Abstract
The Arabidopsis FCLY gene encodes a specific farnesylcysteine (FC) lyase, which is responsible for the oxidative metabolism of FC to farnesal and cysteine. In addition, fcly mutants with quantitative decreases in FC lyase activity exhibit an enhanced response to ABA. However, the enzymological properties of the FCLY-encoded enzyme and its precise role in ABA signaling remain unclear. Here, we show that recombinant Arabidopsis FC lyase expressed in insect cells exhibits high selectivity for FC as a substrate and requires FAD and molecular oxygen for activity. Arabidopsis FC lyase is also shown to undergo post-translational N-glycosylation. FC, which is a competitive inhibitor of isoprenylcysteine methyltransferase (ICMT), accumulates in fcly mutants. Moreover, the enhanced response of fcly mutants to ABA is reversed by ICMT overexpression. These observations support the hypothesis that the ABA hypersensitive phenotype of fcly plants is the result of FC accumulation and inhibition of ICMT.
Keywords: Hormone biology, primary metabolism, seed biology, stomata, membrane proteins, Arabidopsis
INTRODUCTION
Isoprenylated proteins are modified at the carboxyl terminus by a farnesylcysteine (FC) or geranylgeranylcysteine (GGC) moiety (Clarke, 1992; Zhang and Casey, 1996; Rodríguez-Concepción et al., 1999; Crowell, 2000; Galichet and Gruissem, 2003; Crowell and Huizinga, 2009). These modifications are catalyzed by protein farnesyltransferase (PFT) and protein geranylgeranyltransferase type I (PGGT I), respectively, which transfer the prenyl group from farnesyl diphosphate (FPP) or geranylgeranyl diphosphate (GGPP) to the cysteine residue of a carboxyl terminal CaaX motif (C = Cys; a = aliphatic; X = Cys, Ala, Ser, Gln, Met, Leu, Ile). In a similar manner, protein geranylgeranyltransferase type II (PGGT II) transfers geranylgeranyl groups from geranylgeranyl diphosphate to the carboxyl terminal cysteine residues of RAB proteins bound by the RAB escort protein. Isoprenylated CaaX proteins are subsequently proteolyzed, resulting in the removal of the three amino acids downstream of the isoprenylcysteine residue, and carboxyl methylated. These modifications are catalyzed by CaaX endoproteases and isoprenylcysteine methyltransferases (ICMT), respectively (Crowell et al., 1998; Crowell and Kennedy, 2001; Bracha et al., 2002; Narasimha Chary et al., 2002; Cadiñanos et al., 2003; Bracha-Drori et al., 2008). Demethylation of isoprenylated proteins by isoprenylcysteine methylesterase (ICME) has also been reported (Deem et al., 2006; Huizinga et al., 2008).
In Arabidopsis, protein isoprenylation is involved in negative regulation of abscisic acid (ABA) signaling and meristem development. Loss-of-function mutations in the ENHANCED RESPONSE TO ABA1 (ERA1) gene, which encodes the β-subunit of PFT, cause an enhanced response to ABA and enlarged meristems (Cutler et al., 1996; Pei et al., 1998; Running et al., 1998; Bonetta et al., 2000; Yalovsky et al., 2000; Ziegelhoffer et al., 2000). Consequently, era1 mutants exhibit drought resistance and supernumerary floral organs. Loss-of-function mutations in the PLURIPETALA (PLP) gene, which encodes the α-subunit of both PFT and PGGT 1, also cause an enhanced response to ABA and an exaggerated era1-like developmental phenotype (Running et al., 2004). However, to date, a farnesylated negative regulator of ABA signaling has not been reported. Loss-of-function mutations in the GERANYLGERANYLTRANSFERASE BETA (GGB) gene, which encodes the β-subunit of PGGT I, also cause an enhanced response to ABA in guard cells (but not seeds) and an enhanced response to auxin-induced lateral root formation (Johnson et al., 2005). Consistent with these observations, ROP2 and ROP6 have been identified as negative regulators of ABA signaling (Lemichez et al., 2001; Li et al., 2001; Yang, 2002) and AUX2-11 (IAA4), AGG1, and AGG2 have been identified as negative regulators of auxin-induced lateral root formation (Wyatt et al., 1993; Trusov et al., 2007). All five proteins are predicted or known to be geranylgeranylated (Caldelari et al., 2001; Zeng et al., 2007).
Like all proteins, isoprenylated proteins exist in equilibrium between synthesis and turnover; however, whereas the degradation of unmodified proteins liberates free amino acids, the degradation of isoprenylated proteins also liberates isoprenylcysteine compounds (FC or GGC). Consequently, enzymes exist in eukaryotic cells for the metabolism of FC and GGC. From mammalian systems, it is known that proteolysis of isoprenylated proteins generates FC and GGC, which are metabolized to cysteine and either farnesal or geranylgeranial by prenylcysteine lyase, an FAD-dependent thioether oxidase (Zhang et al., 1997; Tschantz et al., 1999, 2001; Digits et al., 2002). However, prenylcysteines and prenylcysteine methyl esters can also be oxidized by flavin-dependent monooxygenases (FMOs) or P450s, or cleaved by cysteine β-lyases (Cashman et al., 1990; Sausen and Elfarra, 1990; Park et al., 1992, 1994). Prenylcysteine lyase is localized to lysosomal membranes, a major site of lipid metabolism, and catalyzes C–S bond cleavage by a mechanism involving removal of the pro-S hydrogen at the C-1 position of the isoprenyl moiety via sequential one-electron transfers to non-covalently bound FAD. This step is followed by hydrolysis of the resulting thiocarbenium ion to a hemithioacetal intermediate (Tschantz et al., 2001; Digits et al., 2002), which breaks down into cysteine and a prenyl aldehyde. Oxidation of FADH2 by O2 generates H2O2 and completes the catalytic cycle. Although mammalian prenylcysteine lyase is specific for substrates with a primary amino group (i.e. it does not recognize N-acetylated isoprenylcysteine substrates), it lacks significant specificity for the isoprenoid tail (i.e. it exhibits similar kinetics for FC and GGC) (Zhang et al., 1997). Disruption of the prenylcysteine lyase gene in fibroblast cells results in greater sensitivity to growth inhibition in the presence of prenylcysteines (Beigneux et al., 2002), and prenylcysteine lyase knockout mice accumulate significant amounts of FC and GGC in brain and liver tissue. Surprisingly, prenylcysteine accumulation is not accompanied by significant developmental or physiological consequences in these mice (Beigneux et al., 2002).
Farnesal and geranylgeranial metabolism is not well understood, but farnesol and geranylgeraniol metabolism has been analyzed in detail. Isoprenyl alcohols, for example, can be degraded via a mechanism similar to fatty acid metabolism or oxidized at the C-1 and ω positions (Christophe and Popjak, 1961; Havel et al., 1986; Gonzalez-Pacanowska et al., 1988; Keung, 1991; Bostedor et al., 1997; Vaidya et al., 1998; DeBarber et al., 2004). Farnesol and geranylgeraniol can also be sequentially phosphorylated by CTP-dependent kinases and the resulting FPP and GGPP used for isoprenoid biosynthesis (Crick et al., 1997; Westfall et al., 1997; Bentinger et al., 1998; Thai et al., 1999).
Unlike mammals, Arabidopsis plants possess an FC lyase (FCLY) with remarkable specificity for FC (Crowell et al., 2007). Moreover, because the Arabidopsis genome contains no genes with significant relatedness to the FCLY (At5g63910) gene, GGC metabolism is thought to proceed by a different mechanism (e.g. S-oxidation by a flavin-dependent monooxygenase, C–S bond cleavage by a pyridoxal 5-phosphate (PLP)-dependent β-lyase, etc.). Reduction of farnesal to farnesol has been shown to be catalyzed by an NAD(P)H-dependent aldehyde reductase/NAD(P)-dependent alcohol dehydrogenase in Arabidopsis (Crowell et al., 2007). Together, these observations suggest the existence of a recycling pathway in plants whereby the farnesal product of FC lyase is reduced to farnesol, which is subsequently phosphorylated to farnesyl diphosphate. Indeed, CTP-dependent phosphorylation of farnesol to farnesyl diphosphate has been described in plants (Thai et al., 1999). Whereas FC and GGC accumulation results in no apparent phenotype in prenylcysteine lyase knockout mice, fcly-1 and fcly-2 mutants of Arabidopsis, which exhibit 20 and 50% of wild-type FC lyase activity, respectively, are hypersensitive to ABA (Crowell et al., 2007). However, the connection between FCLY function and ABA signaling remains obscure. Because FC compounds are competitive inhibitors of ICMT (Tan et al., 1991; Shi and Rando, 1992; Ma et al., 1995; Narasimha Chary et al., 2002) and ICMT is a negative regulator of ABA signaling (Huizinga et al., 2008), we propose that the hypersensitivity of fcly mutants to ABA is caused by FC accumulation and competitive inhibition of ICMT.
