Abstract
Adiponectin is a circulating insulin-sensitizing hormone that homo-oligomerizes into trimers, hexamers, and higher molecular weight (HMW) species. Low levels of circulating HMW adiponectin appear to increase the risk for insulin resistance. Currently, assembly of adiponectin oligomers, and consequently mechanisms responsible for decreased HMW adiponectin in insulin resistance, are not well understood. In the work reported here, we analyzed the re-assembly of the most abundant HMW adiponectin species, the octadecamer, following its collapse to smaller oligomers in vitro. Purified bovine serum adiponectin octadecamer was treated with reducing agents at pH 5 to obtain trimers. These reduced trimers partially and spontaneously reassembled into octadecamers upon oxidative formation of disulfide bonds. Disulfide bonds appear to occupy a greater role in the process of oligomerization than in the structural stabilization of mature octadecamer. Stable octadecamers lacking virtually all disulfide bonds could be observed in abundance using native gel electrophoresis, dynamic light scattering, and collision-induced dissociation nano-electrospray ionization mass spectrometry. These findings indicate that while disulfide bonds help to maintain the mature octadecameric adiponectin structure, their more important function is to stabilize intermediates during the assembly of octadecamer. Adiponectin oligomerization must proceed through intermediates that are at least partially reduced. Accordingly, fully oxidized adiponectin hexamers failed to reassemble into octadecamers at a rate comparable to that of reduced trimers. As the findings from the present study are based on in vitro experiments, their in vivo relevance remains unclear. Nevertheless, they describe a redox environment-dependent model of adiponectin oligomerization that can be tested using cell-based approaches.
Adiponectin is a peptide hormone secreted from adipocytes with insulin-sensitizing and vascular and cardiac protective functions (1–4). Expression of the adiponectin gene and circulating adiponectin levels are subjected to regulation by a variety of hormones, cytokines, and transcription factors (1). Low levels of circulating adiponectin are associated with insulin resistance, coronary artery disease, and obesity, especially visceral obesity, in humans and animals (5, 6). Mice lacking adiponectin display increased proliferation of vascular smooth muscle cells (7) and hypertrophic cardiomyopathy (8), and are predisposed to develop insulin resistance (9–11). Similarly, hypoadiponectinemia appears to be a risk factor for developing insulin resistance or type 2 diabetes in populations from diverse ethnic backgrounds (6).
The primary sequence of adiponectin consists of three domains: an N-terminal region, a collagenous domain, and a C-terminal globular domain (12, 13). Through hydrophobic interactions, three individual globular domains form a globular head that is evidenced by x-ray crystallography (14). The three collagenous domains extending from the globular head presumably adopt a triple-helical structure that appears on electron micrographs as the stick on the lollipop-shaped adiponectin trimer (15, 16). The N-terminal region contains a conserved cysteine at position 22 of mature mouse adiponectin (position 39 including signal peptide) that is required for oligomerization of adiponectin trimers into hexamers and higher molecular weight (HMW) oligomers (15–17). Using different assay methods, different groups have alternatively referred to trimers as low molecular weight (LMW) adiponectin (17) and hexamers as either medium molecular weight (MMW) (17, 18) or LMW (15, 19) adiponectin. Trimers, hexamers, and the largest HMW species, an octadecamer (20), are the three major adiponectin oligomers present in mouse or human serum (15, 17, 21).
Although a consensus is yet to be reached, accumulating evidence indicates that levels of circulating HMW adiponectin correlate more closely with insulin action than total adiponectin (22–26). The cause for the selective decrease of HMW adiponectin in insulin resistance is currently not understood. It is critical to study the biogenesis of adiponectin oligomers because defining the assembly pathways of different oligomers may help us understand the cause of the reduced circulating levels of HMW adiponectin in insulin resistance. In addition, numerous studies have demonstrated discrepancies between changes in adipose tissue adiponectin mRNA levels and circulating adiponectin concentrations (27–29). This suggests that factors other than adiponectin gene expression strongly influence circulating adiponectin levels (30–34). The ability of the endoplasmic reticulum (ER) to undergo oxidative protein folding and properly assemble adiponectin oligomers likely represents one such factor.
Assembly of adiponectin oligomers is poorly understood. The C22 residues in hexameric and HMW adiponectin are fully oxidized to disulfides (16). In addition, replacement of the C22 residue near the N-terminus of adiponectin with alanine or serine precluded formation of hexamers or HMW species (15–17), indicating the importance of disulfide bonds in adiponectin oligomerization beyond trimers. However, while HMW adiponectin is extremely stable under conditions of high salt or pH, it is readily collapsed to hexamers upon modest reduction in pH to 4 or 5 (15, 17). This indicates strong intermolecular forces other than disulfide bonds are needed to maintain the structure of HMW adiponectin. An ER chaperone, ERp44, has been shown to form mixed disulfides with adiponectin via the cysteine residue near the N-terminus (35). Down-regulation of ERp44 in cultured adipocytes led to increased secretion of trimeric adiponectin and decreased secretion of HMW adiponectin (35). Another recently discovered ER chaperone, DsbA-L, has also been shown to promote formation of HMW adiponectin (36). However, the molecular mechanisms by which these chaperones promote HMW adiponectin formation are unclear because a basic understanding of the adiponectin oligomer assembly pathway remains lacking.
Studying adiponectin oligomerization in vivo entails labeling nascent adiponectin followed by isolation of labeled adiponectin in intact oligomeric complexes. Due to the technical difficulties surrounding these types of studies, we have developed an in vitro assay to analyze adiponectin oligomerization using purified bovine serum adiponectin. We show that octadecameric adiponectin could be assembled spontaneously from reduced trimers, but not from fully oxidized hexamers, indicating formation of mature hexamers is a process that is distinct from that of HMW adiponectin. We also identified a non-disulfide bonded octadecameric complex whose oligomerization state was confirmed by multiple analytical methods. This finding underscores the importance of disulfide bond formation in adiponectin assembly because it is not critical for maintaining the octadecameric structure. Indeed, disulfide bond formation accompanied the appearance of octadecameric adiponectin and alkylation of cysteines blocked the assembly of octadecameric adiponectin. Taken together, these findings suggest that free sulfhydryls must be available on adiponectin intermediates in order for disulfide bonds to form between distinct trimer subunits during the oligomerization process. The present study provides a framework for defining the assembly process of adiponectin oligomers, which may assist in understanding the decreased levels of HMW adiponectin observed in insulin resistant states.
Experimental Procedures
Purification of adiponectin octadecamers
Adiponectin octadecamers were purified to homogeneity from fetal bovine serum (Atlanta Biologicals, Atlanta, GA) or calf serum (Invitrogen, Carlsbad, CA) as described previously (37, 38) with one additional chromatography step. The eluate from the zinc chelation column was added to reactive green 19 resin (Sigma, St. Louis, MO) equilibrated with PBS, pH 7.6. This mixture was allowed to rock overnight at 4°C, and the supernatant was recovered and applied to the next chromatography step. The final preparations contained predominantly octadecamers with small amounts of hexamers. The oligomerization state of purified adiponectin octadecamer was confirmed by established gel filtration chromatography and equilibrium sedimentation techniques (20, 21).
Native and denaturing PAGE analysis of adiponectin oligomers
To analyze oligomerization states of adiponectin under native conditions, samples were diluted with a concentrated native loading buffer to a final composition of 31.25 mM Tris, pH 6.8, 12% glycerol, and 0.05% Orange G. Adiponectin oligomers were fractionated in 7% native Tris-acetate gels that were either purchased (Invitrogen, Carlsbad, CA) or prepared from 30% acrylamide stock solution (37.5:1 acrylamide:bis-acrylamide, Bio-Rad Laboratories, Hercules, CA) buffered with 375 mM Tris base titrated to pH 8.5 with glacial acetic acid. The composition of the native running buffer was 25 mM Tris-base and 192 mM glycine at pH 8.3. Gels were run at 18 volts/cm for 2 hrs. The oxidation states of the cysteine residue near the N-terminus of adiponectin were determined by non-reducing denaturing PAGE. Samples were denatured by heating at 85°C for 10 to 20 min in 246 mM Tris pH 8.5, 10% glycerol, 0.51 mM EDTA, 0.2 mM Serva Blue R, 0.175 mM Phenol Red, and 3% SDS. Monomeric (reduced) and dimeric (oxidized) adiponectin molecules were separated by discontinuous (11% separating, 5% stacking) SDS-PAGE (39) or precast 10% Bis-Tris gels in either MOPS- or MES-based SDS running buffer (Invitrogen, Carlsbad, CA). Gels were stained with Krypton IR (Pierce, Rockford, IL) or Coomassie and visualized using the LI-COR Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Densitometry was performed using the Odyssey software to quantify the intensity of the bands corresponding to adiponectin oligomers.
