growth of MAC is not always inhibited by NO generated by immunologically activated M phi; rather, NO generation induced by infection with an NO-resistant MAC strain suppresses phagocytosis of the M phi, which may allow extracellular spreading of such NO-resistant mycobacteria. Therefore, the pathogenic potential of MAC may be partly attributed to its resistance to NO.
Full text
PDF


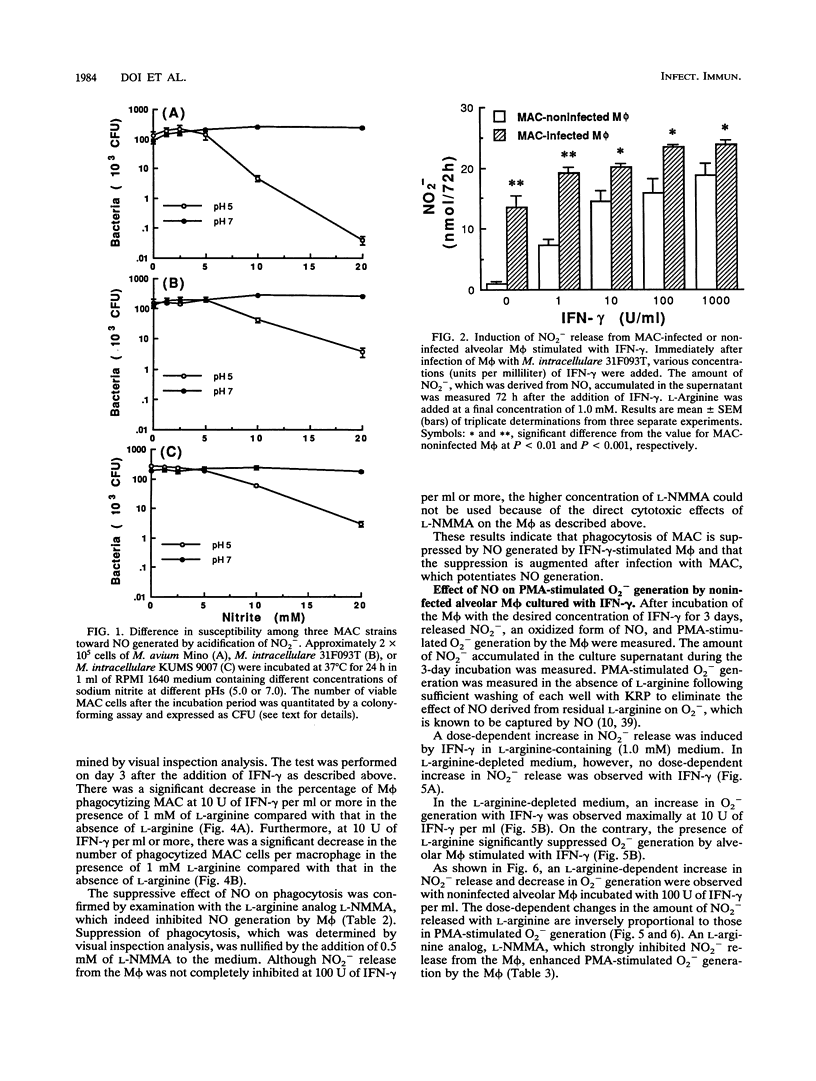


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Molla A., Ando M., Araki S., Maeda H. Molecular mechanism of complex infection by bacteria and virus analyzed by a model using serratial protease and influenza virus in mice. J Virol. 1989 May;63(5):2252–2259. doi: 10.1128/jvi.63.5.2252-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
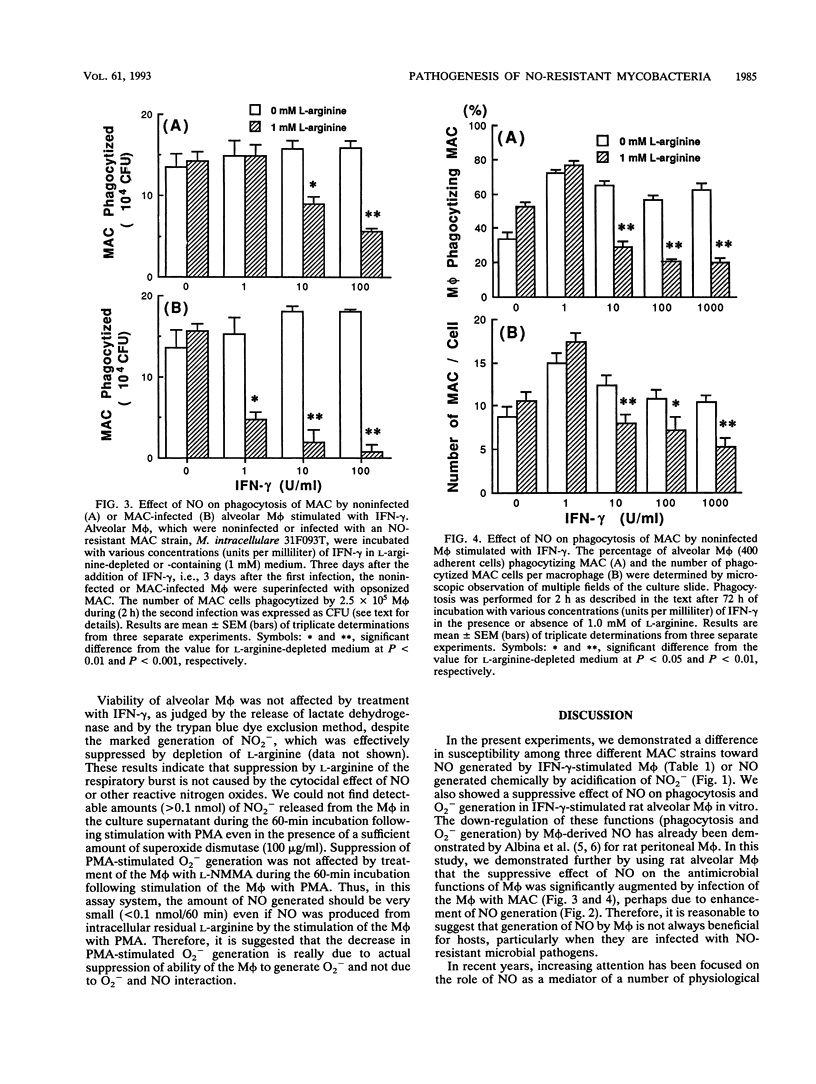
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Albina J. E., Caldwell M. D., Henry W. L., Jr, Mills C. D. Regulation of macrophage functions by L-arginine. J Exp Med. 1989 Mar 1;169(3):1021–1029. doi: 10.1084/jem.169.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Regulation of macrophage physiology by L-arginine: role of the oxidative L-arginine deiminase pathway. J Immunol. 1989 Dec 1;143(11):3641–3646. [PubMed] [Google Scholar]