To confirm the selectivity of Arabidopsis FC lyase for FC and gain insights into the catalytic mechanism of this unique enzyme, we performed kinetic analyses on recombinant Arabidopsis FC lyase expressed in Spodoptera frugiperda (Sf9) cells. Furthermore, to confirm or refute the hypothesis that the enhanced response of fcly mutants to ABA is caused by FC accumulation and concomitant inhibition of ICMT, we compared the FC content of wild-type and fcly plants and tested the prediction that overexpression of ICMT would suppress or reverse the ABA phenotype of fcly plants.
RESULTS
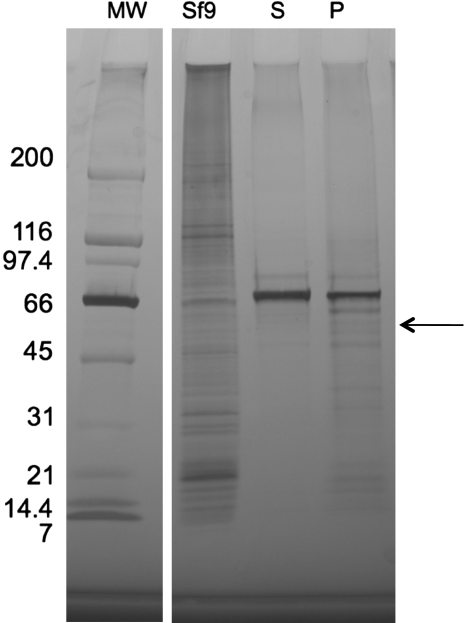
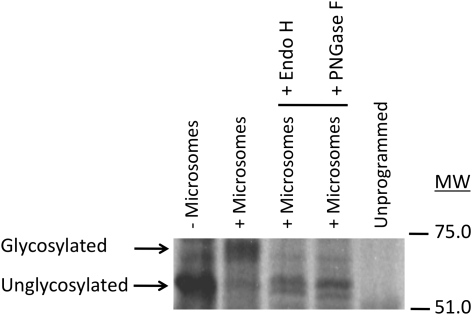
To study the kinetics and catalytic mechanism of Arabidopsis FC lyase, a suitable system for expression of recombinant enzyme was identified. We attempted to express the At5g63910 coding sequence in recombinant yeast cells using the pYES2.1/V5-His-TOPO vector, but were unable to detect galactose-inducible FC lyase activity. Consequently, we expressed the At5g63910 coding sequence in Spodoptera frugiperda (Sf9) cells using recombinant baculovirus—a strategy that was used successfully to express human prenylcysteine lyase (Tschantz et al., 1999). As shown in Figure 1, recombinant Arabidopsis FC lyase was successfully expressed in Sf9 cells. The first sample lane of Figure 1 shows a crude lysate from uninfected Sf9 cells. The second and third lanes show supernatant and pellet fractions, respectively, from Sf9 cells 3 d post infection (dpi) following lysis in the presence of 0.2% CHAPS and centrifugation at 16 000 g. A prominent band at approximately 67 kDA can be seen in both the detergent soluble and insoluble fractions. Interestingly, the protein is approximately 11.7 kDa larger than the predicted molecular mass of the FCLY-encoded protein (55.3 kDa)—a difference that agrees almost exactly with the difference between the predicted and apparent molecular masses of human prenylcysteine lyase (Tschantz et al., 1999). In the case of the human enzyme, N-glycosylation was shown to be responsible for this difference (Tschantz et al., 1999). To test the hypothesis that N-glycosylation accounts for the slow migration of recombinant Arabidopsis FC lyase on SDS-polyacrylamide gels, we searched for potential N-glycosylation sites in the Arabidopsis FC lyase coding sequence. As shown in Figure 2, the protein product of the FCLY gene possesses a predicted amino terminal signal sequence and four putative N-glycosylation sites (N-X-S/T), and a Kyte-Doolittle hydropathy plot predicts three or four transmembrane domains (data not shown). In addition, FC lyase synthesized in vitro in the presence of microsomal membranes migrated at a higher apparent molecular mass than FC lyase synthesized in the absence of microsomal membranes (Figure 3). Treatment of the former with endoglycosidase H (Endo H) or peptide N-glycosidase F (PNGase F) caused the protein to co-migrate with FC lyase synthesized in the absence of microsomes, confirming the N-glycosylation of the enzyme (Figure 3). Treatment of recombinant Arabidopsis FC lyase from Sf9 cells with Endo H also caused the enzyme to migrate at an apparent molecular mass of 55 kDa (data not shown).
Figure 1.
Expression of Recombinant Arabidopsis FC Lyase in Insect Cells.
MW, MW markers are indicated; Sf9, crude extract from uninfected Sf9 cells; S, supernatant fraction from Sf9 cells infected with FCLY-recombinant virus (3 dpi) following lysis in the presence of 0.2% CHAPS and centrifugation at 16 000 g; P, pellet fraction from Sf9 cells infected with FCLY-recombinant virus (3 dpi) following lysis in the presence of 0.2% CHAPS, centrifugation at 16 000 g, and solubilization of the pellet in 0.5% TritonX-100. The predicted molecular mass of Arabidopsis FC lyase is 55.3 kDa (see arrow). Recombinant Arabidopsis FC lyase from insect cells migrates at approximately 67 kDa, suggesting possible post-translational N-glycosylation.
Figure 2.
Features of the FCLY Coding Sequence.
The FCLY coding sequence is predicted to encode a protein with an amino terminal signal peptide and four potential N-glycosylation sites. The arrow represents the probable site of signal peptide cleavage and asterisks mark the positions of putative N-glycosylation sites. A potential sequence-specific vacuolar-sorting signal near the carboxyl terminus is underlined.
Figure 3.
N-Glycosylation of Arabidopsis FC Lyase In Vitro.
Arabidopsis FC lyase was synthesized in vitro using a TnT transcription/translation kit (Promega) in the presence or absence of canine pancreatic microsomal membranes and analyzed by SDS–PAGE and autoradiography. Following in vitro translation in the presence of microsomal membranes, samples were either analyzed directly or solubilized with 0.2% SDS, treated with Endo H or PNGase F, and analyzed. An unprogrammed translation reaction lacking FCLY DNA was also analyzed.
