Abstract
AIM: To investigate the in vivo hepatoprotective effects and mechanisms of Gentiana manshurica Kitagawa (GM) in acetaminophen (APAP)-induced liver injury in mice.
METHODS: GM (200, 150 or 50 mg/kg body weight) or N-acetyl-L-cysteine (NAC; 300 mg/kg body weight) was administrated orally with a single dose 2 h prior to APAP (300 mg/kg body weight) injection in mice.
RESULTS: APAP treatment significantly depleted hepatic glutathione (GSH), increased serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and malonyldialdehyde (MDA) and 4-hydroxynonenal levels, and decreased hepatic activity of glutathione peroxidase (GSH-px) and superoxide dismutase (SOD). However, the pretreatment of GM significantly alleviated APAP-induced oxidative stress by increasing GSH content, decreasing serum ALT, AST and MDA, and retaining the activity of GSH-px and SOD in the liver. Furthermore, GM pretreatment can inhibit caspase-3 activation and phosphorylation of c-Jun-NH2-terminal protein kinase 2 (JNK1/2) and extracellular signal-regulated kinase (ERK). GM also remarkably attenuated hepatocyte apoptosis confirmed by the terminal deoxynucleotidyl transferase mediated dUTP nick end-labeling method.
CONCLUSION: Hepatoprotective effects of GM against APAP-induced acute toxicity are mediated either by preventing the decline of hepatic antioxidant status or its direct anti-apoptosis capacity. These results support that GM is a potent hepatoprotective agent.
Keywords: Gentiana manshurica Kitagawa, Acetaminophen, Oxidative stress, Caspase-3, JNK/ERK MAPK
INTRODUCTION
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug. It is safe at therapeutic doses, however, when taken at high doses, APAP can precipitate severe liver injury that may develop into a liver failure[1,2]. Overdoses of APAP lead to the generation of high amounts of the toxic metabolite N-acetyl-quinoneimine (NAPQI) by the cytochrome P-450 isoenzyme mixed-function oxidase system, which is immediately conjugated with glutathione (GSH), forming the nontoxic metabolites cysteine and mercapturic acid conjugates. Although a number of P450 enzymes can metabolize APAP, the most relevant isoenzyme is CYP2E1[3]. There is an alternative view that oxidative stress plays a role in hepatotoxicity. Oxidative stress in APAP hepatotoxicity is characterized by several features, including lipid peroxidation, mitochondrial damage and ATP depletion in proteins[4]. NAPQI reacts with GSH spontaneously or catalyzed by glutathione-S-transferases to form a GSH-adduct. Thus, GSH depletion and formation of protein adducts are key mechanisms of APAP-induced cell death[5-7].
Many antioxidant agents have been studied in experimental and clinical studies to reduce or prevent APAP-induced hepatotoxicity. The most popular antioxidant for APAP hepatotoxicity is N-acetyl-L-cysteine (NAC)[8]. Protection by NAC is believed to be attributable to its ability to regenerate GSH stores because of its capacity to provide cysteine residues[9].
In recent years, plant-derived natural products have received considerable attention due to their diverse pharmacological properties. A growing interest has been observed in the analysis of these natural entities for their potential benefits to human health on hepatoprotective effects. Gentiana manshurica Kitagawa (GM) is distributed in northeastern China and reputed “Dongbei longdan”, which is one of the most common herbal medicines used by Chinese people suffering from chronic liver diseases. As an iridoid containing plant, GM has various pharmacological activities. Previous phytochemical studies reported that GM includes loganic acid, 6-O-β-d-glucopyranosylgentiopicroside, swertiamarin, gentiopicroside, sweroside and 2-(o,m-dihydroxybenzyl)-sweroside[10]. It was reported that gentiopicroside involves down-regulation of NR2B receptors in the anterior cingulate cortex to persistent inflammatory pain[11]. Animal experiments have revealed adaptogenic[12] and anti-inflammatory[13] activities. GM has also been used traditionally as a folk remedy for healing wound[14,15]. However, no research has been conducted about its hepatoprotective patterns.
Since a high dose APAP-induced hepatotoxicity resulted from the generation of free radicals during its metabolism at liver, the possible protection by GM was evaluated and the results are presented in this paper.
MATERIALS AND METHODS
Chemicals
APAP and NAC were purchased from Sigma Chemicals (St. Louis, MO, USA). Detection kit for GSH and malondialdehyde (MDA), glutathione peroxidase (GSH-px) and superoxide dismutase (SOD) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals used were of analytical grade.