Re-oligomerization of adiponectin following 100 mM dithiothreitol (DTT) and 200 mM β-mercaptoethanol (βME) treatment
Purified adiponectin octadecamers (0.25 mg/ml) dissolved in PBS were treated with 100 mM DTT or 200 mM βME and incubated at 37°C for 60 min. The reaction was then dialyzed against PBS at pH 7.6 using MINI-Dialysis units (Pierce, Rockford, IL) for 3 hrs. Aliquots were removed at 0, 30, 60, 120, and 180 min and placed on ice after addition of concentrated native loading buffer. At each time point, the remaining sample was placed in fresh dialysis buffer. Separate aliquots of octadecamers were kept in 100 mM DTT or 200 mM βME for the duration of the experiment to assess the requirement for dialysis in re-oligomerization. The oligomerization states of the samples were subsequently analyzed using native PAGE.
Effect of reducing agent concentration and pH on collapse of adiponectin octadecamers to smaller oligomers
Purified adiponectin octadecamers in PBS (0.25 mg/ml) were treated with 1, 10, or 100 mM DTT for 30 min at 50°C followed by further incubation at 37°C for 90 min. Alternatively, octadecamers were treated with 2, 20, 50, or 200 mM βME for 15 min at 37°C. At that time an aliquot of each reaction was removed and brought to 50 mM glycine and pH 5. Reactions were further incubated for 15 min at 37°C. The remaining samples were incubated for 15 min at 37°C without glycine and at neutral pH. All samples were immediately analyzed using native PAGE as described above.
Re-oligomerization of octadecameric adiponectin in the presence of hydrogen peroxide
Purified adiponectin octadecamers (0.25 mg/ml) were incubated with 25 mM DTT at 4°C overnight to reduce the disulfide bonds in adiponectin. The following morning, the pH of the reactions was adjusted to 5 by adding glycine, N-acetylneuraminic acid, and glucose to final concentrations of 32.5 mM, 5 mM, and 5 mM, respectively. N-acetylneuraminic acids and glucose were added to reduce aggregation of adiponectin. The reactions were further incubated for 20 min at 37°C to collapse a majority of adiponectin octadecamers to trimers. The reactions were dialyzed against PBS at pH 7.6 supplemented with 10 μM zinc chloride in the presence or absence of 5 mM hydrogen peroxide in MINI-Dialysis units (Pierce, Rockford, IL). Divalent cations were added to facilitate peroxide-mediated formation of disulfide bonds (40). Aliquots were removed and fresh buffer replaced at 0, 30, 60, 120, and 180 min into the dialysis. Half of the aliquots were placed on ice after addition of concentrated native loading buffer while Tris-HCl at pH 7.4 and N-ethylmaleimide (NEM) were added to the other half of the aliquots at final concentrations of 100 mM each and incubated at 37°C for 20 min to fix the redox state of adiponectin. Oligomerization and oxidation states of adiponectin were analyzed by gel electrophoresis as described above.
Iodoacetamide alkylation of adiponectin following βME and low pH treatment
Purified adiponectin octadecamers (0.16 mg/ml) were treated in 50 mM βME for 15 min at 37°C. The pH of the reactions was then lowered to approximately 5 with the addition of glycine, N-acetylneuraminic acid, and glucose to final concentrations of 32.5 mM, 5 mM, and 5 mM, respectively. After an additional incubation for 20 min at 37°C to collapse octadecamers to mostly trimers, the pH of the samples were titrated back to approximately 7 following addition of an equal volume of a solution containing 100 mM Tris base and 100 mM iodoacetamide (IAA). The samples were incubated in the presence or absence of 50 mM iodoacetamide (IAA) at 37°C for 45 min. The purpose for using high concentrations of IAA was to quench βME and to alkylate the cysteines on reduced adiponectin. Reactions with or without IAA were all dialyzed extensively into PBS containing 25 mM βME to remove excess IAA and the highly acidic byproduct of the alkylation reaction hydroiodic acid. Prior to initiation of re-oligomerization by dialysis, the pH of the reactions was measured to ensure they were neutralized. Samples were then dialyzed against PBS at pH 7.6 supplemented with 10 μM zinc chloride and 5 mM hydrogen peroxide for 3 hrs. At 0, 30, 60, 120, and 180 min into dialysis, aliquots of reactions were removed and dialysis buffer was replaced with fresh buffer of the same composition. Oxidation states of adiponectin were fixed with NEM as described above. Oligomerization and oxidation states of adiponectin were analyzed by gel electrophoresis also as described above, except the samples for non-reducing SDS-PAGE analysis were heated at 50°C for 15 min instead of 85°C. While this lower temperature was unable to convert much of the mature octadecamers to smaller subunits, it was sufficient to collapse the vast majority of intermediates to dimers or monomers.
Reduction of adiponectin octadecamers without significant conversion to smaller oligomers
Purified adiponectin octadecamers in PBS (0.16 mg/ml) were incubated in the presence or absence of 4 mM DTT overnight at 4°C. The oligomerization state after treatment was assessed using native PAGE as described above and dynamic light scattering and mass spectrometry as described below. An aliquot of the reaction was removed for redox state assessment using non-reducing denaturing gel electrophoresis. Prior to fractionation in SDS-PAGE, the DTT-treated reaction was incubated at 37°C for 1 hr in the presence of 10 mM NEM to alkylate the reduced cysteines of adiponectin and DTT. This procedure served to prevent re-oxidation of adiponectin and, more importantly, a false positive result due to artificial reduction of adiponectin by DTT during heat and detergent denaturation prior to gel loading. To determine if DTT was completely inactivated by NEM, levels of DTT remaining in DTT- and NEM-treated samples were determined using Ellman’s reagent (41) with two-fold serial dilutions of DTT (from 5 mM to 39 μM) as standards. No DTT was detected in NEM-treated adiponectin samples.
Dynamic light scattering analysis of adiponectin oligomerization state
Purified adiponectin octadecamer dissolved in PBS (0.16 mg/ml) was treated with or without 4 mM DTT at 4°C overnight. Samples were analyzed at 20°C in a Zetasizer Nano instrument equipped with a 4 mW, 633 nm He-Ne laser (Malvern Instruments, Worcestershire, United Kingdom). The apparent molecular mass was estimated from empirical relationships between the hydrodynamic radii and known molecular masses of a series of globular proteins. Following data collection, the samples were retrieved from light scattering cuvettes. The oligomerization state of adiponectin was analyzed again using native PAGE. The oxidation state of adiponectin in the samples were fixed by treating them first with 2% SDS for 2 hrs at 4°C and then with 25 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) for 15 min at 50°C. Non-reducing SDS-PAGE was used to assess oxidation states as described above.
Mass spectrometry of octadecameric adiponectin
The monomeric molecular mass within bovine adiponectin octadecamers was determined by matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) through the University of Arizona Proteomics Consortium facilities. MALDI-TOF mass spectra were acquired using an Applied Biosystems Voyager DE-STR (Framingham, MA), operating a 337 nm nitrogen laser. An aliquot of sample was mixed with an equal volume of a saturated α-cyano-4-hydroxy-cinnamic acid solution in 50% acetonitrile/50% water containing 0.1% TFA and then 1 ml was spotted on the target plate and allowed to air-dry prior to mass analysis. Mass spectra were collected in linear mode with an accelerating voltage of 25000 V. The grid voltage was set at 92.5% with an extraction delay time of 1050 nsec. A minimum of 400 laser shots at 20 Hz were combined per mass spectra recorded. Octadecamers were partially heat denatured at 75°C in 1% SDS and 50 mM DTT in PBS and subsequently dialyzed into deionized water supplemented with 0.1% SDS and 50 mM DTT before being mixed with matrix. The oligomerization state of adiponectin was determined by native nano-electrospray mass spectrometry. Purified HMW adiponectin was buffer exchanged into 200 mM ammonium acetate at pH 7 using microconcentrators (Bio-rad, Hecules, CA). An aliquot of purified octadecameric adiponectin was also treated with 1 mM DTT at 4°C for 24 hrs. Samples were ionized via the nanospray method and analyzed in a Q-Tof 2™ quadrupole-time-of-flight mass spectrometer (Waters Corp, Milford, MA). The cone voltage was varied between 150 to 200 volts DC. Charge state distributions were analyzed using the MassLynx software system. For collision-induced dissociation (CID) experiments, ions with a given mass-to-charge ratio were selected with the quadrupole mass analyzer, fragmented through multiple low energy collisions with argon gas in the collision cell, and the product ions were analyzed in the TOF. The voltage difference between the source hexpole and the collision cell was 175 to 200 volts and analyzer pressure was 3.5 ×10−4 mbar. Additional details for native mass spectrometry and CID have been described previously (42)
Collapse of adiponectin octadecamers to hexamers and subsequent re-oligomerization studies
Purified adiponectin octadecamers (0.16 mg/ml) were converted to hexamers by reducing the pH of the samples to 5 with addition of glycine to a final concentration of 50 mM and incubated for 30 to 40 min at 37°C. Duplicate samples were also treated in 50 mM βME at 37°C for 15 min to obtain trimers. Both samples were dialyzed against PBS supplemented with 10 μM zinc chloride and 5 mM hydrogen peroxide at pH 7.6 as described above for 1 to 2 hrs in a microdialyzer system (Pierce, Rockford, IL). Samples for native PAGE were removed at 0, 30, and 60 min into dialysis. Buffer chamber was replenished with fresh dialysis buffer every 10–20 min. To determine if the hexamers could re-oligomerize into octadecameric adiponectin following reduction to trimers, the once glycine-treated hexamer samples were placed in 50 mM βME at the end of a 2 hr dialysis period and incubated for 30 min at 37°C. The samples were again dialyzed for 2 hrs against the same buffer as the first dialysis. Oligomerization and oxidation states of adiponectin were analyzed by gel electrophoresis as described above. Oxidation states of adiponectin were fixed in 25 mM NEM at 37°C for 45 min.