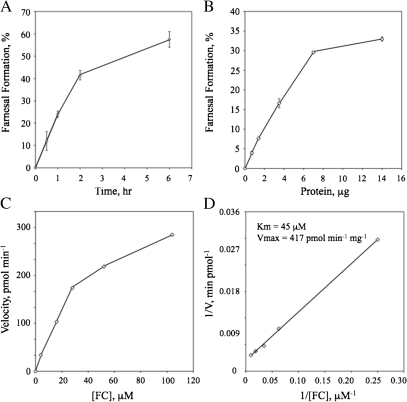
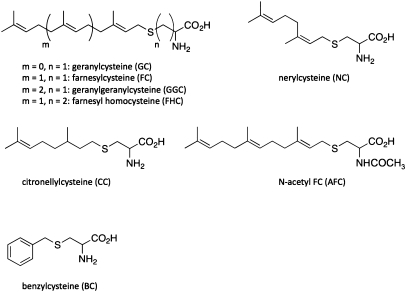
Recombinant Arabidopsis FC lyase activity ([1-3H]FC conversion to [1-3H]farnesal) was linear with time up to 2 h in the presence of 5 μg protein and linear with the amount of protein in the assay up to 7 μg in a 1-h assay (Figure 4). Moreover, the enzyme exhibited saturation kinetics, and a Lineweaver-Burke plot revealed an apparent Km of 45 μM for FC and an apparent Vmax of 417 pmol min−1 mg−1. To test the substrate specificity of recombinant Arabidopsis FC lyase, the ability of unlabeled FC, GGC, and other prenylcysteine analogs to inhibit the conversion of [1-3H]FC to [1-3H]farnesal was measured. As shown in Figure 5 and Table 1, unlabeled FC effectively competed with [1-3H]FC and exhibited an IC50 of 50 μM. Substrate analogs with alterations on the amino acyl side of the sulfur atom (N-acetyl FC and farnesyl homocysteine) also competed, but with IC50 values 4–10-fold higher than that of unlabeled FC. In contrast, analogs with alterations in the prenyl moiety were significantly less competitive. In fact, geranylgeranylcysteine, nerylcysteine, citronellylcysteine, and benzylcysteine did not compete (no inhibition of [1-3H]FC oxidation was observed at concentrations as high as 500 μM). The exception to this was geranylcysteine, which exhibited an IC50 four-fold higher than that of unlabeled FC. These data demonstrate the specificity of Arabidopsis FC lyase, which appears to have no detectable activity toward geranylgeranylated substrates, and support the hypothesis that FC and GGC metabolism proceed by different mechanisms. Further support for this hypothesis can be found in Supplementary Data, where it is shown that [1-3H]GGC metabolism does not involve a geranylgeranial intermediate.
Figure 4.
Michaelis-Menten Kinetics of Recombinant Arabidopsis FC Lyase Expressed in Sf9 Cells.
FC lyase reactions were carried out as described in the Methods section. The standard error of the mean is indicated in (A)–(D); however, the errors bars are obscured by the plot symbols in (C) and (D).
(A) Linearity of FC lyase activity with time. Reactions contained 5 μg of protein and 5 μM [1-3H]FC.
(B) Linearity of FC lyase activity with protein. Reactions contained 4 μM [1-3H]FC and were incubated for 1 h.
(C) Substrate saturation kinetics of the FC lyase reaction. Reactions contained 3 μg of protein and were incubated for 1 h.
(D) Lineweaver-Burke plot of FC lyase substrate saturation kinetics. The data in Figure 4C were used for Lineweaver-Burke analysis.
Figure 5.
Structures of the Prenylcysteine Analogs used in Table 1.
Table 1.
Inhibition of FC Lyase by Diphenyl Iodonium Chloride and the Prenylcysteine Analogs Shown in Figure 5.
| Substrate/inhibitor | IC50, μM* | Notes |
| FC | 50 ± 6 | |
| N-acetyl FC | 512 ± 0 | |
| FHC | 187 ± 5 | |
| GGC | – | No inhibition at 325 μM |
| GC | 194 ± 9 | |
| NC | – | No inhibition at 500 μM |
| CC | – | No inhibition at 500 μM |
| BC | – | No inhibition at 500 μM |
| Diphenyl iodonium chloride | 124 ± 8 |
The standard error of the mean is indicated.
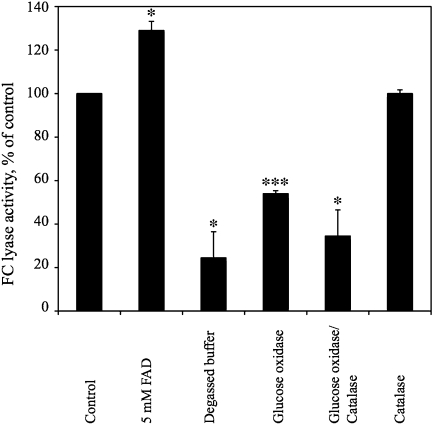
As shown in Table 1, diphenyl iodonium inhibited the FC lyase reaction. This observation suggests that, like mammalian prenylcysteine lyase, Arabidopsis FC lyase is a flavoprotein (Coves et al., 1999; Shiemke et al., 2004). To further examine the mechanism of action of Arabidopsis FC lyase, its dependence on FAD and molecular oxygen was tested. As shown in Figure 6, the addition of excess FAD to the reaction enhanced the FC lyase reaction by 30% (reactions were performed at substrate saturation as described in Methods). This result is consistent with the hypothesis that FAD is tightly, but non-covalently, bound to FC lyase and is required for FC lyase activity. To determine whether Arabidopsis FC lyase requires molecular oxygen, experiments were performed to monitor [1-3H]FC metabolism under anaerobic and aerobic (control) conditions. As expected, degassing the buffer solution caused a significant decrease in FC lyase activity (Figure 6). Moreover, the addition of glucose and glucose oxidase, which catalyzes the O2-dependent oxidation of β-D-glucose to D-glucono-1,5-lactone and H2O2, caused a 46% decrease in FC lyase activity, and the addition of glucose, glucose oxidase, and catalase caused a 66% decrease in activity (the latter prevents product inhibition of glucose oxidase by converting H2O2 to H2O and O2; however, two molecules of O2 are consumed by glucose oxidase for every molecule of O2 regenerated by catalase). These results support the mechanism proposed by Tschantz et al. (2001) for enzymes in the prenylcysteine lyase family. Hydrogen peroxide production by FC lyase was detected spectrophotometrically at 405 nm using ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) and horseradish peroxidase and was slightly less than stoichiometric relative to farnesal production, presumably due to the presence of a peroxidase activity in the membrane fraction of recombinant Sf9 cells (Table 2).
Figure 6.
Dependence of Arabidopsis FC Lyase on FAD and Molecular Oxygen.
FC lyase reactions were performed as described in the Methods section (3.5 μg protein, 4.5 μM [1-3H]FC, incubation for 0.5 or 1 h) in the presence of 5 mM FAD, degassed buffer, 2 U glucose oxidase, 2 U glucose oxidase plus 11 U catalase, and 11 U catalase. 20 mM glucose was added to all reactions containing glucose oxidase. * and *** represent significant differences compared with the control of P < 0.05 and P < 0.001, respectively, as determined by student's t-test.
Table 2.
Generation of Isoprenoid and Hydrogen Peroxide Products by Recombinant Arabidopsis FC Lyase.
| Product | Production, pmol min−1 mg−1*** |
| Farnesal + farnesol* | 1,333 ± 0 |
| H2O2** | 1,195 ± 28 |
* NADase pretreatments were not performed. Consequently, farnesal and farnesol were resolved and quantified by gas chromatography and the amounts summed. Because this experiment was performed with a different preparation of recombinant Arabidopsis FC lyase (i.e. a different baculovirus infection) from that described in Figure 4, the activity is higher. ** Peroxide formation was slightly less than farnesal and farnesol, presumably due to peroxidase activity in Sf9 cell extracts. *** The standard error of the mean is indicated.
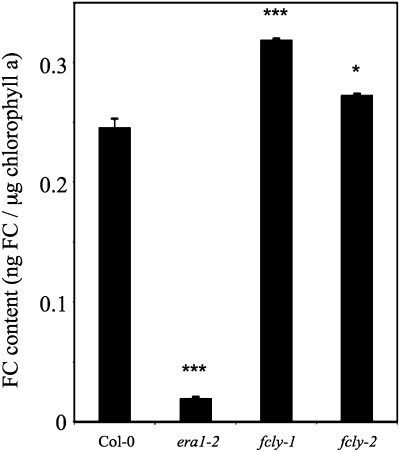
As stated above, fcly mutants of Arabidopsis exhibit reduced FC lyase activity and an enhanced response to ABA (Crowell et al., 2007). One hypothesis to explain this phenotype is that FC accumulation in fcly plants leads to competitive inhibition of ICMT activity. Because ICMT is a negative regulator of ABA signaling (Huizinga et al., 2008), it follows that inhibition of ICMT would result in an enhanced response to ABA. To test this hypothesis, Col-0, era1-2, fcly-1, and fcly-2 plants were analyzed for FC accumulation. era1-2 plants were included in this experiment as a negative control and were expected to contain little or no FC (i.e. because era1-2 plants lack the β-subunit of PFT). As shown in Figure 7, extracts of era1-2 plants contained significantly lower levels of FC than extracts of Col-0 plants (normalized to chlorophyll a, which co-extracts with FC). That era1-2 plants contained measurable amounts of FC, albeit greatly reduced, confirms the isoprenoid cross-specificity of PFT and PGGT1 and provides an explanation for the partial compensation of the PFT defect in era1-2 plants by PGGT1 (i.e. PGGT1 has a weak protein farnesyltransferase activity) (Trueblood et al., 1993; Yokoyama et al., 1995; Johnson et al., 2005). fcly-1 and fcly-2 plants, on the other hand, contained significantly higher levels of FC than Col-0 plants (Figure 7). fcly-1, which exhibits 20% of wild-type FC lyase activity (Crowell et al., 2007), contained 30% more FC than Col-0, whereas fcly-2, which exhibits 50% of wild-type FC lyase activity (Crowell et al., 2007), contained 11% more FC than Col-0. Similar results were obtained when the data were normalized to fresh weight.