Preparation of GM
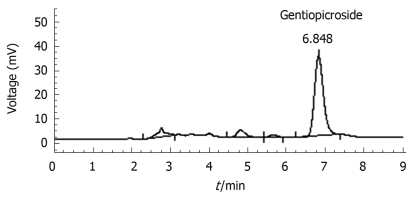
The rhizomes and roots GM were purchased from Yanbian Puhe, China in March 2006 and authenticated by Prof. Hui-Zi Lv of College of Pharmacy, Yanbian University. The rhizomes and roots of GM (1 kg) were extracted three times with methanol (10 L) and boiled under reflux for 4 h at 40°C, and then the percolated was concentrated in a rotary vacuum evaporator followed by lyophilization. The yield (w/w) of extract was about 22.35%. The freeze-dried extract was used in both chemical analysis and pharmacological studies. GM extract was analyzed on HPLC to confirm the presence of gentiopicroside (Figure 1). The content of gentiopicroside was 2.48% in GM extract. The extract was pre-solubilized in distilled saline for the in vivo studies.
Figure 1.
HPLC chromatograms of Gentiana manshurica Kitagawa (GM). Column: Diamonsil C18 (250 mm × 4.6 mm); Flow rate: 1 mL/min; Mobile phase: Methanol and H2O (35:65).
Animals and treatment
Male Kunming mice (20-22 g) were provided by Yanbian University Laboratory Animal Center, fed with a standard chow diet and given tap water ad libitum. Animals were housed in plastic cages and maintained at 22 ± 2°C and 50%-60% relative humidity, with a 12-h light-dark cycle throughout the experiment. Animal experiments were performed under the latest edition of “Guiding Principles in the Use of Animals in Toxicology” adopted by the Society of Toxicology (USA).
The mice were fasted overnight (16 h) prior to administration of a single intraperitoneal dose (300 mg/kg) of APAP dissolved in sterile phosphate buffered saline (PBS, pH 7.4) warmed to 40°C[16]. Animals were divided into 7 groups of ten animals each. Animals of group 1 received vehicle only and served as normal and group 2 treated intraperitoneally with a single dose of APAP (300 mg/kg) was kept as control. Groups 3, 4 and 5 were administered orally with GM extract (50, 100 or 200 mg/kg) 2 h before APAP injection, and served as GM per se. Group 6 was treated with NAC (300 mg/kg) 2 h before APAP injection, and served as positive control. Group 7 received only GM extract (200 mg/kg) 2 h after saline injection, instead of APAP injection. Animals were sacrificed and blood was collected from the carotid artery 12 h after administration of APAP. Serum was then separated by centrifugation at 4°C, 3000 r/min for 30 min. The liver was removed immediately from each mouse, and kept at -80°C until analyzed.
Blood biochemistry
Blood was collected at 12 h after APAP administration. Serum levels of AST and ALT were detected using an Autodry Chemistry Analyzer (SPOTCHEM™ SP4410, Arkray, Japan).
GSH, SOD, GSH-px activities and hepatic lipid peroxidation assay
The removed liver tissue was homogenized in 9 volumes of cold buffer (0.01 mol/L Tris-HCl, 0.0001 mol/L EDTA-2Na, 0.01 mol/L sucrose, and 0.8% saline, pH 7.4) on ice. The homogenate was centrifuged at 4°C (3000 r/min, 15 min) and the supernatant was used for the determination of GSH and MDA, GSH-px and SOD following the manufacturer’s instructions. Briefly, the MDA was detected using the thiobarbituric acid reactive substances (TBARS) methods; 4-hydroxynonenal (4-HNE) was detected as a fluorimetric derivative[17]; the GSH activity was detected through yellow tetramethyl-benzidine and oxidised GSH produced by the combination of GSH and dithio-nitrobenzene; SOD activity was examined through nitroblue tetrazolium coloration; and GSH-px activity was determined through detecting selenium cysteine, the active centre of GSH-px. Protein content was determined with a Bio-Rad Protein Assay Kit (Bio-Rad, USA).
Histopathological analysis
Liver samples obtained at different time points after the APAP injection were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin for histopathological analysis. The sections were examined under light microscopy and graded for the presence and intensity of lesions using a scale from 0 to 5 (0 = no lesions; 1 = minimal lesions involving a single or few necrotic cells; 2 = mild lesions, 10%-25% necrotic cells or mild diffuse degenerative changes; 3 = moderate lesions, 25%-40% necrotic or degenerative cells; 4 = marked lesions, 40%-50% necrotic or degenerative cells; and 5 = severe lesions, more than 50% necrotic or degenerative cells)[18,19].