Results
Octadecameric adiponectin re-oligomerizes from reduced trimer
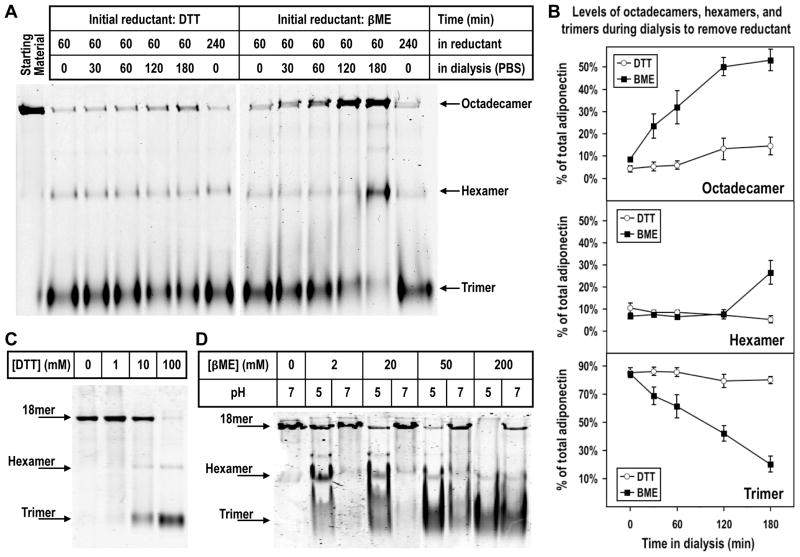
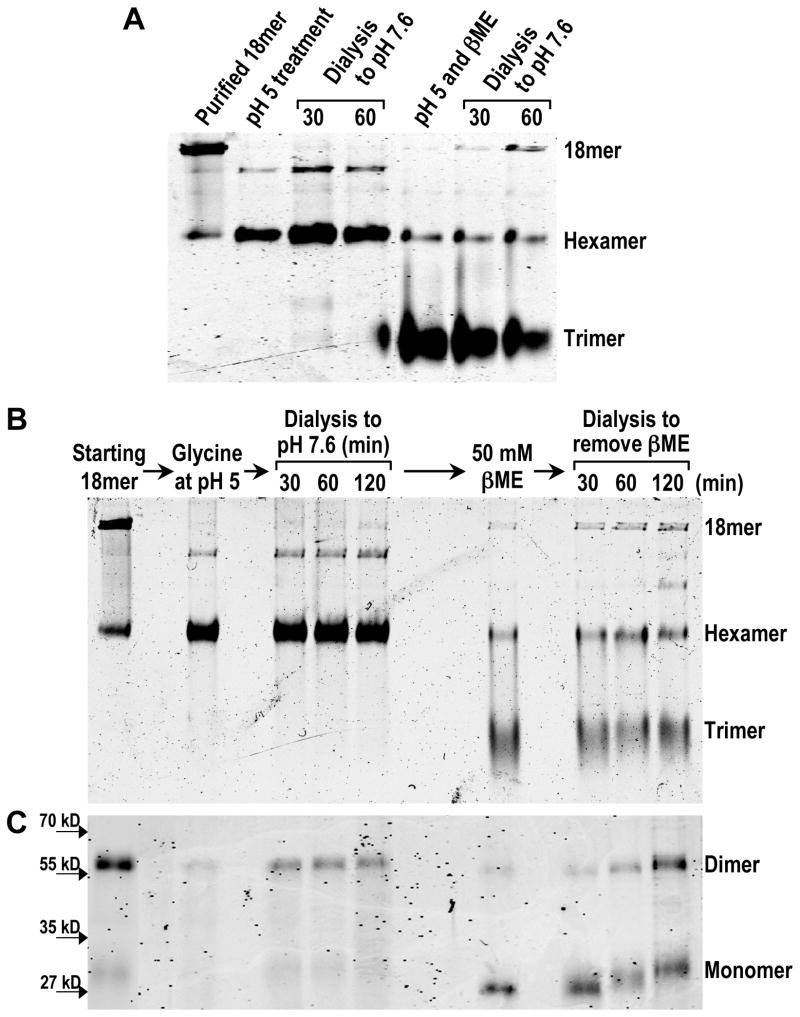
In order to resolve the molecular details of adiponectin oligomerization from trimers to hexamers and HMW species, we used trimers from purified adiponectin octadecamers as starting substrates for in vitro oligomerization assays. As shown in Fig 1A, DTT (100 mM) and βME (200 mM) treatment at 37°C for 60 min converted the majority of octadecamers to mostly trimers and a small amount of hexamers. Titration experiments showed that high concentrations of reducing agents were needed to significantly collapse octadecamers to trimers at neutral pH (Fig. 1C and 1D). The collapsing activities of βME were more pronounced at pH 5 (Fig. 1D), indicating the effects of reducing agents and the acidic pH treatment are additive. Following octadecamer breakdown by reducing agents, we attempted to generate stable trimers by removing the reducing agents. Surprisingly, as either reducing agent was gradually removed by dialysis against PBS at pH 7.6, increasing amounts of octadecamers were observed along with decreasing amounts of trimers (Fig. 1A and 1B). Our experimental procedures did not allow us to determine if trimers were stoichiometrically converted to octadecamers because of unequal accessibility to the protein stain dyes among different oligomers in native gels. To determine if removal of the reductants is necessary for oligomerization, an aliquot of octadecamer was kept in 100 mM DTT or 200 mM βME for the duration of the entire experiment including the dialysis period. Unlike the samples that were dialyzed against PBS, neither sample exhibited re-oligomerization to octadecamer (Fig. 1A). These data indicate that octadecameric adiponectin can be spontaneously assembled from reduced trimers, but removal of the reductant is necessary for re-oligomerization to occur.
Figure 1. (A) Native PAGE analysis of adiponectin oligomeric state following reduction and collapse of octadecamers and subsequent removal of reductant by dialysis.
Purified bovine serum octadecamers were collapsed to trimers in 100 mM DTT or 200 mM βME and subsequently dialyzed against PBS over a 3 hr period to remove DTT or βME. Following fractionation in PAGE, the proteins were detected by fluorescence from an infrared stain as described in Experimental Procedures. Two samples, one in DTT and the other in βME, were not dialyzed against PBS to assess if re-oligomerization to octadecamers depended upon removal of reducing agents. (B) Densitometric analysis of adiponectin octadecamer (top panel), hexamer (middle panel), and trimer (bottom panel) levels during removal of reductant by dialysis following collapse of purified octadecamers. The integrated intensity of each band corresponding to a specific oligomer at a particular time point was normalized to the sum of the integrated intensities of all three major oligomers at the same time point to obtain a value for the percentage of total adiponectin. Average percentage values and standard error of the mean from four and five independent experiments whose initial reductant was, respectively, DTT (open circle) and βME (closed square) were plotted. (C) Oligomerization state of adiponectin following titration with different concentrations of DTT. Purified octadecamers were treated with 1, 10, and 100 mM DTT and resulting products’ oligomerization states were analyzed using native PAGE as described above and in Experimental Procedures. (D) Effect of pH and βME concentration on adiponectin oligomerization state. Adiponectin octadecamers were treated with 2, 20, 50 or 200 mM βME at pH 5 or 7 as described in Experimental Procedures. Native PAGE was used to analyze oligomerization states following the treatments.