Figure 7.
Quantification of FC Accumulation in Col-0, era1-2, fcly-1, and fcly-2 Seedlings.
The standard error of the mean is shown. * and *** represent significant differences compared with the Col-0 control of P < 0.05 and P < 0.001, respectively, as determined by student's t-test.
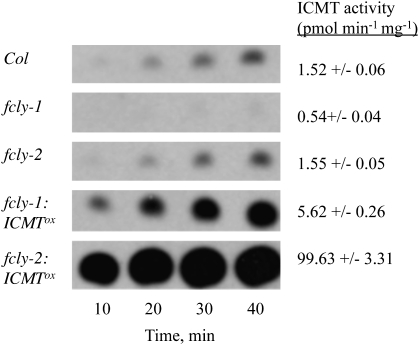
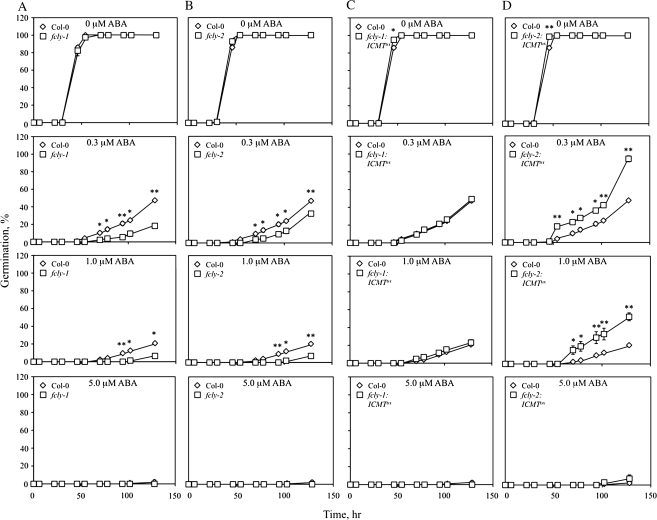
The next test was to determine whether overexpression of AtSTE14B (ICMT) suppresses or reverses the ABA phenotype of fcly-1 and fcly-2 plants—a result that would be expected if the ABA hypersensitivity of fcly-1 and fcly-2 plants was the result of competitive inhibition of ICMT by accumulated FC (FC is a substrate of ICMT and competes with prenylated proteins for the ICMT active site; Tan et al., 1991; Shi and Rando, 1992; Ma et al., 1995; Narasimha Chary et al., 2002). As shown in Figure 8, ICMT overexpression was achieved in both fcly-1 and fcly-2 plants. These lines, called fcly-1:ICMT°x and fcly-2:ICMT°x, exhibited significantly increased ICMT activity compared to the corresponding fcly-1 and fcly-2 lines. As shown in Figure 9, both fcly-1 and fcly-2 exhibited an enhanced response to ABA-induced inhibition of seed germination. As expected, fcly-1 exhibited the more dramatic phenotype. Overexpression of ICMT suppressed the ABA hypersensitivity of the fcly-1 line and, consequently, germination of fcly-1:ICMT°x seeds was identical to that of wild-type Col-0 seeds (Figure 9). Interestingly, overexpression of ICMT in the fcly-2 line effectively reversed the ABA hypersensitive phenotype and caused an ABA-insensitive phenotype in fcly-2:ICMT°x seeds (Figure 9). This observation is most likely due to two factors: (1) the relatively modest ABA hypersensitivity of fcly-2 seeds, and (2) the strong overexpression of ICMT achieved in the fcly-2:ICMT°x line.
Figure 8.
ICMT Activity in Col, fcly-1, fcly-2, fcly-1:ICMT°x, and fcly-2:ICMT°x Lines of Arabidopsis.
ICMT activity is shown as a function of time for Col, fcly-1, fcly-2, fcly-1:ICMT°x, and fcly-2:ICMT°x (membranes were prepared from approximately 100 seedlings for each sample). Assays were performed as described in the Methods section. ICMT activity is indicated as the mean plus or minus the standard error of the mean (n = 4).
Figure 9.
Overexpression of ICMT Suppresses or Reverses the Enhanced Response of fcly-1 and fcly-2 Seeds to ABA.
Seed germination was scored for Col-0, fcly-1, fcly-2, fcly-1:ICME°x, and fcly-2:ICME°x lines as a function of time in the presence of 0, 0.3, 1.0, or 5.0 μM cisABA (each data point represents four independent trials, 65 < n < 101). The standard error of the mean is indicated for all data points; for most, the error bars are hidden by the symbols. * and ** represent significant differences compared with the Col-0 control of P < 0.05 and P < 0.01, respectively, as determined by student's t-test.
(A) Germination of Col and fcly-1 seeds.
(B) Germination of Col and fcly-2 seeds.
(C) Germination of Col and fcly-1:ICMT°x seeds.
(D) Germination of Col and fcly-2:ICMT°x seeds.
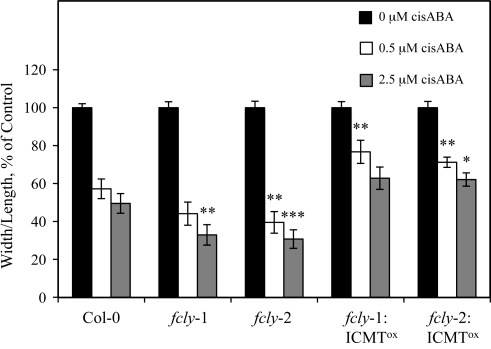
To further examine the effect of ICMT overexpression on the response of fcly-1 and fcly-2 plants to ABA, we examined ABA-induced stomatal closure in Col-0, fcly-1, fcly-2, fcly-1:ICMT°x, and fcly-2:ICMT°x plants. As shown in Figure 10, fcly-1 and fcly-2 plants exhibited an enhanced response to ABA-induced stomatal closure. However, overexpression of ICMT caused a decreased response to ABA in fcly-1:ICMT°x and fcly-2:ICMT°x guard cells. These results, together with the results shown in Figures 7 and 9, provide strong support for the hypothesis that the enhanced response of fcly-1 and fcly-2 plants to ABA is due to FC accumulation and competitive inhibition of ICMT. Further support for the hypothesis that FC competes with ICMT substrates can be found in Supplementary Data, where it is shown that FC competitively inhibits ICMT-mediated methylation of N-acetyl-S-trans, trans-farnesyl-L-cysteine with an apparent IC50 of 50 μM.
Figure 10.
Overexpression of ICMT Reverses the Enhanced Response of fcly-1 and fcly-2 Stomata to ABA.
Stomatal apertures were measured for Col-0, fcly-1, fcly-2, fcly-1:ICMT°x, and fcly-2:ICMT°x lines after incubation of excised leaves for 2 h in the presence of 0, 0.5, or 2.5 μM cisABA. Stomatal apertures were measured from photomicrographs of epidermal peels and reported as the average of 50 stomata per data point. The standard error of the mean is shown. *, **, and *** represent significant differences compared with the Col-0 control of P = 0.05, P < 0.05, and P < 0.01, respectively, as determined by student's t-test.