Transferase-mediated dUTP nick end-labeling (TUNEL) assay
Apoptotic cells were detected by the terminal deoxynucleotidyl TUNEL method using an in situ cell detection kit (Roche, Mannheim, Germany) for the detection and quantification of apoptosis at a single-cell level. Staining of tissue sections was performed according to the manufacturer’s protocol, as follows. Paraffin-embedded sections were dewaxed in xylene and rehydrated by passing through a graded series of ethanol solutions, ending with phosphate-buffered saline. Sections were permeabilized with proteinase K (20 μg/mL in 10 mmol/L Tris-HCl, pH 7.4-8.0) at 37°C for 15 min. After washing, sections were stained with fluorescent anti-TdT. Sections were viewed and photographed using standard fluorescent microscopic techniques.
Western blotting analysis
The total protein extracts were made by pulverization in a grinder with liquid nitrogen, then using a ratio of 1 mL lysis buffer (150 mmol/L NaCl, 1.0% NP-40, 0.5% NaVO4, 0.1% SDS, 50 mmol/L Tris, pH 7.5) containing 1 mmol/L PMSF for each 100 mg powdered liver sample. Liver lysates (40 μg) were electroblotted onto a PVDF membrane following separation on 8%-12% SDS-polyacrylamide gel electrophoresis. Blotted membranes were blocked with 5% skim milk in incubation buffer at room temperature, followed by incubation overnight at 4°C with 1:1000 dilution of caspase-3, JNK, ERK (Santa Cruz biotechnology, CA, USA) and phospho-JNK, phospho-ERK (Cell Signaling Technology, MA, USA) primary antibody. Bound antibody was detected with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, CA, USA) and immunodetected proteins were visualized using WEST-ZOL™ (plus) Western blotting detection system (iNtRON Biotechnology, Gyeonggi, Korea). Loading accuracy was evaluated by membrane rehybridization with monoclonal antibodies against α-tubulin (Sigma, St. Louis, MO, USA). Densities of the immunoreactive bands were analyzed with the Image Master 1D Elite software (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Statistical analysis
All values were expressed as mean ± SD. All other results, except pathological findings, were evaluated by one-way ANOVA and Tukey’s multiple comparison tests. Liver histopathological examination data were analyzed by the Kruskall-Wallis nonparametric test, followed by a Mann-Whitney test. Statistically significant differences between groups were defined as P values less than 0.05. Calculations were performed with the GraphPad Prism program (Graphpad Software, Inc, San Diego, USA).
RESULTS
Effects of GM on serum AST and ALT levels
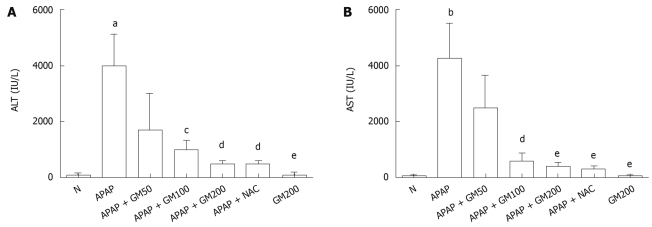
Serum activities of ALT and AST are shown in Figure 2. A single dose of APAP significantly elevated the serum ALT (P < 0.05) and AST (P < 0.01) activities when compared with the normal animals. Pretreatment with GM 2 h prior to APAP administration lowered markedly both serum ALT and AST levels. Serum ALT levels were significantly decreased to 43%, 26%, 13% or 13% in GM groups (200, 100 and 50 mg/kg) or NAC group (300 mg/kg) compared with APAP alone group, respectively. Serum AST levels were significantly decreased to 58%, 14%, 10% or 8% in GM groups (200, 100 and 50 mg/kg) or NAC group (300 mg/kg) compared with APAP alone group, respectively.
Figure 2.
Protective effects of GM on serum biochemical parameters after acetaminophen (APAP) administration. Mice were intraperitoneally injected with APAP (300 mg/kg body weight). GM (200, 100 or 50 mg/kg body weight) was orally administered at 2 h before APAP injection. All data are presented as mean ± SD, n = 10/group. aP < 0.05, bP < 0.01, significantly different when compared with normal controls. cP < 0.05, dP < 0.01, eP < 0.001 significantly different when compared with APAP alone group.
