Abstract
Actinomycosis is an uncommon chronic infectious disease. Common sites of involvement include the cervicofacial, thoracic and abdominopelvic regions. In abdominopelvic actinomycosis, the ileocecal region, including the appendix, is the most commonly involved site. In some reports, limited appendiceal actinomycosis has revealed a thickened appendiceal wall with peri-appendiceal inflammation as acute appendicitis or perforated appendicitis. We experienced pathologically confirmed intraluminal limited appendiceal actinomycosis without peri-appendiceal infiltration. Here, we report the computed tomography and ultrasound findings.
Keywords: Actinomyces, Actinomycosis, Appendiceal neoplasms, Appendicitis
INTRODUCTION
Actinomycosis is a chronic progressive suppurative disease caused by Actinomyces israelii, and is characterized by the formation of multiple abscesses, draining sinus, abundant granulation, and dense fibrous tissue[1]. Several reports have described the radiological findings of abdominopelvic actinomycosis. The infiltrative mass with unusual aggressiveness is the one of important radiological findings[1-3]. Also, some reports of appendiceal actinomycosis have described wall thickening and peri-appendiceal inflammation, with contrast enhancement[4,5]. We experienced pathologically confirmed appendiceal actinomycosis that presented as a small intraluminal mass without peri-appendiceal infiltration. Here, we report the computed tomography (CT) and ultrasound (US) findings.
CASE REPORT
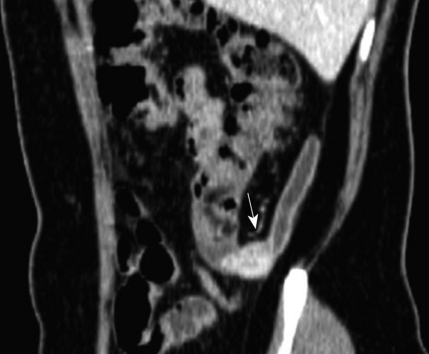
A 50-year-old woman was found incidentally to have a small appendiceal mass during routine screening. Past clinical history, physical examination, and laboratory examination were all unremarkable. Contrast-enhanced abdominal CT showed a well-defined small mass at the origin of the appendix. The length of the mass on CT images was 2 cm. This mass showed dense enhancement of 100 HU at the arterial phase and 125 HU at the portal phase. Infiltration around the meso-appendix and peri-appendiceal area was not demonstrated by CT, nor was regional lymph node enlargement detected (Figure 1). Positron emission tomography (PET) with [F18]-2-fluoro-2-deoxy-D-glucose (FDG) showed a high metabolic rate, with a maximum standardized uptake value (SUV) of 3.64.
Figure 1.
Contrast-enhanced CT revealed a well-defined solid mass with strong enhancement in the base of the appendix (arrow). Peri-appendiceal infiltration was not seen.
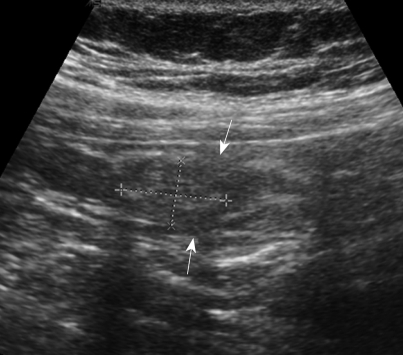
US images obtained with a 9.4-MHz linear probe showed a well-defined intraluminal mass with heterogeneous echogenicity in the base of the appendix. The diameter of the mass was 1.6 cm. Peri-appendiceal infiltration was not demonstrated by US. US revealed focal defects at the echogenic inner mucosal layer. However, relatively well preserved mural echogenicity was noted (Figure 2).
Figure 2.
US showed a heterogeneous, hyperechoic, intraluminal mass at the base of the appendix, without peri-appendiceal infiltration. We also noted focal defects at the echogenic inner mucosal layer (arrows).
From these findings, appendiceal neoplasms of mucosal origin, such as mucinous adenocarcinoma and carcinoid, were diagnosed, and a laparoscopic partial cecectomy was performed. Surgical findings revealed an intraluminally growing small mass with no sign of macroscopic serosal invasion. Also, there was no sign of infiltration or invasion of the meso-appendix and other surrounding organs.