DISCUSSION
In this paper, recombinant Arabidopsis FC lyase expressed in Sf9 cells is shown to migrate by SDS–PAGE at an apparent molecular mass approximately 11.7 kDa greater than its predicted molecular mass (Figure 1). This difference was recapitulated by in vitro translation of Arabidopsis FC lyase in the presence or absence of microsomal membranes—an observation that strongly suggests the enzyme is proteolytically processed at the amino terminus and post-translationally N-glycosylated (Figure 3). Treatment with Endo H or PNGase F caused FC lyase translated in the presence of microsomal membranes, or expressed in Sf9 cells, to co-migrate with FC lyase translated in the absence of microsomes, supporting the hypothesis that it is N-glycosylated (Figure 3). Recombinant Arabidopsis FC lyase exhibited classical Michaelis-Menten kinetics, with an apparent Km for FC of 45 μM (Figure 4). Moreover, the enzyme selectively oxidized FC, and exhibited a requirement for FAD and molecular oxygen (Table 1 and Figure 6). fcly-1 and fcly-2 mutant plants, which possess approximately 20 and 50% of wild-type FC lyase activity, respectively (Crowell et al., 2007), accumulated significant amounts of FC compared to wild-type plants and exhibited a greater response to ABA in seed germination and stomatal closure assays (Figures 7, 9, and 10). Overexpression of ICMT suppressed the enhanced response of fcly-1 seeds to ABA and reversed the enhanced response of fcly-2 seeds to ABA (Figure 9). Moreover, the ABA hypersensitivity of fcly-1 and fcly-2 stomata was reversed by ICMT overexpression (Figure 10). These and other data (Crowell et al., 2007) demonstrate that, like ICMT, FCLY is a negative regulator of ABA signaling.
The enhanced response of fcly mutants to ABA could be due to FC over-accumulation and competitive inhibition of ICMT, which would be expected to cause incomplete processing of prenylated proteins, or farnesal and farnesol under-accumulation, which would potentially cause reduced protein farnesylation. The data presented here favor the former hypothesis because: (1) FC accumulates in fcly mutant plants, and (2) overexpression of ICMT suppresses or reverses the ABA phenotype of fcly plants (i.e. ICMT overexpression would not reverse the effects of decreased protein farnesylation). Nevertheless, it is surprising that the modest accumulation of FC observed in fcly mutants results in significant inhibition of ICMT. This observation suggests that prenylated protein substrates do not effectively compete with excess FC for the ICMT active site in fcly-1 and fcly-2 mutants. Furthermore, loss of FC lyase function may cause FC to accumulate in the endoplasmic reticulum, where ICMT is located.
In mammalian cells, prenylcysteine lyase is localized to lysosomes. This is an interesting observation in view of the fact that the enzyme generates hydrogen peroxide (i.e. the enzyme is not localized to peroxisomes where hydrogen peroxide can be metabolized). FC lyase is similarly localized in Arabidopsis and has been detected in proteomic analyses of vacuolar proteins (Shimaoka et al., 2004; Jaquinod et al., 2007). In addition to a predicted amino terminal signal sequence, the FCLY coding region also contains two potential sequence-specific vacuolar sorting signals [N/L]-[P/I/L]-[I/P]-[R/N/S] (Carter et al., 2004; Jolliffe et al., 2005; Vitale and Hinz, 2005), one of which is near the carboxyl terminus of the protein. In contrast, neither of two possible peroxisome targeting signals is found on the FCLY protein (PTS1 consists of the carboxyl terminal tripeptide [S/C/A]-[K/R]-[L/M/I/F], whereas PTS2, which is typically found within 30 amino acids of the amino terminus, consists of the nonapeptide R-[X]6-[H/K]-[L/I/F] (Hayashi and Nishimura, 2003; Baker and Sparkes, 2005)).
Arabidopsis FC lyase is unique in many respects. First, reduced FC lyase activity causes a measureable phenotype in fcly-1 and fcly-2 mutants of Arabidopsis. Second, unlike the mammalian ortholog, Arabidopsis FC lyase specifically oxidizes FC (Zhang et al., 1997; Crowell et al., 2007). Why the plant enzyme would have evolved to exhibit specificity for FC is a matter of conjecture, but perhaps geranylgeranial and geranylgeraniol are more toxic to plant cells than farnesal and farnesol, which would result in evolutionary pressures to metabolize GGC by an alternative pathway. Consistent with the hypothesis that FC and GGC are metabolized by different mechanisms, we detected no aldehyde product of [1-3H]GGC in the presence of Arabidopsis membranes (i.e. no metabolite was detected that co-migrated by either HPLC or GC with geranylgernial, see Supplementary Data). Rather, we detected three metabolites, one of which co-migrated by HPLC with GGC sulfoxide, but the identities of these metabolites have yet to be definitively established.
Isoprenylcysteine methylation and demethylation were recently shown to regulate ABA signaling in Arabidopsis (Huizinga et al., 2008). ICMT overexpressing lines of Arabidopsis exhibited an ABA-insensitive phenotype in seed germination and stomatal closure assays, whereas ICME overexpressing lines exhibited an ABA-hypersensitive phenotype in these assays. ABA was also shown to induce the expression of the ICME gene (Huizinga et al., 2008). Thus, like ABA biosynthesis, ABA signaling is under positive feedback regulation (i.e. ABA increases ABA sensitivity of plant cells via induction of ICME expression and demethylation (i.e. inactivation) of isoprenylated negative regulators of ABA signaling). The balance between methylation and demethylation of isoprenylated negative regulators of ABA signaling can be perturbed in a number of ways. In this report, it is shown that this balance is perturbed in fcly mutants by the accumulation of FC, a competitive inhibitor of ICMT (Tan et al., 1991; Shi and Rando, 1992; Ma et al., 1995; Narasimha Chary et al., 2002). Consequently, in fcly plants, decreased isoprenylcysteine methyltransferase function results in an enhanced response to ABA. This is unlike the situation in mice lacking a functional prenylcysteine lyase gene because, despite the accumulation of FC and GGC in these mice, no developmental or pathological defects were observed (Beigneux et al., 2002). This observation is unexpected because icmt–/– knockout mice do not survive (Bergo et al., 2001). Thus, it is reasonable to speculate that the accumulated FC and GGC in prenylcysteine lyase knockout mice must be sequestered in an intracellular compartment that prevents inhibition of ICMT.
METHODS
Plant Materials and Growth Conditions
Surface sterilization of Arabidopsis (Arabidopsis thaliana) seeds was accomplished using the following protocol: 95% ethanol for 5 min, 20–50% bleach for 5–20 min, five washes in sterile de-ionized water. Sterile seeds were suspended in 0.1% agar, stratified on 1/2X Murashige-Skoog (MS) plates containing 1% sucrose and 0.8% agar for 3 d at 4°C, and germinated at 22°C under long day conditions (18 h of white light at 100 μmol m−2 sec−1 followed by 6 h of dark) in a vertical orientation. Seedlings were harvested after 4 d for extraction of membranes or transferred to ProMix and propagated under the same conditions for analysis of stomatal function or collection of seed. Plants were fertilized with a standard mixture of macro- and micro-nutrients from below.
Expression of Recombinant FC Lyase
The FCLY cDNA sequence was PCR-amplified using the primers 5′-AGA TCT ATG AAA GAT TTC CCG ATA GCA ATC TC-3’ (containing a Bgl11 site) and 5′-GGA TCC TTA TGA GTC TGA GTG CAG ACC AGA A-3’ (containing a BamH1 site), inserted into the pCR2.1–TOPO vector, and subjected to DNA sequence analysis. The 1.5-kbp FCLY cDNA was then recloned into the Bgl11 and BamH1 sites of the Baculovirus transfer vector pVL1392, confirmed by DNA sequence analysis, and transfected into Sf9 insect cells using the BD BaculoGold kit (BD Biosciences/Pharmingen, San Diego, CA). Recombinant baculovirus expressing FCLY was amplified using the supplier's protocol. A 30-ml suspension culture of Sf9 cells (106 cells ml−1 in Grace's Medium supplemented with 10% fetal bovine serum) was infected with 1 ml of recombinant baculovirus (107–108 pfu ml−1). Cells were then incubated on a rotary shaker at 100 rpm for 3 d at room temperature. At 3 d post infection (3 dpi), cells were sedimented at 3000 g and re-suspended in 3 ml of 40 mM Tris-HCl, pH 7.5, 1 mM DTT containing Complete® protease inhibitors (Roche Diagnostics, Indianapolis, IN). The suspension was then homogenized on ice for 3 min and centrifuged at 16 000 g for 10 min. The resulting supernatant was centrifuged at 100 000 g for 1 h and membranes were re-suspended in 300 μl of 40 mM Tris-HCl, pH 7.5, 1 mM DTT, 15% glycerol and stored at –80°C.