Effects of GM on GSH, SOD, GSH-px activities, MDA and 4-HNE contents
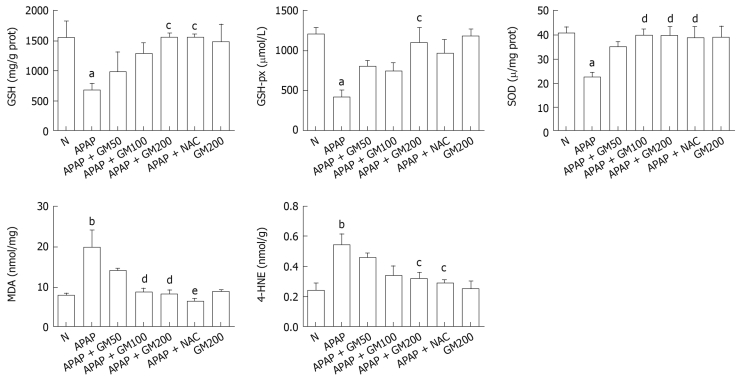
Twelve hours after APAP administration, GSH, GSH-px and SOD concentrations were significantly decreased to 44% (P < 0.05), 35% (P < 0.05) and 55% (P < 0.05) respectively in APAP group compared with the normal group. However, pretreatment with a high does of GM (200 mg/kg) significantly alleviated subsequent APAP-induced GSH depletion to 1561 ± 186 (P < 0.05). SOD (P < 0.01) and GSH-px activities (P < 0.05) were significantly enhanced in GM 200 mg/kg plus APAP treated group. MDA levels increased by 252% in mice treated with APAP, indicating that APAP administration significantly increased lipid peroxidation in liver (P < 0.01). Briefly, in mice receiving GM (200 or 100 mg/kg) plus APAP, the MDA levels were significantly reduced to 42% (P < 0.01) or 43% (P < 0.01) as compared with the APAP treated mice. In mice receiving GM (200 mg/kg) plus APAP, the 4-HNE levels were significantly reduced to 59% (P < 0.05) compared with the APAP treated mice (Figure 3).
Figure 3.
Protective effects of pretreatment with GM against APAP-induced glutathione (GSH) depletion, malonyldialdehyde (MDA) levels, 4-hydroxynonenal (4-HNE) levels and superoxide dismutase (SOD), glutathione peroxidase (GSH-px) activity in liver of mice. APAP was given intraperitoneally with a single dose of 300 mg/kg. GM was given orally with a single dose of 200, 100 or 50 mg/kg. All data are presented as mean ± SD, n = 10/group. aP < 0.05, bP < 0.01, significantly different when compared with normal controls. cP < 0.05, dP < 0.01, eP < 0.001 significantly different when compared with APAP alone group.
Effects of GM on histopathology
Histopathological analysis of the APAP alone treated animal showed severe centrilobular necrosis, fatty infiltration and lymphocytes infiltration (data not shown), which were significantly less in the GM plus APAP treated groups with mild sinusoidal congestion, less inflammatory cell infiltration, and well preserved hepatocytes with less area of necrosis (Table 1 and Figure 4).
Table 1.
Effects of GM on hepatic damage induced by APAP in mice
| Treatment | Dose (mg/kg) |
Histopathological scores |
||||||
| 0 | 1 | 2 | 3 | 4 | 5 | Average | ||
| Saline + Saline | - | 10 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| APAP + Saline | - | 0 | 0 | 1 | 3 | 4 | 2 | 3.7 |
| APAP + GM | 200 | 0 | 4 | 3 | 2 | 1 | 0 | 2.0b |
| 100 | 0 | 3 | 3 | 2 | 2 | 0 | 2.3a | |
| 50 | 0 | 2 | 3 | 2 | 2 | 1 | 2.7 | |
| APAP + NAC | 300 | 0 | 3 | 4 | 2 | 1 | 0 | 2.1b |
APAP was given intraperitoneally with a single dose of 300 mg/kg. GM was given orally with a single dose of 200, 100 or 50 mg/kg. Livers were graded from 0 to 5 as described in Materials and Methods. Each value is the number of animals with grading changes.
P < 0.05,
P < 0.01 significantly different when compared with APAP alone group. GM: Gentiana manshurica Kitagawa; APAP: Acetaminophen.