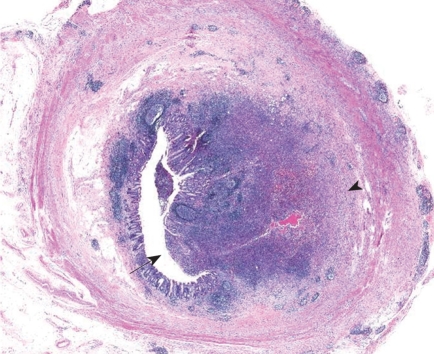
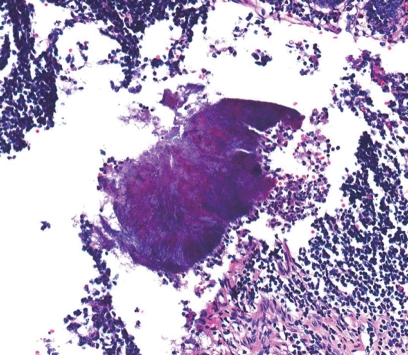
Gross pathology revealed an ill-defined, yellowish white, protruding mass lesion, which originated from the basal portion of the appendix. Adjacent appendiceal and cecal wall showed edema, and the serosal surface was relatively clear. Upon microscopy, the appendix showed a localized, mass-like abscess formation of the appendiceal wall (Figure 3). With higher magnification, a typical sulfur granule surrounded by neutrophils was found (Figure 4), which was confirmed as appendiceal actinomycosis. At the submucosa, the outer portion of the abscess showed evidence of organization, with vascularized granulation tissue and fibrosis in addition to chronic inflammatory cell infiltration.
Figure 3.
Microscopy of appendiceal actinomycosis. An abscess composed of chronic and acute inflammatory cells was observed in a mass-like lesion (arrow), from the mucosal surface to the superficial submucosa (arrowhead) (HE, × 10).
Figure 4.
Higher magnification showed a typical sulfur granule surrounded by neutrophils in the clefted abscess center (HE, × 200).
DISCUSSION
Actinomycosis has a worldwide distribution and is found with equal frequency in urban and rural dwellers. In humans, Actinomyces israelii is the most common cause of the disease. These organisms are indigenous in the oral cavity, gastrointestinal tract, and genital tract, with opportunistic infection occurring when the mucosal barrier is broken, which leads to multiple abscess formation, fistula, or mass lesions[6,7]. Actinomycosis commonly occurs in three distinct forms that may occasionally overlap; most clinical disease is cervicofacial (55%), with only 20% occurring in the abdominopelvic form and 15% in the thoracic form[8]. Although the clinical features depend on which organs are involved, common symptoms and signs include fever and leukocytosis[3,6].
Abdominopelvic actinomycosis is associated with abdominal surgery (such as appendectomy), bowel perforation, or trauma[9]. In addition, the presence of a long-standing intrauterine device (IUD) is a reported risk factor in young women[10]. Although the pathogenesis of abdominopelvic actinomycosis is not well understood, the appendix is the most commonly involved intra-abdominal organ, the colon, stomach, liver, gallbladder, pancreas, small intestine, pelvis, and abdominal wall may also be involved[6]. However, development of abdominopelvic actinomycosis after acute appendicitis has decreased because of early diagnosis, a lower incidence of perforated appendicitis, and improved antibiotic therapy[8].
As a result of its resemblance to other diseases such as appendicitis, diverticulitis, colon carcinoma, Crohn’s disease, ulcerative colitis, and tubo-ovarian abscess, the diagnosis of abdominopelvic actinomycosis is difficult[11]. A definite diagnosis is generally based on histological identification of actinomycotic granules or culture of the Actinomyces species, or both. High-dose intravenous penicillin injection is the treatment of choice and has a favorable response[11]. Therefore, early diagnosis is important to minimize morbidity of the disease and prevent unnecessary surgery.