FC Lyase Assays
FC lyase reactions were performed in a total volume of 20 μl and consisted of 5 μl of membrane protein from recombinant Sf9 cells (0–15 μg of protein) and 0–100 μM [1-3H]farnesylcysteine (12.5 Ci mmol−1) in 40 mM Tris-HCl, pH 7.5. One or more of the following were added, as required: 5 mM FAD, 20 mM glucose, 2 U glucose oxidase, 11 U catalase. To remove oxygen from the system, 40 mM Tris-HCl, pH 7.5, was repeatedly frozen and thawed under vacuum, sparged with nitrogen gas, and FC lyase reactions were started under a nitrogen atmosphere. Reactions were incubated at 30°C for various times (0–6 h), terminated with 5 μl of methanol, and a 10-μl portion was analyzed, along with authentic farnesol and farnesal standards, by thin layer chromatography using a silica gel plate and hexane:tetrahydrofuran (3:1) as a mobile phase. TLC plates were cut into six sections and analyzed by liquid scintillation. Alternatively, the assay mixture was diluted with 100 μl of water and extracted with 200 μl of hexane, after which the organic and aqueous layers were partitioned and analyzed by liquid scintillation. Radioactivity detected by liquid scintillation was multiplied by 2 before calculating enzyme activity because FC lyase is stereospecific at the C-1 position, whereas [1-3H]FC is racemic. Consequently, only 50% of the reaction products were expected to be radioactive.
Unlabeled FC and prenylcysteine analogs were synthesized according to published procedures (Brown et al., 1991) from cysteine or homocysteine and the appropriate prenyl halides, which were prepared from the corresponding prenyl alcohols (Aldrich) by sequential mesylation (MsCl, Et3N) and reaction with LiCl or by treatment with phosphorus tribromide (PBr3). Farnesal and geranylgeranial standards were synthesized by Dess-Martin periodinane oxidation of farnesol and geranylgeraniol, respectively. GGC sulfoxide was prepared by treatment of GGC with hydrogen peroxide as described (Cashman et al., 1990).
Hydrogen Peroxide Determination
Extracts of infected Sf9 cells (106 cells ml−1, 4 dpi) were prepared by centrifugation of infected cells at 3000 g, after which the cell pellet was lysed in 40 mM Tris-HCl, pH 7.5, containing 1% Triton X-100, 150 mM NaCl and Complete® protease inhibitors (Roche Diagnostics, Indianapolis, IN). After standing on ice for 30 min, the mixture was centrifuged at 16 000 g for 10 min and the supernatant was used to analyze the production of farnesal and hydrogen peroxide by recombinant Arabidopsis FC lyase (to account for product reduction, farnesal and farnesol were quantified by GC and summed). Peroxide formation was determined spectrophotometrically at 405 nm using ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) and horseradish peroxidase.
In Vitro Transcription/Translation and Analysis of Protein N-Glycosylation
In vitro transcription/translation using a reticulocyte-based translation system and canine pancreatic microsomal membranes is a common method for demonstrating in vitro N-glycosylation of plant proteins (Dellapenna and Bennett, 1988; Devoto et al., 1999; Abell et al., 2002; McCartney et al., 2004). In vitro transcription/translation was performed in the presence of [35S]methionine (10 mCi ml−1, 1000 Ci mmol−1, PerkinElmer, Boston, MA) using the TnT Quick Coupled Transcription/Translation System (Promega, Madison, WI) and canine pancreatic microsomal membranes (Promega, Madison, WI) according to the manufacturer's specifications. Endoglycosidase H and peptide N-glycosidase F were from Sigma-Aldrich (St Louis, MO) and were used according to the manufacturer's instructions following solubilization of microsomal membranes in 0.2% SDS.
Analysis of FC Content
Arabidopsis seedlings were pulverized in liquid nitrogen and stored at –80°C. Pulverized seedlings were then placed in a mortar and pestle and homogenized with 3 mL of cold ethanol. The material was transferred to a conical vial, sonicated (12 W, 1 min), filtered, and the solvent was removed under reduced pressure. The resulting residue was dissolved in 300 μL of ethanol and a portion (25 μL) analyzed for chlorophyll content (i.e. diluted to 1 mL in ethanol, and absorbances determined at 464 and 646 nm). To the remainder was sequentially added 450 μL of water, 60 μL of pyridine, and 100 μL of ethyl chloroformate. The mixture was vortexed and allowed to stand for 15 min at room temperature, at which time it was extracted three times with 500 μL of dichloromethane. The organic extracts were combined, filtered through a short silica gel pad, and concentrated. The residue was then re-dissolved in isopropanol (50–100 μL) and subjected to GC analysis (CP-Sil 19 CB column, Varian) using the following GC conditions: flow rate = 1.5 mL min−1; column temperature = 150°C, 1 min; 15°C min−1 to 250°C; 250°C, 20 min. Under these conditions, the N-ethoxycarbonyl derivative of FC and the N-ethoxycarbonyl ethyl ester of FC eluted at 13.0 and 13.75 min, respectively. FC levels were quantified by correlation with a GC calibration curve using a synthesized FC derivative.
Plant Transformation
To generate the fcly-1:ICMT°x and fcly-2:ICMT°x lines, fcly-1 and fcly-2 mutants of Arabidopsis were transformed with a recombinant binary vector (pCL108) containing the CaMV 35S promoter and the AtSTE14B (ICMT, At5g08335) coding sequence (Huizinga et al., 2008). Agrobacterium tumefaciens strain GV3101 (pMP90) (Koncz and Schell, 1986) and the floral dip method (Clough and Bent, 1998) were used for plant transformation. T1 transformants were selected on 1/2X Murashige-Skoog plates containing 1% sucrose, 0.8% agar, and 200 μg ml−1 gentamycin. Transgenic plant lines were propagated to the T3 generation and scored for homozygosity by PCR and non-segregation of the antibiotic resistance marker.
ICMT Assays
Four-day-old Arabidopsis seedlings were pulverized in a mortar and pestle at 4°C in homogenization buffer containing 50 mM Hepes, pH 7.4, 500 mM mannitol, 5 mM EDTA, 5 mM DTT, and Complete® protease inhibitors (Roche Diagnostics, Indianapolis, IN). Extracts were filtered through cheesecloth, centrifuged at 8000 g for 10 min at 4°C, and membranes were sedimented from extract supernatants at 100 000 g for 60 min at 4°C. Membranes were then re-suspended in a buffer containing 2.5 mM Hepes, pH 7.0, 250 mM mannitol, and 1 mM DTT and aliquots were stored in the presence of 15% glycerol at −80°C. ICMT assays were performed as described (Crowell et al., 1998) in the presence of 100 mM Hepes, pH 7.0, 60 μg of membrane protein, 24 μM (2.5 Ci mmol−1, 60 μCi ml−1) S-adenosyl-L-[3H methyl]methionine as a methyl donor (GE Healthcare Life Sciences, Piscataway, NJ), and 200 μM AFC (BioMol, Plymouth Meeting, PA) as a methyl acceptor in a total volume of 50 μl. Reactions were incubated at 30°C for 10–40 min, terminated by the addition of 50 μl of 90% v/v methylene chloride, 9.75% v/v methanol, 0.25% v/v acetic acid, mixed vigorously, and centrifuged in a microcentrifuge for 1 min. A 10-μl portion of the organic phase was spotted onto a plastic-backed silica gel plate and developed in 90% methylene chloride, 9.75% methanol, 0.25% acetic acid. Plates were then sprayed with En3hance fluorographic reagent (NEN/DuPont, Boston, MA) and fluorography was performed at –80°C using X-Omat AR film (Eastman Kodak, Rochester, NY). Liquid scintillation of excised TLC spots was performed to quantify ICMT activity.
Seed Germination Assays
Seeds used for germination assays were harvested from control and experimental plants, which were grown together under identical conditions. Seeds were surface-sterilized, suspended in sterile 0.1% agar, and placed on 1/2X Murashige-Skoog plates containing 1% sucrose and 0.8% agar in the dark at 22°C (Cutler et al., 1996). Seeds from control and experimental plants were sown on the same plates and germination (radical emergence) was scored in the presence of various concentrations of exogenous ABA under a dissecting microscope.