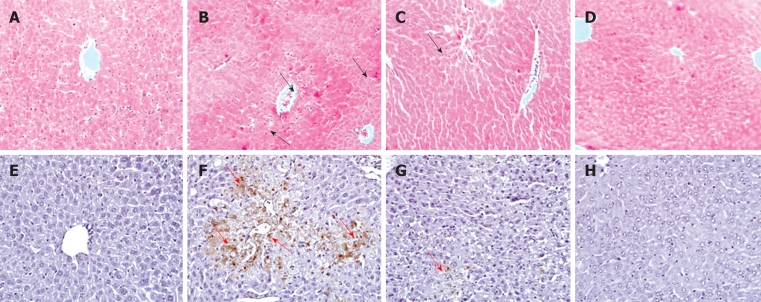
Figure 4.
Liver histopathology and transferase-mediated dUTP nick end-labeling (TUNEL) assay. APAP was given intraperitoneally with a single dose of 300 mg/kg. GM was given orally with a single dose of 200 mg/kg. A and E: Normal group; B and F: APAP group; C and G: APAP plus GM 200 mg/kg; D and H: Only GM 200 mg/kg. Upper panels stand for HE staining and the lower panels for TUNEL assay. The regions by black arrows in the upper figures indicate the hepatocyte necrosis or hepatocyte degeneration; the regions by red arrows in the lower figures indicate the TUNEL-positive apoptotic cells (× 100 magnification).
GM protects against APAP-induced hepatocyte apoptosis in mice via inhibiting caspase-3 and JNK/ERK MAPK pathway
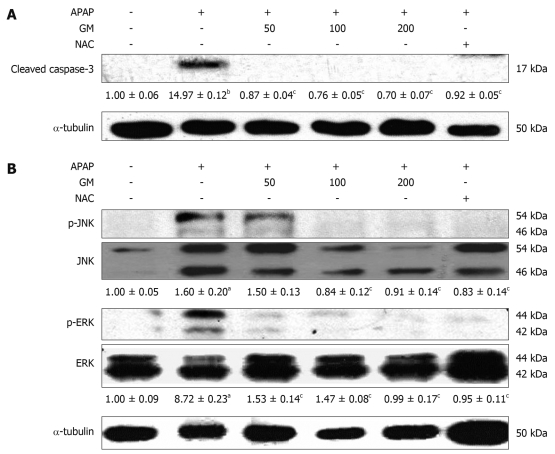
Apoptotic hepatocytes were detected by TUNEL staining. A large number of TUNEL-positive hepatocytes were seen in the livers of APAP-treated mice 12 h after injection, however, a few TUNEL-positive hepatocytes were found in livers from animals pretreated with GM (Figure 4). The protease activity of caspase-3 was measured in APAP-induced liver injury mice treated with and without GM. Casapase-3 was proteolytically processed to the active p17 fragment 12 h after APAP treatment in mice (Figure 5). However, GM significantly inhibited caspase-3 activity. It has recently been reported that c-Jun-NH2-terminal protein kinase 2 (JNK1/2) plays a critical role in mediating APAP hepatotoxicity in mice[20]. JNK1/2 activation is an early key signal in mediating mitochondria-mediated lethal cell injury triggered by toxicants in hepatocytes[21]. Therefore, we investigated whether APAP-induced JNK activation was attenuated by GM. Our data revealed that phosphorylated JNK and phosphorylated ERK, significantly increased after treatment with APAP when oxidative stress in the liver had been significantly enhanced as described above, while the JNK and ERK total protein level were almost normal 12 h after APAP treatment (Figure 5). After administrated with various doses of GM 2 h via APAP injection, phosphorylated JNK and phosphorylated ERK levels were declined (Figure 5). These data are consistent with our hypothesis that GM inhibits JNK/ERK MAPK signaling pathway.
Figure 5.
Western blotting analysis of caspase-3 and JNK/ERK MAPK. GM (200, 100 or 50 mg/kg), NAC (300 mg/kg) or saline was orally administered 2 h before APAP injection. Active form of caspase-3, phospho-JNK and phospho-ERK were detected by Western blot. The active form of caspase-3 levels corresponding to each immunoreactive band were digitized and expressed as a percentage of the α-tubulin levels. Densitometric scanning data of phospho-JNK and phospho-ERK levels were expressed as the ratio of JNK or ERK, respectively. The ratio of the normal group band was set to 1.00. Data of three independent experiments are expressed as mean ± SD. aP < 0.01, bP < 0.001, significantly different when compared with the normal group. cP < 0.001 significantly different when compared with APAP alone group.