Direct spread into adjacent tissue is the most common primary route of propagation after penetration of the organism through the mucosal barrier. Therefore, infiltration has been well described as an important radiological characteristic[1,3]. CT is an important imaging modality for suggesting the diagnosis and determining the anatomical location and extent of the disease, as well as monitoring the effectiveness of treatment[2,3,6]. Important CT features are an infiltrative mass (predominantly cystic or solid) adjacent to the other involved organs or anatomical structures, and the main CT feature when the gastrointestinal tract is involved is bowel wall thickening[1-3]. After infusion of contrast material, dense contrast enhancement in the mass or involved bowel has been reported, which may be caused by abundant granulation and dense fibrous tissue[1,3,10]. Although these findings are nonspecific, actinomycosis should be included in the differential diagnosis, especially in patients with abdominal pain, fever, leukocytosis, or long-term IUD use[6,9]. CT features of abdominopelvic actinomycosis closely resemble complicated gastrointestinal malignancy or other chronic inflammatory disease (such as intestinal tuberculosis or Crohn’s disease). However, because of the size of the bacterium, it usually does not spread via the lymphatic system; therefore, regional lymphadenopathy is uncommon or develops late[8,9].
In our case, appendiceal actinomycosis showed dense contrast enhancement, especially at the portal phase, and vascularized granulation tissue with fibrosis in the submucosa. There was no sign of regional lymphadenopathy. However, appendiceal actinomycosis presented as an intraluminal limited mass without peri-appendiceal infiltration, which is an unusual finding. A review of the literature about abdominopelvic actinomycosis did not reveal any similar cases.
Footnotes
Peer reviewer: Clifford S Cho, MD, Assistant Professor, Department of Surgery, Section of Surgical Oncology, University of Wisconsin School of Medicine and Public Health, H4/724 Clinical Sciences Center, 600 Highland Avenue, Madison, WI 53792, United States
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Lee IJ, Ha HK, Park CM, Kim JK, Kim JH, Kim TK, Kim JC, Cho KS, Auh YH. Abdominopelvic actinomycosis involving the gastrointestinal tract: CT features. Radiology. 2001;220:76–80. doi: 10.1148/radiology.220.1.r01jl1376. [DOI] [PubMed] [Google Scholar]
- 2.Shah HR, Williamson MR, Boyd CM, Balachandran S, Angtuaco TL, McConnell JR. CT findings in abdominal actinomycosis. J Comput Assist Tomogr. 1987;11:466–469. doi: 10.1097/00004728-198705000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Ha HK, Lee HJ, Kim H, Ro HJ, Park YH, Cha SJ, Shinn KS. Abdominal actinomycosis: CT findings in 10 patients. AJR Am J Roentgenol. 1993;161:791–794. doi: 10.2214/ajr.161.4.8372760. [DOI] [PubMed] [Google Scholar]
- 4.Maternini M, Saucy F, Sandmeier D, Vuilleumier H. Simple appendicitis. Can J Surg. 2008;51:E54–E55. [PMC free article] [PubMed] [Google Scholar]
- 5.Yiğiter M, Kiyici H, Arda IS, Hiçsönmez A. Actinomycosis: a differential diagnosis for appendicitis. A case report and review of the literature. J Pediatr Surg. 2007;42:E23–E26. doi: 10.1016/j.jpedsurg.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Berardi RS. Abdominal actinomycosis. Surg Gynecol Obstet. 1979;149:257–266. [PubMed] [Google Scholar]
- 7.Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973;4:319–330. doi: 10.1016/s0046-8177(73)80097-8. [DOI] [PubMed] [Google Scholar]
- 8.Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198–1217. doi: 10.1288/00005537-198409000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Yegüez JF, Martinez SA, Sands LR, Hellinger MD. Pelvic actinomycosis presenting as malignant large bowel obstruction: a case report and a review of the literature. Am Surg. 2000;66:85–90. [PubMed] [Google Scholar]
- 10.Peitsidis P, Papadimitriou C, Rodolakis A, Peitsidou A. Actinomycosis of the appendix and pelvis: a case report. J Reprod Med. 2008;53:711–713. [PubMed] [Google Scholar]
- 11.Allen HA 3rd, Scatarige JC, Kim MH. Actinomycosis: CT findings in six patients. AJR Am J Roentgenol. 1987;149:1255–1258. doi: 10.2214/ajr.149.6.1255. [DOI] [PubMed] [Google Scholar]