Stomatal Closure Assays
Mature rosette leaves were excised and incubated in the presence of various concentrations of ABA or an equivalent volume of DMSO (as a solvent control) in 10 ml of water for 2 h. Abaxial epidermal peels were then prepared as described (Huizinga et al., 2008), stained with toluidine blue, mounted on a microscope slide, and visualized with a Nikon Eclipse E600 microscope interfaced to a SPOT digital camera. In random order, K.G.K. performed leaf excision and incubation in the presence of various concentrations of ABA. D.H.H. prepared epidermal peels and performed photography and measurement of stomatal apertures without knowledge of sample identities. Data are recorded as the average width/length of individual apertures (n = 50 per sample).
Statistical Methods
Throughout this paper, data are presented as the mean plus or minus the standard error of the mean. Unlike the standard deviation, the standard error of the mean takes into account sample size and is, thus, a more meaningful measure of error. In all data figures, experimental plants are compared to the Col-0 control at the same time and/or concentration of ABA. Because these are pair-wise comparisons, a student's t-test was performed to assess the statistical significance of these differences and only differences with a P-value less than or equal to 0.05 (95% confidence) were considered to be significant.
SUPPLEMENTARY DATA
Supplementary Data are available at Molecular Plant Online.
FUNDING
This work was supported by NSF grant MCB-0900962. The authors also acknowledge NIH Grant P20RR16454 from the INBRE program of the National Center for Research Resources, which provided funds for the Molecular Research Core Facility at Idaho State University, and the Mentored Undergraduate Summer Experience (MUSE) program at The College of New Jersey.
No conflict of interest declared.
Supplementary Material
References
- Abell BM, High S, Moloney MM. Membrane protein topology of oleosin is constrained by its long hydrophobic domain. J. Biol. Chem. 2002;277:8602–8610. doi: 10.1074/jbc.M103712200. [DOI] [PubMed] [Google Scholar]
- Baker A, Sparkes IA. Peroxisome protein import: some answers, more questions. Curr. Opin. Plant Biol. 2005;8:640–647. doi: 10.1016/j.pbi.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Beigneux A, Withycombe SK, Digits JA, Tschantz WR, Weinbaum CA, Griffey SM, Bergo M, Casey PJ, Young SG. Prenylcysteine lyase deficiency in mice results in the accumulation of farnesylcysteine and geranylgeranylcysteine in brain and liver. J. Biol. Chem. 2002;277:38358–38363. doi: 10.1074/jbc.M205183200. [DOI] [PubMed] [Google Scholar]
- Bentinger M, Grunler J, Peterson E, Swiezewska E, Dallner G. Phosphorylation of farnesol in rat liver microsomes: properties of farnesol kinase and farnesyl phosphate kinase. Arch. Biochem. Biophys. 1998;353:191–198. doi: 10.1006/abbi.1998.0611. [DOI] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 2001;276:5841–5845. doi: 10.1074/jbc.C000831200. [DOI] [PubMed] [Google Scholar]
- Bonetta D, Bayliss P, Sun S, Sage T, McCourt P. Farnesylation is involved in meristem organization in Arabidopsis. Planta. 2000;211:182–190. doi: 10.1007/s004250000283. [DOI] [PubMed] [Google Scholar]
- Bostedor RG, Karkas JD, Arison BH, Bansal VS, Vaidya S, Germershausen JI, Kurtz MM, Bergstrom JD. Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. J. Biol. Chem. 1997;272:9197–9203. doi: 10.1074/jbc.272.14.9197. [DOI] [PubMed] [Google Scholar]
- Bracha K, Lavy M, Yalovsky S. The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J. Biol. Chem. 2002;277:29856–29864. doi: 10.1074/jbc.M202916200. [DOI] [PubMed] [Google Scholar]
- Bracha-Drori K, Shichrur K, Lubetzky TC, Yalovsky S. Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 2008;148:119–131. doi: 10.1104/pp.108.120477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Milano PD, Lever DC, Epstein WW, Poulter CD. Prenylated proteins: a convenient synthesis of farnesyl cysteinyl thioethers. J. Am. Chem. Soc. 1991;113:3176–3177. [Google Scholar]
- Cadiñanos J, Varela I, Mandel DA, Schmidt WK, Díaz-Perales A, López-Otín C, Freije JM. AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J. Biol. Chem. 2003;278:42091–42097. doi: 10.1074/jbc.M306700200. [DOI] [PubMed] [Google Scholar]
- Caldelari D, Sternberg H, Rodríguez-Concepción M, Gruissem W, Yalovsky S. Efficient prenylation by a plant geranylgeranyltransferse-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol. 2001;126:1416–1429. doi: 10.1104/pp.126.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman JR, Olsen LD, Bornheim LM. Enantioselective S-oxygenation by flavin-containing and cytochrome P-450 monooxygenases. Chem. Res. Toxicol. 1990;3:344–349. doi: 10.1021/tx00016a012. [DOI] [PubMed] [Google Scholar]
- Christophe J, Popjak G. Studies on the biosynthesis of cholesterol: XIV. The origin of prenoic acids from allyl pyrophosphates in liver enzyme systems. J. Lipid Res. 1961;2:244–257. [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Coves J, Lebrun C, Gervasi G, Dalbon P, Fontecave M. Overexpression of the FAD-binding domain of the suphite reductase flavoprotein component from Escherichia coli and its inhibition by iodonium diphenyl chloride. Biochem. J. 1999;342:465–472. [PMC free article] [PubMed] [Google Scholar]
- Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem. Biophys. Res. Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- Crowell DN. Functional implications of protein isoprenylation in plants. Prog. Lipid Res. 2000;39:393–408. doi: 10.1016/s0163-7827(00)00010-2. [DOI] [PubMed] [Google Scholar]
- Crowell DN, Huizinga DH. Protein isoprenylation: the fat of the matter. Trends Plant Sci. 2009;14:163–170. doi: 10.1016/j.tplants.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Crowell DN, Kennedy M. Identification and functional expression in yeast of a prenylcysteine alpha-carboxyl methyltransferase gene from Arabidopsis thaliana. Plant Mol. Biol. 2001;45:469–476. doi: 10.1023/a:1010671202925. [DOI] [PubMed] [Google Scholar]
- Crowell DN, Huizinga DH, Deem AK, Trobaugh C, Denton R, Sen SE. Arabidopsis thaliana plants possess a specific farnesylcysteine lyase that is involved in detoxification and recycling of farnesylcysteine. Plant J. 2007;50:839–847. doi: 10.1111/j.1365-313X.2007.03091.x. [DOI] [PubMed] [Google Scholar]
- Crowell DN, Sen SE, Randall SK. Prenylcysteine alpha-carboxyl methyltransferase in suspension-cultured tobacco cells. Plant Physiol. 1998;118:115–123. doi: 10.1104/pp.118.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- DeBarber AE, Bleyle LA, Roullet JB, Koop DR. Omega-hydroxylation of farnesol by mammalian cytochromes p450. Biochim. Biophys. Acta. 2004;1682:18–27. doi: 10.1016/j.bbalip.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Deem AK, Bultema RL, Crowell DN. Prenylcysteine methylesterase in Arabidopsis thaliana. Gene. 2006;380:159–166. doi: 10.1016/j.gene.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Dellapenna D, Bennett AB. In vitro synthesis and processing of tomato fruit polygalacturonase. Plant Physiol. 1988;86:1057–1063. doi: 10.1104/pp.86.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto A, Piffanelli P, Nilsson I, Wallin E, Panstruga R, von Heijne G, Schulze-Lefert P. Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J. Biol. Chem. 1999;274:34993–35004. doi: 10.1074/jbc.274.49.34993. [DOI] [PubMed] [Google Scholar]
- Digits JA, Pyun HJ, Coates RM, Casey PJ. Stereospecificity and kinetic mechanism of human prenylcysteine lyase, an unusual thioether oxidase. Biol. Chem. 2002;277:41086–41093. doi: 10.1074/jbc.M208069200. [DOI] [PubMed] [Google Scholar]
- Galichet A, Gruissem W. Protein farnesylation in plants: conserved mechanisms but different targets. Curr. Opin. Plant Biol. 2003;6:530–535. doi: 10.1016/j.pbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pacanowska D, Arison B, Havel CM, Watson JA. Isopentenoid synthesis in isolated embryonic Drosophila cells: farnesol catabolism and omega-oxidation. J. Biol. Chem. 1988;263:1301–1306. [PubMed] [Google Scholar]