Formation of disulfide bonds accompanies assembly of octadecameric adiponectin
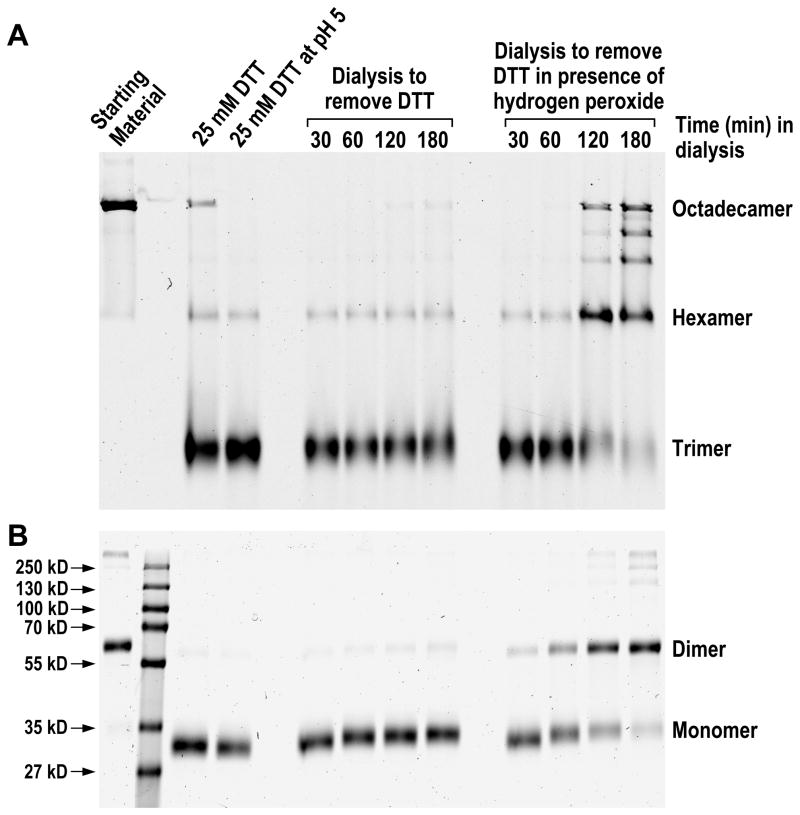
The secreted HMW and hexameric isoforms of adiponectin are composed of disulfide-bonded dimers (16). Studies of adiponectin in which the cysteine residue near the N-terminus was replaced with alanine or serine have demonstrated that oxidation of that cysteine residue is critical for the existence of hexameric and HMW adiponectin (15–17). To understand the role of cysteine oxidation in adiponectin assembly in vitro, we examined whether the re-oligomerization of octadecameric adiponectin from reduced trimers is accompanied by formation of disulfide bonds. Non-reducing denaturing PAGE was the method of choice for assessing the oxidation states of adiponectin oligomers, with reduced adiponectin migrating as monomers and oxidized adiponectin as dimers. Prior to denaturation by heat and detergent, the sulfhydryl-alkylating agent NEM was added to protect the oxidation states of adiponectin and to quench the remaining reducing agents that had not been removed by dialysis. In order to maintain a sufficient ratio of NEM to the reductant, octadecameric adiponectin was collapsed to trimers by a decreased DTT concentration of 25 mM combined with a mild acid treatment at pH 5. Data in Fig. 1D showed that octadecamers could be collapsed to trimers using lower reducing agent concentrations at pH 5 than at pH 7. As shown in Fig. 2A and 2B, while 25 mM DTT was able to reduce nearly all adiponectin to monomers from dimers, significant amounts of adiponectin remained as octadecamers. Further incubation of DTT-treated octadecamer at pH 5 resulted in near complete collapse to trimers with a minor amount of hexamers (Fig. 2A). The hexamers that remained likely represent adiponectin cross-linked at the collagenous domain, a known phenomenon in collagens. The putatively cross-linked adiponectin is also the probable source of the dimers in Fig. 2B that could not be converted to monomers by 25 mM DTT in denaturing gels. Fig. 2A also shows that as DTT was slowly removed by dialysis, increasing amounts of octadecamers reappeared. This was associated with increasing levels of oxidized adiponectin that migrated as dimers in non-reducing denaturing gel (Fig. 2B). Currently, the identity of the electron acceptor for the oxidation of adiponectin is unclear. To further address the role of oxidation in adiponectin oligomerization, we examined whether inclusion of a strong oxidizer could accelerate adiponectin oxidation and re-formation of octadecameric adiponectin. Hydrogen peroxide causes the oxidation of cysteines to cystines through a cysteine sulfenic acid intermediate (40). Addition of 5 mM hydrogen peroxide during dialysis increased the rate of octadecamer assembly (Fig. 2A) and formation of disulfide-bonded adiponectin that was reflected in the increasing ratios of dimers to monomers (Fig. 2B). Intermediate oligomers between hexamers and octadecamers were also observed in the presence of hydrogen peroxide (Fig. 2A). These results indicate that disulfide bond formation is an important feature in the assembly of adiponectin octadecamer.
Figure 2. Effect of oxidizing agent hydrogen peroxide on (A) re-oligomerization of octadecameric adiponectin and (B) oxidation of cysteines near N-terminus to form disulfide bonds.
Purified adiponectin octadecamers were first reduced by DTT followed by lowering the pH to 5 as described in Experimental Procedures to produce trimers. The samples were subsequently dialyzed against PBS at pH 7.6 to remove DTT in the absence or presence of 5 mM hydrogen peroxide over a period for 3 hrs as indicated. Samples were removed at different time points during the dialysis and fractionated in native gels to monitor oligomerization states and in non-reducing denaturing gels to distinguish the oxidized (dimer) and reduced (monomer) forms of adiponectin. Oxidation states were fixed by NEM treatment.
Assembly of octadecameric adiponectin requires formation of disulfide bonds
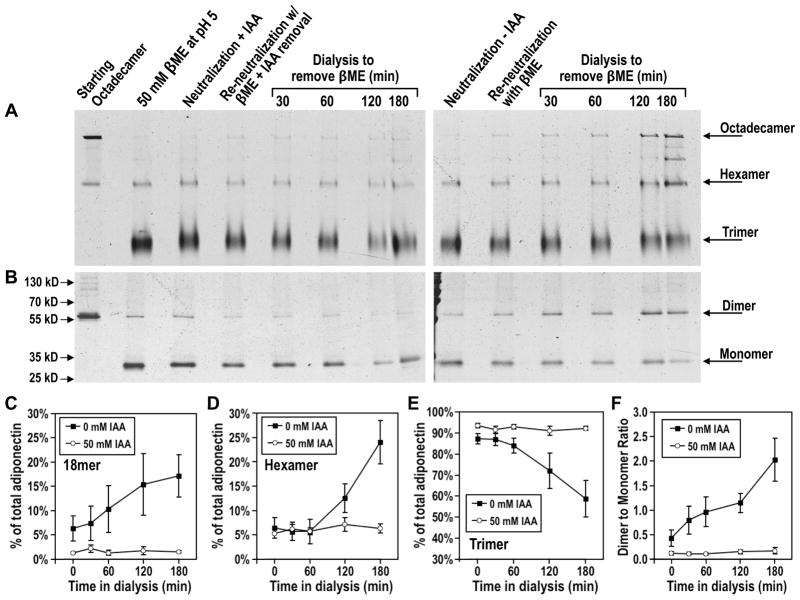
To test if disulfide bond formation is required for octadecamer re-oligomerization, we used the thiol-specific alkylating agent iodoacetamide (IAA) to block disulfide formation and examined the effects on assembly of octadecameric adiponectin. Octadecamers were collapsed to trimers by treatment with βME under mildly acidic conditions as described in Experimental Procedures. IAA and βME were chosen because of their relatively low reactivity with each other (43). Following near complete collapse of octadecamers, the samples were neutralized and alkylated with 50 mM IAA at 37°C for 45 min. Reaction of IAA with thiol groups (in cysteine residues and in βME) results in formation of hydroiodic acid, a strong acid that may interfere with the subsequent re-oligomerization of octadecamers. The samples were therefore further dialyzed against PBS at pH 7.6 supplemented with 25 mM βME to remove hydroiodic acid and unreacted IAA. Upon returning the pH to neutral, samples were placed in the same dialysis buffer except the 25 mM βME was replaced with 5 mM hydrogen peroxide in order for re-oligomerization to proceed. Similar to the results shown in Figs. 1 and 2, reduced adiponectin trimers that had not been treated with IAA were able to re-oligomerize into octadecamers (Fig. 3A, right panel, Fig. 3C, and Fig. 3E) and to re-form disulfide bonded dimers as evidenced by an increase in the dimer to monomer ratio (Fig. 3B, right panel, and Fig. 3F). In contrast, IAA-treated adiponectin trimers showed a greatly diminished propensity to undergo oxidation (Fig. 3B, left panel, and Fig. 3F). This resulted in a lack of re-oligomerization from reduced trimers to octadecamers in samples treated with IAA (Fig. 3A, left panel, Fig. 3C, and Fig. 3E). These findings indicate that assembly of octadecameric adiponectin requires disulfide formation.
Figure 3. Effect of sulfhydryl alkylating agent iodoacetamide (IAA) on (A) re-oligomerization and (B) oxidation of adiponectin.