DISCUSSION
Progress has been achieved in research of the chemical constituents and pharmacological activities of genus Gentiana. It is reported that gentianine from Gentiana Macrophylla Radix, one of Gentiana species, might express anti-inflammatory activities at least partly through preventing the immune cells, including macrophages, from producing TNF-α and IL-6, pro-inflammatory cytokines in male Sprague Dawley rats treated with LPS[22]. Hepatoprotective effects of Gentiana olivieri Griseb, flowering herbs on subacute administration were studied using in vivo models in rats, and the remarkable hepatoprotective activity of Gentiana olivieri might be due to the potent antioxidant activity of iso-orientin[23]. However, this is the first study to report the hepatoprotective effects of GM against APAP-related liver toxicity.
APAP is a safe and effective analgesics when used at therapeutic doses, an overdose of APAP, however, can induce severe hepatotoxicity in experimental animals and in human[24,25]. Liver injury induced by APAP is commonly used for the screening of hepatoprotective drugs[16]. NAC is used currently in clinical treatment for APAP overdose. Hereby, we used NAC as positive control, to compare with GM on hepatoprotective effects.
Administration of a single high dose of APAP significantly (P < 0.01) elevated the serum transaminase activities compared with the normal controls (Figure 2). The significantly decreased serum transaminases activities in the GM administered groups prior to APAP demonstrated its hepatoprotective effects. Thus, a single dose of methanol extract of GM could render a complete protection.
Cytochrome P-450 enzymes are the major catalysts involved in the metabolism of drugs. APAP is mainly metabolized by cytochrome P-450 to form an electrophilic metabolite, N-acetyl-p-benzoquinonimine, which is primarily inactivated by conjugation with GSH[26,27]. A large number of the metabolites produced by APAP are found to generate superoxide anion and other free radicals in the biological systems[28]. However, at a higher dose of APAP, intermediate metabolites accumulate and cause liver damage. Depletion of GSH beyond certain critical level can lead to oxidative stress and development of overt hepatotoxicity[29].
The decreased hepatic antioxidant status is related to oxidative stress and elevation of lipid peroxidation that lead to the leakage of hepatic enzymes to serum in APAP alone treated animals. To determine whether GM could inhibit APAP-induced GSH depletion, we measured the hepatic GSH levels. Our results showed that co-treatment with GM and APAP inhibited APAP-induced GSH depletion (Figure 3). NAC reduced APAP hepatic toxicity by increasing hepatic GSH levels. The increasing GSH levels had no significant differences between the group pretreated with GM (200 mg/kg) and NAC. The elevated hepatic reduced GSH level could partially explain the hepatoprotective mechanism of GM. Reduced GSH can function as a reductant in the metabolism of hydrogen peroxide and various organic peroxides. The GSH-px present in the cells can catalyze this reaction[30]. It is reported that depletion of GSH below a threshold value was associated with a significant conversion of xanthine dehydrogenase to reversible xanthine oxidase, a superoxide radical generation reaction catalyzing enzyme. Therefore, the enhanced hepatic GSH-px and SOD activities in the GM plus APAP treated group further support its hepatoprotective effects (Figure 3). MDA and HNE are major end-products of oxidation of polyunsaturated fatty acids, and are frequently measured as indicators of lipid peroxidation and oxidative stress in vivo. Thereby, the elevated antioxidant status in the liver of GM plus APAP treated group is related to the decreased MDA level and 4-HNE level, could maintain the membrane integrity and prevented lipid peroxidation and was comparable to NAC (Figure 3). The histopathological analysis of liver section indicates a moderate centrilobular necrosis, fatty infiltration and lymphocytic infiltration in the GM plus APAP treated animals with respect to the APAP alone treated animals (Table 1 and Figure 4).
APAP was believed to induce apoptosis based on the observation that APAP treatment results in the activation of caspase-3. In this study, pretreatment with GM prior to APAP inhibited caspase-3 cleavage (Figure 5). Thus, the active form of caspase-3 was not observed in GM or NAC pretreated groups. Microscopic observation on TUNEL-stained sections demonstrated that GM significantly decreased the TUNEL-positive apoptotic hepatocytes. Furthermore, it was reported that p38 MAPK, JNK, and ERK were activated by APAP[31]. JNK2 plays a protective role against APAP-induced liver injury in mice, in part, by modulating hepatocellular regeneration and repair, which further suggests the use of JNK inhibitors as a potential treatment for APAP-induced liver injury[32]. In both cultured hepatocytes and in vivo livers, treatment with APAP induced a sustained activation of JNK as reflected in increased phospho-c-jun levels[33]. A number of studies have suggested that oxidative stress leads to JNK activation, either through redox alteration of the sequestration of JNK or through inhibition of JNK phosphatase[34,35]. Our data showed that phospho-JNK1/2 expression was greatly increased 12 h after APAP administration (Figure 5). Coinciding with the expression of phospho-JNK1/2, APAP also increased the level of phosphor-ERK1/2. However, GM pretreatment can effectively inhibit phosphorylation of ERK1/2 (Figure 5). These data suggest that GM inhibits the JNK/ERK signaling pathway at a more proximal regulatory step, resulting in inhibition of its downstream effector mechanisms.