- Havel C, Rector ER, 2nd, Watson JA. Isopentenoid synthesis in isolated embryonic Drosophila cells: possible regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by shunted mevalonate carbon. J. Biol. Chem. 1986;261:10150–10156. [PubMed] [Google Scholar]
- Hayashi M, Nishimura M. Entering a new era of research on plant peroxisomes. Curr. Opin. Plant Biol. 2003;6:577–582. doi: 10.1016/j.pbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Huizinga DH, Omosegbon O, Omery B, Crowell DN. Isoprenylcysteine methylation and demethylation regulate abscisic acid signaling in Arabidopsis. Plant Cell. 2008;20:2714–2728. doi: 10.1105/tpc.107.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteom. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Chary SN, Chernoff EA, Zeng Q, Running MP, Crowell DN. Protein geranylgeranyltransferase I is involved in specific aspects of abscisic acid and auxin signaling in Arabidopsis. Plant Physiol. 2005;139:722–733. doi: 10.1104/pp.105.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe NA, Craddock CP, Frigerio L. Pathways for protein transport to seed storage vacuoles. Biochem. Soc. Trans. 2005;33:1016–1018. doi: 10.1042/BST20051016. [DOI] [PubMed] [Google Scholar]
- Keung WM. Human liver alcohol dehydrogenases catalyze the oxidation of the intermediary alcohols of the shunt pathway of mevalonate metabolism. Biochem. Biophys. Res. Commun. 1991;174:701–707. doi: 10.1016/0006-291x(91)91474-q. [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of the TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 1986;204:383–396. [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001;15:1808–1816. doi: 10.1101/gad.900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Phyisol. 2001;126:670–684. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YT, Gilbert BA, Rando RR. Farnesylcysteine analogs to probe role of prenylated protein methyltransferase. Methods Enzymol. 1995;250:226–234. doi: 10.1016/0076-6879(95)50075-8. [DOI] [PubMed] [Google Scholar]
- McCartney AW, Dyer JM, Dhanoa PK, Kim PK, Andrews DW, McNew JA, Mullen RT. Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. Plant J. 2004;37:156–173. doi: 10.1111/j.1365-313x.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- Narasimha Chary S, Bultema RL, Packard CE, Crowell DN. Prenylcysteine alpha-carboxyl methyltransferase expression and function in Arabidopsis thaliana. Plant J. 2002;32:735–747. doi: 10.1046/j.1365-313x.2002.01463.x. [DOI] [PubMed] [Google Scholar]
- Park SB, Howald WN, Cashman JR. S-oxidative cleavage of farnesylcysteine and farnesylcysteine methyl ester by the flavin-containing monooxygenase. Chem. Res. Toxicol. 1994;7:191–198. doi: 10.1021/tx00038a012. [DOI] [PubMed] [Google Scholar]
- Park SB, Osterloh JD, Vamvakas S, Hashmi M, Anders MW, Cashman JR. Flavin-containing monooxygenase-dependent stereoselective S-oxygenation and cytotoxicity of cysteine S-conjugates and mercapturates. Chem. Res. Toxicol. 1992;5:193–201. doi: 10.1021/tx00026a008. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schoeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Yalovsky S, Gruissem W. Protein prenylation in plants: old friends and new targets. Plant Mol. Biol. 1999;39:865–870. doi: 10.1023/a:1006170020836. [DOI] [PubMed] [Google Scholar]
- Running MP, Fletcher JC, Meyerowitz EM. The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development. 1998;125:2545–2553. doi: 10.1242/dev.125.14.2545. [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc. Natl Acad. Sci. U S A. 2004;101:7815–7820. doi: 10.1073/pnas.0402385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausen PJ, Elfarra AA. Cysteine conjugate S-oxidase: characterization of a novel enzymatic activity in rat hepatic and renal microsomes. J. Biol. Chem. 1990;265:6139–6145. [PubMed] [Google Scholar]
- Shi YQ, Rando RR. Kinetic mechanism of isoprenylated protein methyltransferase. J. Biol. Chem. 1992;267:9547–9551. [PubMed] [Google Scholar]
- Shiemke AK, Arp DJ, Sayavedra-Soto LA. Inhibition of membrane-bound methane monooxygenase and ammonia monooxygenase by diphenyliodonium: implications for electron transfer. J. Bacteriol. 2004;186:928–937. doi: 10.1128/JB.186.4.928-937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka T, Ohnishi M, Sazuka T, Mitsuhashi N, Hara-Nishimura I, Shimazaki K, Maeshima M, Yokota A, Tomizawa K, Mimura T. Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:672–683. doi: 10.1093/pcp/pch099. [DOI] [PubMed] [Google Scholar]
- Tan EW, Pérez-Sala D, Cañada FJ, Rando RR. Identifying the recognition unit for G protein methylation. J. Biol. Chem. 1991;266:10719–10722. [PubMed] [Google Scholar]
- Thai L, Rush JS, Maul JE, Devarenne T, Rodgers DL, Chappell J, Waechter CJ. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc. Natl Acad. Sci. U S A. 1999;96:13080–13085. doi: 10.1073/pnas.96.23.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueblood CE, Ohya Y, Rine J. Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993;13:4260–4275. doi: 10.1128/mcb.13.7.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR. Heterotrimeric G protein gamma subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschantz WR, Digits JA, Pyun HJ, Coates RM, Casey PJ. Lysosomal prenylcysteine lyase is a FAD-dependent thioether oxidase. J. Biol. Chem. 2001;276:2321–2324. doi: 10.1074/jbc.C000616200. [DOI] [PubMed] [Google Scholar]
- Tschantz WR, Zhang L, Casey PJ. Cloning, expression, and cellular localization of a human prenylcysteine lyase. J. Biol. Chem. 1999;274:35802–35808. doi: 10.1074/jbc.274.50.35802. [DOI] [PubMed] [Google Scholar]
- Vaidya S, Bostedor R, Kurtz MM, Bergstrom JD, Bansal VS. Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch. Biochem. Biophys. 1998;355:84–92. doi: 10.1006/abbi.1998.0704. [DOI] [PubMed] [Google Scholar]
- Vitale A, Hinz G. Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 2005;10:316–323. doi: 10.1016/j.tplants.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Westfall D, Aboushadi N, Shackelford JE, Krisans SK. Metabolism of farnesol: phosphorylation of farnesol by rat liver microsomal and peroxisomal fractions. Biochem. Biophys. Res. Commun. 1997;230:562–568. doi: 10.1006/bbrc.1996.6014. [DOI] [PubMed] [Google Scholar]
- Wyatt RE, Ainley WM, Nagao RT, Conner TW, Key JL. Expression of the Arabidopsis AtAux2-11 auxin-responsive gene in transgenic plants. Plant Mol. Biol. 1993;22:731–749. doi: 10.1007/BF00027361. [DOI] [PubMed] [Google Scholar]
- Yalovsky S, Kulukian A, Rodríguez-Concepción M, Young CA, Gruissem W. Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell. 2000;12:1267–1278. doi: 10.1105/tpc.12.8.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Small GTPases: versatile signaling switches in plants. Plant Cell. 2002;14(Suppl.):S375–S388. doi: 10.1105/tpc.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, McGeady P, Gelb MH. Mammalian protein geranylgeranyltransferase-I: substrate specificity, kinetic mechanism, metal requirements, and affinity labeling. Biochemistry. 1995;34:1344–1354. doi: 10.1021/bi00004a029. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Wang X, Running MP. Dual lipid modification of Arabidopsis Ggamma subunits is required for efficient plasma membrane targeting. Plant Physiol. 2007;143:1119–1131. doi: 10.1104/pp.106.093583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tschantz WR, Casey PJ. Isolation and characterization of a prenylcysteine lyase from bovine brain. J. Biol. Chem. 1997;272:23354–23359. doi: 10.1074/jbc.272.37.23354. [DOI] [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM. Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc. Natl Acad. Sci. U S A. 2000;97:7633–7638. doi: 10.1073/pnas.130189397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.