Purified adiponectin octadecamers were first reduced by βME followed by lowering of pH to 5 as described in Experimental Procedures to produce trimers. Samples were neutralized in the absence or presence of IAA as indicated, and dialyzed against PBS containing 25 mM βME to quench IAA and remove hydroiodic acid, a product of the alkylation reaction. Samples were then dialyzed against PBS to remove βME and allow re-oligomerization to occur. Native PAGE analysis was performed to distinguish between the different oligomeric states of adiponectin and assess the extent to which oligomerization was inhibited by lack of disulfide formation following alkylation of reduced cysteines. Oxidation states were fixed by NEM treatment and determined using non-reducing SDS-PAGE. (C–E) Densitometric analysis of (C) octadecameric, (D) hexameric, and (E) trimeric adiponectin during removal of βME following alkylation of cysteines by IAA. Levels of each oligomer as percentages of total adiponectin were calculated as described in legend to Fig. 1B. Average percentage values and standard error of the mean from three independent experiments in which collapsed octadecamers were treated with (open circle) or without (closed square) IAA were plotted. (F) Densitometric analysis of disulfide bond formation in adiponectin complexes. The integrated intensities of protein bands corresponding to adiponectin dimers in non-reducing denaturing gels were normalized to the integrated intensities of bands corresponding to monomers in order to obtain a dimer to monomer ratio. Average ratios and standard error of the mean from three independent experiments in which collapsed octadecamers were treated with (open circle) or without (closed square) IAA were plotted.
Stable octadecameric adiponectin in native gels following reduction of disulfide bonds
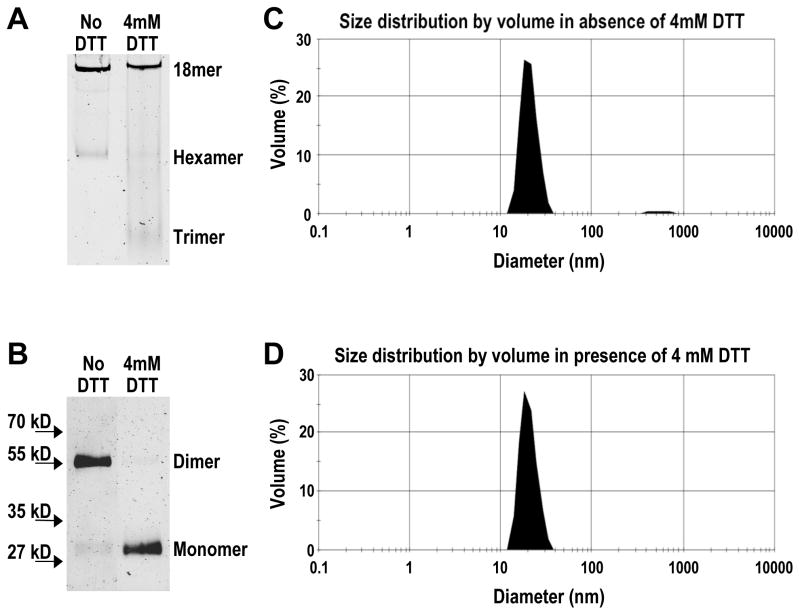
Disulfide bonds between correct pairs of cysteine residues are known to have stabilizing effects on the structures of many proteins. It is possible that disulfide formation is critical for adiponectin oligomerization because it is needed to stabilize the structure of hexameric and octadecameric adiponectin. However, extreme molar excesses of DTT and βME were needed to collapse most octadecamers to trimers (Fig. 1), raising the possibility that octadecameric adiponectin lacking disulfide bonds could be stable in solution. The presence of reduced octadecamers argues against a vital structural role for disulfide bonds and instead supports a role in the assembly process per se. To examine the possible existence of non-disulfide bonded octadecameric adiponectin, we treated purified octadecamers with or without 4 mM DTT at 4°C overnight. Native PAGE analysis of the DTT-treated sample revealed that a majority of the adiponectin remained as octadecamers along with minor amounts as hexamers and trimers (Fig. 4A). Following complete inactivation of DTT by NEM, as detailed in Experimental Procedures, analysis of the oxidation states of adiponectin by non-reducing denaturing PAGE showed that the vast majority of the DTT-treated octadecameric adiponectin existed in the reduced state lacking disulfide bonds (Fig. 4B). As controls, the non-DTT treated octadecamers consisted mostly of dimers, indicating they were disulfide-bonded and oxidized (Fig. 4B).
Figure 4. Effect of low concentrations of DTT on (A) oligomerization and (B) oxidation states of adiponectin octadecamers using gel electrophoresis and confirmation of oligomerization state in solution by dynamic light scattering under (C) oxidized and (D) reduced conditions.
Purified adiponectin octadecamers in PBS were treated with or without 4 mM DTT at 4°C overnight. Oligomerization state was assessed using native and oxidation state using non-reducing denaturing PAGE. AMS was used to quench DTT and alkylate reduced cysteines to prevent their oxidation during sample denaturation. Samples identically treated as those loaded onto native gels were analyzed using dynamic light scattering as described in Experimental Procedures.
Stable octadecameric adiponectin in solution following reduction of disulfide bonds
To eliminate the possibility that the presence of reduced octadecameric adiponectin in native gels was an artifact caused by aberrant migration of a smaller oligomer, the oligomerization state of DTT-treated adiponectin in solution was assessed using dynamic light scattering. Purified adiponectin octadecamers were treated with 4 mM DTT at 4°C overnight in the same manner as described above for comparative native and non-reducing denaturing electrophoresis. The hydrodynamic parameters of octadecamers in the absence and presence of DTT are listed in Table 1. The hydrodynamic sizes of oxidized and reduced adiponectin were virtually identical and also in agreement with previous reports (20). With the appearance of small amounts of trimers and hexamers following DTT treatment (Fig. 4A), the mean diameter of the reduced adiponectin sample was expectedly lower (by 1.7%) than that of oxidized adiponectin octadecamer. The increased oligomer heterogeneity in the reduced sample also led to a slightly higher standard deviation and polydispersity as well as slightly lower apparent molecular mass (Table 1). The mode diameter, a measurement that is less sensitive to the presence of small amounts of trimers and hexamers, was identical between DTT-treated adiponectin and untreated octadecamer (Table 1). The size distributions of both oxidized and reduced adiponectin samples were relatively narrow (Fig. 4C and 4D), reflecting the overall low degree of polydispersity exhibited by both samples (Table 1). Following light scattering analysis, the oligomerization and oxidation states of the samples were confirmed again using, respectively, native and non-reducing denaturing PAGE (data not shown). These results indicate the presence of abundant and fully reduced octadecameric adiponectin lacking disulfide bonds in a physiological solution.
Table 1.
Hydrodynamic parameters of native and reduced adiponectin octadecamer.
| Sample | % Mass | Mean diameter | Mode diameter | Standard deviation | % Poly- dispersity | Predicted mass* |
|---|---|---|---|---|---|---|
| 18mer | 97.6 | 20.36 nm | 18.17 nm | 4.163 nm | 20.5 | 767 kDa |
| 18mer + DTT | 99.2 | 20.02 nm | 18.17 nm | 4.281 nm | 21.4 | 738 kDa |
The apparent molecular mass was predicted from the known hydrodynamic sizes of a series of globular protein standards.