In conclusion, in this study, GM can significantly prevent the APAP-induced acute hepatotoxicity by enhancing the hepatic antioxidant activity, and inhibiting the caspase-3 cleavage and JNK/ERK MAPK activation. GM exerts some effects which resemble those of an antidote of acetaminophen such as NAC. However, further detailed studies are required to confirm its clinical application.
COMMENTS
Background
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug that is safe at therapeutic doses, however, when taken at high doses, APAP can precipitate severe liver injury that can develop into a liver failure. Overdoses of APAP lead to the generation of high amounts of the toxic metabolite N-acetyl-quinoneimine (NAPQI). NAPQI reacts with glutathione (GSH) spontaneously or catalyzed by glutathione-S-transferases to form a GSH-adduct. Thus, GSH depletion and formation of protein adducts are key mechanisms of APAP-induced cell death.
Research frontiers
In recent years, plant-derived natural products have received considerable attention due to their diverse pharmacological properties. Further studies on hepatoprotective effect of these natural entities and its possible mechanism are important for understanding the mechanism of APAP-induced liver injury.
Innovations and breakthroughs
The authors investigated the effects of Gentiana manshurica Kitagawa (GM) on APAP-induced liver injury in mice and whether GM prevents APAP-induced hepatocyte apoptosis in vivo. The present study concluded that GM can significantly prevent the APAP-induced acute hepatotoxicity by enhancing the hepatic antioxidant activity, and inhibiting the caspase-3 cleavage and JNK/ERK MAPK activation, and GM exerts some effects which resemble those of an antidote of acetaminophen such as NAC.
Applications
The results provide significant evidence illustrating the key feature of recovery from APAP-induced acute liver injury.
Peer review
This is an interesting paper that, although descriptive, points out to the potential hepatoprotective effect of a herbal compound (Gentiana manshurica Kitagawa:GM) on the acetaminophen-induced acute hepatotoxicity. Results are clear and manuscript is well written.
Footnotes
Supported by National Natural Science Foundation of China, No. 30660225 and No. 30960511
Peer reviewers: Isabel Fabregat, PhD, Associate Professor, Laboratory of Molecular Oncology, Institute of Biomedical Research of Bellvitge, Gran Via Km 2, 7, L’Hospitalet, 08907 Barcelona, Spain; Maurizio Parola, Professor, Department of Experimental Medicine and Oncology, University of Turin, Corso Raffaello 30, 10125 Torino, Italy
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Sener G, Toklu HZ, Sehirli AO, Velioğlu-Oğünç A, Cetinel S, Gedik N. Protective effects of resveratrol against acetaminophen-induced toxicity in mice. Hepatol Res. 2006;35:62–68. doi: 10.1016/j.hepres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM, Ostapowicz G. Acetaminophen: pathology and clinical presentation of hepatotoxicity. Drug-Induced Liver Disease. New York: Marcel Dekker; 2003. pp. 327–344. [Google Scholar]
- 3.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 6.Schwabe RF. Cell death in the liver-all roads lead to JNK. Gastroenterology. 2006;131:314–316. doi: 10.1053/j.gastro.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 8.Buckley NA, Whyte IM, O‘Connell DL, Dawson AH. Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose. J Toxicol Clin Toxicol. 1999;37:753–757. doi: 10.1081/clt-100102452. [DOI] [PubMed] [Google Scholar]
- 9.Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology. 2006;43:454–463. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]