- Alspaugh J. A., Granger D. L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991 Jul;59(7):2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Suga M., Shima K., Sugimoto M., Tokuomi H. Activation of alveolar macrophages exposed to lavage-procured immunoglobulin G obtained from normal rabbit lungs. Infect Immun. 1978 May;20(2):476–484. doi: 10.1128/iai.20.2.476-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson A. L., Phillips G. E. A rapid colorimetric assay for serum lactic dehydrogenase. Clin Chim Acta. 1965 Aug;12(2):210–215. doi: 10.1016/0009-8981(65)90032-x. [DOI] [PubMed] [Google Scholar]
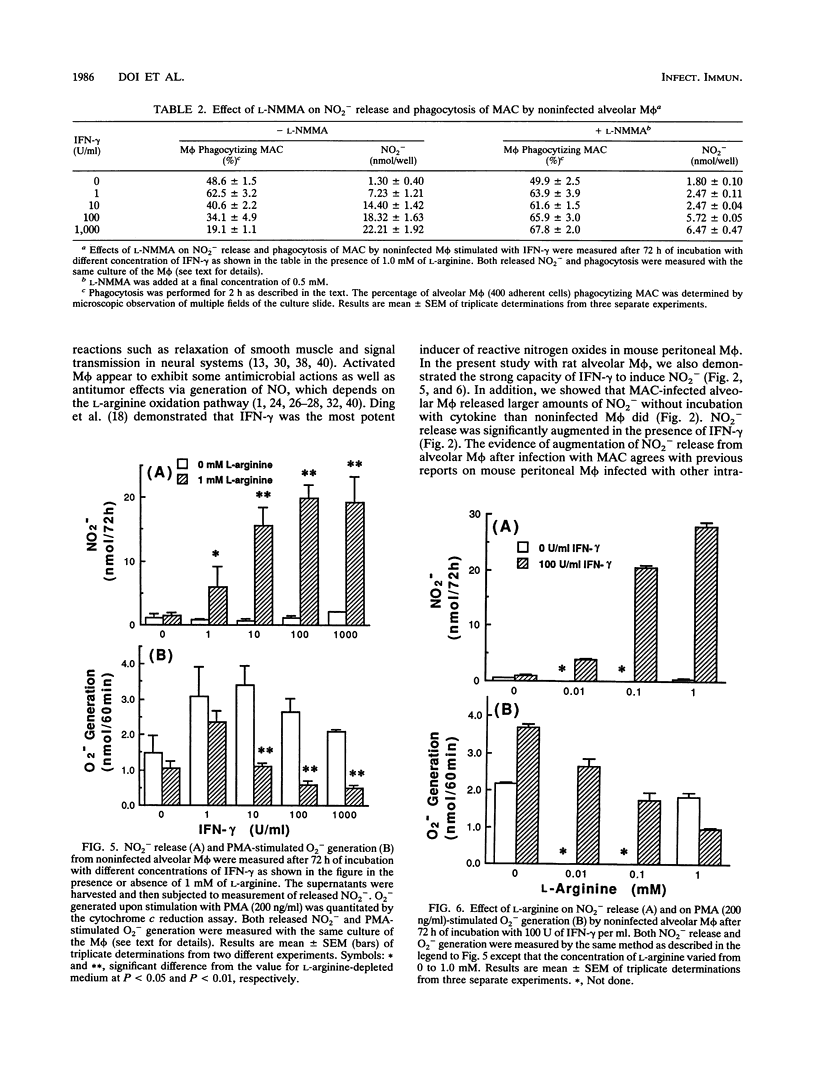
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Djeu J. Y. Interferon decreases the growth inhibition of Mycobacterium avium-intracellulare complex by fresh human monocytes but not by culture-derived macrophages. J Infect Dis. 1991 Jul;164(1):152–157. doi: 10.1093/infdis/164.1.152. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Nakane A., Minagawa T. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect Immun. 1989 Aug;57(8):2345–2349. doi: 10.1128/iai.57.8.2345-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- James S. L., Kipnis T. L., Sher A., Hoff R. Enhanced resistance to acute infection with Trypanosoma cruzi in mice treated with an interferon inducer. Infect Immun. 1982 Feb;35(2):588–593. doi: 10.1128/iai.35.2.588-593.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuze F. Experimental chemotherapy in chronic Mycobacterium avium-intracellulare infection of mice. Am Rev Respir Dis. 1984 Mar;129(3):453–459. doi: 10.1164/arrd.1984.129.3.453. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Mor N., Goren M. B., Crowle A. J. Enhancement of growth of Mycobacterium lepraemurium in macrophages by gamma interferon. Infect Immun. 1989 Aug;57(8):2586–2587. doi: 10.1128/iai.57.8.2586-2587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett E. L., Pruett S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992 Jul 31;186(2):775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. A. The indirect fluorescent antibody test for the detection of antibody in human cryptococcal disease. J Infect Dis. 1966 Dec;116(5):573–580. doi: 10.1093/infdis/116.5.573. [DOI] [PubMed] [Google Scholar]