Simultaneous demonstration of reduced and octadecameric adiponectin by mass spectrometry
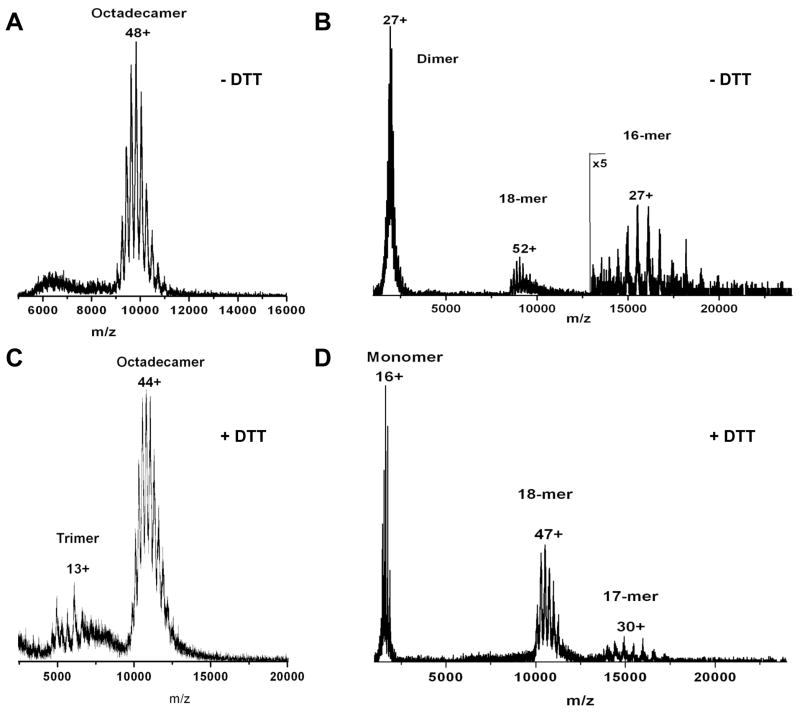
While native electrophoresis and dynamic light scattering experiments demonstrated the presence of abundant octadecameric adiponectin following reduction by DTT, the redox state of adiponectin had to be verified separately using non-reducing denaturing PAGE. Ideally, proof of reduced octadecameric adiponectin’s existence is best confirmed in a single experiment in which the redox state and oligomerization status of adiponectin are determined simultaneously. We used collision-induced dissociation nano-electrospray ionization tandem mass spectrometry to accomplish this goal. In agreement with the results in Fig. 4A, the native mass spectrum of purified bovine serum adiponectin octadecamers contains a single dominant species centered on the +48 charge state and corresponding to a molecular mass of approximately 475 kDa (Fig. 5A). Independently, the molecular weight of monomeric units within purified bovine serum octadecamers was assessed after reduction by DTT and heat denaturation using MALDI-TOF mass spectrometry. The mass spectrum of denatured and reduced adiponectin octadecamers showed a single, but broad, species with a mass-to-charge ratio of 26162.4 (data not shown). Based on the monomeric adiponectin mass determined by MALDI-TOF, the predicted mass of an octadecamer is approximately 471 kDa. This number differs from the native mass spectrometry-derived mass of 475 kDa by 0.8%, consistent with the fact that the native mass spectrometry conditions used typically lead to adducts of the oligomer with solvent and buffer salts. This confirms that the octadecameric structure of adiponectin is maintained in the gaseous environment of a nanospray mass spectrometer. The minimal covalent structure within the native octadecamer was determined by acceleration into an argon-filled collision cell to dissociate the octadecamer. The collision-induced dissociation (CID) spectrum (Fig. 5B) shows a dissociation pathway in which the octadecamers predominantly fragmented into dimers and hexadecamers (16mers). This indicates that the smallest covalent subunits within an octadecamer complex are dimers linked by disulfide bonds. Similar to the results obtained in Figs. 1B and 4, treating octadecamers with low concentrations of DTT led to the conversion of only small amounts of octadecamers to lower molecular mass oligomers including trimers (Fig. 5C). The octadecamers that survived the DTT treatment were selected with a quadrupole mass analyzer and dissociated through collisions with argon. In contrast to native octadecamers (Fig. 5B), the CID spectrum of DTT-treated octadecamers (Fig. 5D) showed ejection of single monomeric subunits from octadecamers, resulting in the appearance of monomers and heptadecamers (17mers). This indicates that the minimal covalent structure within a DTT-treated octadecamer is a monomer. Using an analytical technique capable of detecting octadecamers, dimers, and monomers simultaneously, the CID of DTT-treated octadecamers into monomers reinforces the results shown in Fig. 4 and Table 1 to further confirm the existence of octadecameric adiponectin lacking disulfide bonds.
Figure 5. (A, C) Native and (B, D) collision-induced dissociation (CID) mass spectra of adiponectin octadecamers in the (A, B) absence or (C, D) presence of 1 mM DTT.
Purified bovine serum octadecamers in 200 mM ammonium acetate at pH 7 were treated with or without 1 mM DTT at 4°C overnight. Native and reduced adiponectin samples were ionized via nano-electrospray and their mass-to-charge ratios determined in a quadrupole-time-of-flight mass spectrometer as described in Experimental Procedures. To assess the oxidation states of octadecameric adiponectin detected in the mass spectrometer, a single charged octadecamer species was selected by the quadrupole mass analyzer and fragmented by collision with argon gas. The mass-to-charge ratios of the fragments were determined in TOF and the charge state distributions were calculated using the MassLynx software system. The peaks corresponding to the monomers in the CID spectrum in panel D contain a predominant species with a molecular mass of 26206.7. As dimers consist of monomers with different posttranslational modifications, the peaks corresponding to the dimers in the CID spectrum in panel B are broader than those in panel D. The median molecular mass calculated from the range of dimer peaks is 52412.3.
Impaired octadecamer re-oligomerization from fully oxidized hexamers
The ability of adiponectin to exist as octadecamers without disulfide bonds argues against structure stabilization of octadecamers as the primary function of forming disulfides during assembly of octadecameric adiponectin. Yet data in Figs. 2 and 3 indicate a requirement for disulfide bond formation during octadecamer oligomerization. The most likely conclusion that could account for both lines of experimental observations is a need for disulfide bonds to link intermediates together during octadecamer assembly. This suggests that only the intermediate oligomers that possess free thiols near their N-terminus have the ability to further oligomerize and eventually grow into octadecamers. If this hypothesis is correct, fully oxidized intermediate oligomers without free thiols should lack the ability to oligomerize to octadecamers. Previous studies have shown that HMW adiponectin could be easily converted to hexamers with a modest decrease in pH (15, 17). We thus compared the rate of low pH-derived hexamers to re-assemble into octadecameric adiponectin in vitro with that of low pH and βME treatment-derived trimers. Without the additive effects of βME or DTT, purified adiponectin octadecamers in PBS were collapsed to mostly hexamers by decreasing the pH of the solution to 5 with glycine alone (Fig. 6A). Minor amounts of an oligomer intermediate between hexamers and octadecamers were also observed (Fig. 6A). While the oligomerization state of this minor oligomer remains to be defined, its levels could be decreased by adding high concentrations of NaCl (data not shown). Upon dialysis against PBS at 7.6, negligible amounts of octadecameric adiponectin re-formed from hexamers by 1 hr (Fig. 6A). In contrast, addition of βME to otherwise identically treated octadecamers at pH 5 resulted in appearance of trimers and, following dialysis, re-assembly of octadecameric adiponectin (Fig. 6A). To confirm that glycine-mediated lowering of pH alone did not produce modifications or conformational changes that disrupted the potential of hexamers to re-oligomerize, the fully oxidized hexamers that remained at the end of a 2 hr dialysis period were treated in 50 mM βME and dialyzed again under identical conditions and duration as the first dialysis procedure (Fig. 6B and 6C). Following reduction of disulfide bonds by βME treatment to produce trimers (Fig. 6B and 6C) and dialysis, significant amounts of octadecameric adiponectin had re-assembled from the same hexamers that had previously failed to re-oligomerize under the same conditions (Fig. 6B). As shown previously in Figs. 2 and 3, the re-assembly of octadecameric adiponectin was accompanied by formation of disulfide bonds (Fig. 6C). These results indicate that fully oxidized adiponectin hexamers can re-assemble into octadecamers at only a very slow rate. They support the idea that disulfide bond formation can facilitate the assembly of adiponectin octadecamers from reduced intermediates by accelerating the rate of oligomerization.
Figure 6. (A) Comparison of re-oligomerization rates between oxidized hexamers and reduced trimers using native PAGE.
Samples of adiponectin octadecamers were converted to mostly trimers by βME treatment at pH 5 while a decrease of pH to 5 alone resulted in mostly hexamers. Samples were dialyzed against PBS at pH 7.6 as described in Experimental Procedures to allow re-oligomerization to occur over a 1 hr period. (B) Native and (C) non-reducing denaturing PAGE analysis of the low pH-derived hexamers’ potential to re-oligomerize to octadecamers. Samples of hexamers generated originally by glycine treatment were dialyzed against PBS for 2 hrs as described in Experimental Procedures. After 2 hrs of dialysis, the samples were treated with βME to convert oxidized hexamers to reduced trimers, and the abilities of these trimers to re-oligomerize to octadecamers and to re-form disulfide bonds over a period of 2 hrs were monitored in native and non-reducing denaturing gels.
Discussion
The importance of decreased serum levels of HMW adiponectin in insulin resistance is well documented (22, 44, 45). As recently reviewed (46, 47), mounting evidence indicates that this decrease could be the consequence of defects in adiponectin oligomerization. Currently, it is known that adiponectin oligomerization involves molecular chaperones ERp44 and DsbA-L (35, 36) and ER oxidoreductase Ero1-Lα (33). It also requires disulfide bonds between adjacent cysteine residues near the N-terminus (15–17). However, a detailed molecular description of adiponectin oligomer assembly remains lacking. Without such knowledge, it is impossible to understand the mechanisms by which ERp44, DsbA-L, and Ero1-Lα influence adiponectin oligomerization and why levels of HMW adiponectin are reduced in insulin resistance. Results from the present study indicate that oligomerization of octadecamers, the largest and most abundant of HMW isoforms, occur spontaneously from trimers produced under specific reducing conditions (Fig. 1). The only necessary condition is formation of disulfide bonds (Figs. 2 and 3). While formation of disulfide bonds is required for re-oligomerization of adiponectin octadecamers, we were able to demonstrate the presence of stable and non-disulfide bonded octadecameric adiponectin using multiple analytical methods (Figs. 4 to 5). In addition, fully oxidized hexamers were unable to re-oligomerize into octadecamers at a rate comparable to reduced trimers (Fig. 6). These in vitro data led us to propose that while octadecamers can be formed through spontaneous aggregation of smaller oligomers, the rate of formation is greatly accelerated by stabilization of intermediate oligomers through disulfide bonds. This conclusion may have potential implications in disease processes. In insulin resistance associated with type 2 diabetes, reduced levels of circulating HMW adiponectin is likely due in part to an impaired oligomerization process (46, 47). If the ER redox condition becomes excessively oxidizing, or if chaperones shielding the unpaired cysteines are lacking, the intermediate oligomers could be trapped in a fully oxidized state that could only oligomerize slowly. The decreased levels of octadecamers associated with insulin resistance could be the result of an abnormally oxidizing state in the ER or decreased levels of chaperones. Indeed, levels of DsbA-L, an adiponectin-interacting ER resident protein with thioredoxin-like domains, have been shown to be reduced in humans and mice exhibiting insulin resistance (36).