- 10.Jiang RW, Wong KL, Chan YM, Xu HX, But PP, Shaw PC. Isolation of iridoid and secoiridoid glycosides and comparative study on Radix gentianae and related adulterants by HPLC analysis. Phytochemistry. 2005;66:2674–2680. doi: 10.1016/j.phytochem.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Liu JC, Zhang XN, Guo YY, Xu ZH, Cao W, Sun XL, Sun WJ, Zhao MG. Down-regulation of NR2B receptors partially contributes to analgesic effects of Gentiopicroside in persistent inflammatory pain. Neuropharmacology. 2008;54:1175–1181. doi: 10.1016/j.neuropharm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Oztürk N, Başer KH, Aydin S, Oztürk Y, Caliş I. Effects of Gentiana lutea ssp. symphyandra on the central nervous system in mice. Phytother Res. 2002;16:627–631. doi: 10.1002/ptr.998. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Takano F, Hojo H. Suppression of chemically and immunologically induced hepatic injuries by gentiopicroside in mice. Planta Med. 1994;60:414–416. doi: 10.1055/s-2006-959521. [DOI] [PubMed] [Google Scholar]
- 14.Duke J. Handbook of medicinal plants. 1st ed. Boca Raton: CRC Press; 1985. pp. 207–208. [Google Scholar]
- 15.Duke J, Bogenschuts-Godwin MJ, duCellier J, Duke PAK. Handbook of medicinal plants. 2nd ed. Boca Raton: CRC Press; 2002. pp. 324–325. [Google Scholar]
- 16.Wu YL, Piao DM, Han XH, Nan JX. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol Pharm Bull. 2008;31:1523–1529. doi: 10.1248/bpb.31.1523. [DOI] [PubMed] [Google Scholar]
- 17.Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartolone JB, Beierschmitt WP, Birge RB, Hart SG, Wyand S, Cohen SD, Khairallah EA. Selective acetaminophen metabolite binding to hepatic and extrahepatic proteins: an in vivo and in vitro analysis. Toxicol Appl Pharmacol. 1989;99:240–249. doi: 10.1016/0041-008x(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 19.Lai JT, Fang HL, Hsieh WT, Lin WC. Protective effect of a fermented substance from Saccharomyces cerevisiae on liver injury in mice caused by acetaminophen. Biosci Biotechnol Biochem. 2008;72:2514–2520. doi: 10.1271/bbb.70681. [DOI] [PubMed] [Google Scholar]
- 20.Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- 21.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 22.Kwak WJ, Kim JH, Ryu KH, Cho YB, Jeon SD, Moon CK. Effects of gentianine on the production of pro-inflammatory cytokines in male Sprague-Dawley rats treated with lipopolysaccharide (LPS) Biol Pharm Bull. 2005;28:750–753. doi: 10.1248/bpb.28.750. [DOI] [PubMed] [Google Scholar]
- 23.Orhan DD, Aslan M, Aktay G, Ergun E, Yesilada E, Ergun F. Evaluation of hepatoprotective effect of Gentiana olivieri herbs on subacute administration and isolation of active principle. Life Sci. 2003;72:2273–2283. doi: 10.1016/s0024-3205(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 24.Hwang HJ, Kwon MJ, Kim IH, Nam TJ. Chemoprotective effects of a protein from the red algae Porphyra yezoensis on acetaminophen-induced liver injury in rats. Phytother Res. 2008;22:1149–1153. doi: 10.1002/ptr.2368. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Abril ER, Bethea NW, McCuskey RS. Ethanol binging enhances hepatic microvascular responses to acetaminophen in mice. Microcirculation. 2004;11:625–632. doi: 10.1080/10739680490503456. [DOI] [PubMed] [Google Scholar]
- 26.Orrenius S, Moldéus P. The multiple roles of glutathione in drug metabolism. Trends Pharmacol Sci. 1984;5:432–435. [Google Scholar]
- 27.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries J. Hepatotoxic metabolic activation of paracetamol and its derivatives phenacetin and benorilate: oxygenation or electron transfer. Biochem Pharmacol. 1981;30:399–402. doi: 10.1016/0006-2952(81)90622-5. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 30.Cighetti G, Debiasi S, Paroni R. Effect of glutathione depletion on the conversion of xanthine dehydrogenase to oxidase in rat liver. Biochem Pharmacol. 1993;45:2359–2361. doi: 10.1016/0006-2952(93)90213-g. [DOI] [PubMed] [Google Scholar]
- 31.Lacour S, Antonios D, Gautier JC, Pallardy M. Acetaminophen and lipopolysaccharide act in synergy for the production of pro-inflammatory cytokines in murine RAW264.7 macrophages. J Immunotoxicol. 2009;6:84–93. doi: 10.1080/15476910902938250. [DOI] [PubMed] [Google Scholar]
- 32.Bourdi M, Korrapati MC, Chakraborty M, Yee SB, Pohl LR. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun. 2008;374:6–10. doi: 10.1016/j.bbrc.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]