Thermodynamics of adiponectin oligomerization
Our findings have four implications. First, assembly of adiponectin octadecamers could proceed spontaneously through self-association of at least partially reduced trimers. No additional proteins are needed for assembly of octadecamers in vitro. Oxidation of cysteine residues near the N-terminus of adiponectin molecules into disulfides also appears to be spontaneous. While reducing equivalents in the form of DTT or βME are needed to break up octadecamers, no energy input in the form of ATP, reducing equivalents, or heat, is necessary for re-oligomerization of adiponectin. This suggests that both oligomerization of adiponectin and oxidative formation of disulfide bonds are energetically favorable processes. If oligomerization is thermodynamically favorable needing only reduced trimers as starting substrates, the most likely role of ER chaperones and oxidoreductases is to influence the rate of adiponectin oligomerization and the relative distribution of different oligomers. If the in vitro findings in this study holds true in vivo, future studies on the regulation of adiponectin oligomerization can focus on the mechanisms by which ER chaperones and oxidoreductases can influence the distribution of hexamers versus HMW adiponectin. Regulation of ER redox state is not well understood but appears to be tightly controlled (48–51). If the reduction of HMW adiponectin associated with insulin resistance is a characteristic of impaired oxidative protein folding in the ER, understanding the causes of decreased HMW adiponectin formation will likely provide insight into the regulation of ER redox state.
A major question surrounding the present study is whether the spontaneity of oligomerization is restricted to bovine adiponectin and not shared by mouse or human adiponectin. In a pioneering study using size-fractionated human plasma, Peake et al observed conversion of adiponectin present in HMW plasma fractions to trimers following treatment with 100 mM DTT at 37°C for 60 min (18). Upon removal of DTT by gel filtration, there was substantial re-formation of HMW adiponectin, even though the authors described the re-formed HMW adiponectin as “not fully assembled to the size of the HMW isoform present in plasma” (18). These data suggest that human HMW adiponectin could undergo re-oligomerization similar to bovine adiponectin octadecamers. The inability of size-fractionated plasma containing HMW adiponectin to reassemble into bona fide HMW species perhaps reflects presence of proteins found in the same fractions that interfered with the oligomerization process.
Role of disulfide bond formation in oligomer assembly
Another implication of our in vitro findings is that disulfide bonds may be critical for the oligomerization process of adiponectin octadecamer in the ER in addition to having a structural role in the maintenance of fully assembled octadecamer. In Figs. 4 and 5, treating mature adiponectin octadecamers purified from serum with low concentrations of DTT resulted in near total reduction of disulfide bonds but only partial collapse of small amounts of octadecamers to trimers. These data indicate that while disulfide bonds do have a stabilizing effect in maintaining mature octadecamers, their presence is nevertheless not an obligatory requirement for the octadecameric structure. At first glance, this finding appears to contradict the data showing the necessity of disulfide bond formation in octadecamer assembly (Figs. 2 and 3). A hypothesis that is consistent with both findings is a need for disulfide bonds to stabilize intermediate oligomers during octadecamer assembly. Indeed, while fully reduced octadecamer was abundantly present in adiponectin treated with DTT, no intermediate species other than small amounts of trimers and hexamers were observed (Figs. 4 and 5). This suggests the predicted intermediate nonameric, dodecameric, and pentadecameric species are unstable without any disulfide bonds. In contrast, what appears to be nonameric and docadecameric adiponectin species were clearly visible in Fig. 2A and Fig. 3A along with octadecamer as disulfide-bonded adiponectin became more prevalent (Fig. 2B and Fig. 3B). Interestingly, addition of hydrogen peroxide in dialysis buffer accelerated the oxidation of adiponectin (Fig. 2B) and formation of octadecamers (Fig. 2A). While peroxide has been known to induce cysteine oxidation in aqueous solution (40), it was recently shown to accelerate disulfide bond formation in bovine pancreatic trypsin inhibitor (52).
The ability to form stable octadecamers lacking disulfide bonds is likely not an exclusive property of bovine adiponectin. Schraw and colleagues successfully demonstrated the presence of an HMW-like complex in addition to trimers in a solution of recombinant mouse C39S adiponectin (C22 residue excluding signal sequence) lacking the disulfide-forming cysteine residue near the N-terminus (19). While the authors described the C39S HMW-like complex as having a gel filtration elution profile that was not identical to that of recombinant wild type mouse HMW adiponectin, their data support the possibility of a higher-ordered mouse adiponectin structure without disulfide bonds.
Assembly pathways of adiponectin octadecamers and hexamers
The third implication of our in vitro findings is that assembly of mature adiponectin hexamers and octadecamers are distinct processes. Mature adiponectin hexamers are fully oxidized (16), and fully oxidized hexamers failed to re-assemble into octadecamers in the time frame during which reduced trimers re-assembled into octadecamers in significant quantities (Fig. 6). Fully oxidized hexamers may represent a dead end product that is secreted rather than retained inside the cell as precursors for HMW adiponectin. Using disulfide bonds to stabilize intermediates may be the reason why fully oxidized hexamers do not assemble into octadecamers to a significant extent. Fully oxidized hexamers do not have free sulfhydryls that could form additional disulfide bonds with other oligomers. In contrast, fully or partially reduced adiponectin oligomers have the potential to further oligomerize into larger species by forming disulfide bonds with other oligomers. This indicates that while partially overlapping, assembly pathways of hexamers and octadecamers are distinct. Fully oxidized hexamers cannot serve as building blocks for octadecamers. Relative flux through the distinct assembly pathways for adiponectin hexamer and octadecamer could account for differences in the ratio of HMW to hexameric adiponectin among individuals or various physiological states (22–25, 44).
If disulfide bonds indeed are responsible for bridging smaller oligomers and stabilizing larger intermediate structures, as our in vitro results indicate, these disulfide bonds must be rearranged prior to secretion of mature octadecamers. Mature octadecamers are not composed of trimers that are all connected to each other through disulfides. Octadecamers could be converted to hexamers without reduction of disulfide bonds (15). This indicates that the intermediate oligomers are held together only temporarily by disulfide bonds during assembly. These transient disulfide bonds are subsequently broken and rearranged in a manner that leads to the appearance of three oxidized hexamers within a mature octadecamer. The presence of both oxidized and reduced cysteine residues within a single trimer may facilitate trans-thiolation reactions within the octadecameric intermediate to achieve this maturation. The potential combination of disulfide formation and rearrangement suggests a proper redox environment in the ER is crucial for octadecamer assembly. An excessively oxidizing redox environment, characterized, for example, by high levels of reactive oxygen species, may prematurely lead to fully oxidized hexamers that are unable to oligomerize further. It may also make the predicted rearrangement of disulfide bonds in the final octadecameric intermediate difficult to achieve, leading to decreased secretion of octadecamers. On the other hand, if the redox environment is too reducing, disulfide bonds needed to bridge intermediate oligomers together may not occur at all.
The ER is more oxidizing than the surrounding cytosol (53). A crucial question is whether there is a need to protect the cysteine residues of adiponectin from premature oxidation. ERp44 and DsbA-L are ER chaperones that have been shown to influence the oligomerization of adiponectin and/or promote its secretion (35, 36). It is possible that these proteins can shield the cysteine residues of adiponectin, in the case of ERp44 by directly forming mixed disulfides, from premature oxidation and as a result facilitate formation of HMW adiponectin.
Molecular forces holding adiponectin octadecamers together
Lastly, the presence of octadecameric adiponectin lacking virtually all disulfide bonds in vitro demonstrated strong non-covalent interactions among constituent subunits within octadecamers. HMW adiponectin was previously shown to be easily converted to hexamers under mildly acidic conditions without reduction of disulfides within HMW adiponectin (15, 17). In addition, HMW adiponectin has never been observed to convert to other oligomers in circulation (15, 19, 54) or in vitro under normal storage conditions (21). Combined, these two lines of evidence suggest that molecular interactions other than disulfide bonds likely contribute to the stability of HMW adiponectin. Nevertheless, there has been no solid experimental evidence showing the existence of such molecular interactions. Hydroxylation and subsequent glycosylation of lysine residues in the collagen domain of adiponectin has been shown to be important for HMW adiponectin formation (18, 55). However, while glycosylation has been shown to stabilize the triple helical structure of collagen domains (56), it is not known to facilitate clustering of multiple collagen triple helices. Based upon electron micrographs, the region showing the most contact among trimers within the octadecamer is the collagen tail (16, 20, 57). While stability of long collagen fibrils is conferred by hydration forces and entropy associated with hydrophobic forces (58, 59), it is not clear if these interactions are sufficient to cluster short collagen domains like those in adiponectin (only 22 Gly-X-Y or Gly-X-Pro repeats). Data from this study provided unambiguous proof of strong non-covalent interactions that can sustain an octadecameric structure for adiponectin molecules lacking disulfide bonds. We propose that the short collagen domains in adiponectin serve to provide the non-covalent interactions which maintain octadecamers, representing a novel mechanism of protein-protein interaction.
In summary, the present study attempted to address the mechanisms of adiponectin oligomerization using an in vitro approach based on octadecamers purified from serum. The results demonstrated abundant presence of stable and non-disulfide bonded octadecameric adiponectin, implying the existence of strong non-covalent interactions that could drive the spontaneous assembly of octadecamers using disulfide bonds as intermediate-stabilizing forces. We hope these findings will provide the groundwork for developing future hypotheses that can be tested both in vitro and in vivo to understand the cause of decreased HMW adiponectin in insulin resistance and other disease states.
Acknowledgments
We thank Drs. Vahe Bandarian, David Lee, Megan McEvoy, and Lisa Rezende for valuable discussions throughout this study, and Ms. Kiki M. Moriguchi and Shiana Ferng for expert technical assistance.
Abbreviations
- IAA
iodoacetamide
- NEM
N-ethylmaleimide
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- DTT
dithiothreitol
- βME
β-mercaptoethanol
- EDTA
ethylenediaminetetraacetic acid
- SDS
sodium dodecyl sulfiate
- PAGE
polyacrylamide gel electrophoresis
- MS
mass spectrometry
- CID
collision-induced dissociation
- HMW
higher molecular weight
- PBS
phosphate buffered saline
- kDa
kilodaltons
- ER
endoplasmic reticulum
Footnotes
This work was supported by a Junior Faculty Award from American Diabetes Association (1-08-JF-54) and by a grant from the Arizona Biomedical Research Commission to T.-S.T. We also acknowledge financial support from NSF (DBI Grant CHE 024447) for development of the QToF instrument and NIH (R01 GM-051387) for support of experiments on fragmentation. C.M.J. is the recipient of a Pfizer Graduate Research Fellowship in Analytical Chemistry. MALDI-TOF mass spectrometric data were acquired by the Arizona Proteomics Consortium with support from NIEHS (ES06694) to the University of Arizona Southwest Environmental Health Sciences Center, from NIH/NCI (CA023074) to the Arizona Cancer Center, and from the BIO5 Institute of the University of Arizona.
References
- 1.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(Suppl 7):S13–18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–221. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 6.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 8.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 10.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 12.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 13.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Current Biology. 1998;8:335–340. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 15.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 16.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 17.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 18.Peake PW, Hughes JT, Shen Y, Charlesworth JA. Glycosylation of human adiponectin affects its conformation and stability. J Mol Endocrinol. 2007;39:45–52. doi: 10.1677/JME-07-0030. [DOI] [PubMed] [Google Scholar]
- 19.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–814. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 22.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 23.Engl J, Bobbert T, Ciardi C, Laimer M, Tatarczyk T, Kaser S, Weiss H, Molnar C, Tilg H, Patsch JR, Spranger J, Ebenbichler CF. Effects of pronounced weight loss on adiponectin oligomer composition and metabolic parameters. Obesity (Silver Spring) 2007;15:1172–1178. doi: 10.1038/oby.2007.627. [DOI] [PubMed] [Google Scholar]
- 24.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the High-Molecular Weight Form of Adiponectin in Plasma Is Useful for the Prediction of Insulin Resistance and Metabolic Syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 25.O’Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 26.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 27.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab. 2006;290:E42–E46. doi: 10.1152/ajpendo.00240.2005. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin CC, Finck BN, Eagon JC, Halpin VJ, Magkos F, Mohammed BS, Klein S. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity (Silver Spring) 2007;15:640–645. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 31.Pereira RI, Leitner JW, Erickson C, Draznin B. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci. 2008;83:638–643. doi: 10.1016/j.lfs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab. 2008;295:E842–850. doi: 10.1152/ajpendo.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase ero1-l alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding Y-Y, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZV, Schraw TD, Kim J-Y, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the Adipocyte-Specific Secretory Protein Adiponectin Critically Depends on Thiol-Mediated Protein Retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276:28849–28856. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Lu G, Wong WP, Vliegenthart JF, Gerwig GJ, Lam KS, Cooper GJ, Xu A. Proteomic and functional characterization of endogenous adiponectin purified from fetal bovine serum. Proteomics. 2004;4:3933–3942. doi: 10.1002/pmic.200400826. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Luo D, Smith SW, Anderson BD. Kinetics and mechanism of the reaction of cysteine and hydrogen peroxide in aqueous solution. J Pharm Sci. 2005;94:304–316. doi: 10.1002/jps.20253. [DOI] [PubMed] [Google Scholar]
- 41.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 42.Galhena AS, Dagan S, Jones CM, Beardsley RL, Wysocki VH. Surface-Induced Dissociation of Peptides and Protein Complexes in a Quadrupole/Time-of-Flight Mass Spectrometer. Analytical Chemistry. 2008;80:1425–1436. doi: 10.1021/ac701782q. [DOI] [PubMed] [Google Scholar]
- 43.Shafer DE, Inman JK, Lees A. Reaction of Tris(2-carboxyethyl)phosphine (TCEP) with maleimide and alpha-haloacyl groups: anomalous elution of TCEP by gel filtration. Anal Biochem. 2000;282:161–164. doi: 10.1006/abio.2000.4609. [DOI] [PubMed] [Google Scholar]
- 44.Seino Y, Hirose H, Saito I, Itoh H. High molecular weight multimer form of adiponectin as a useful marker to evaluate insulin resistance and metabolic syndrome in Japanese men. Metabolism. 2007;56:1493–1499. doi: 10.1016/j.metabol.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 46.Wolf G. New insights into thiol-mediated regulation of adiponectin secretion. Nutr Rev. 2008;66:642–645. doi: 10.1111/j.1753-4887.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci U S A. 2008;105:18077–18078. doi: 10.1073/pnas.0810027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sitia R, Molteni SN. Stress, protein (mis)folding, and signaling: the redox connection. Sci STKE 2004. 2004:pe27. doi: 10.1126/stke.2392004pe27. [DOI] [PubMed] [Google Scholar]
- 49.Molteni SN, Fassio A, Ciriolo MR, Filomeni G, Pasqualetto E, Fagioli C, Sitia R. Glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum. J Biol Chem. 2004;279:32667–32673. doi: 10.1074/jbc.M404992200. [DOI] [PubMed] [Google Scholar]
- 50.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Freedman RB. A non-catalytic disulphide bond regulating redox flux in the ER oxidative folding pathway. Embo J. 2009;28:169–170. doi: 10.1038/emboj.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karala AR, Lappi AK, Saaranen M, Ruddock LW. Efficient Peroxide Mediated Oxidative Refolding of a Protein at Physiological Ph and Implications for Oxidative Folding in the Endoplasmic Reticulum. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2326. [DOI] [PubMed] [Google Scholar]
- 53.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 54.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol. 2005;153:409–417. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Lam KSL, Chan L, Chan KW, Lam JBB, Lam MC, Hoo RCL, Mak WWN, Cooper GJ, Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 56.Bann JG, Bachinger HP. Glycosylation/Hydroxylation-induced stabilization of the collagen triple helix. 4-trans-hydroxyproline in the Xaa position can stabilize the triple helix. J Biol Chem. 2000;275:24466–24469. doi: 10.1074/jbc.M003336200. [DOI] [PubMed] [Google Scholar]
- 57.Radjainia M, Wang Y, Mitra AK. Structural Polymorphism of Oligomeric Adiponectin Visualized by Electron Microscopy. J Mol Biol. 2008;381:419–430. doi: 10.1016/j.jmb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Piez KA, Trus BL. Microfibrillar structure and packing of collagen: hydrophobic interactions. J Mol Biol. 1977;110:701–704. doi: 10.1016/s0022-2836(77)80086-7. [DOI] [PubMed] [Google Scholar]
- 59.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]