Abstract
The gut contents of larval mosquitoes are alkalinized by the anterior midgut and reacidified by the posterior midgut. In the present study the cellular mechanisms of reacidification were studied in isolated, perfused posterior midgut by measuring the transepithelial voltage (Vte) and the rate of acid secretion as indicated by the color change of m-cresol purple during intervals of perfusion stop. The lumen-positive Vte and reacidification were significantly increased by serotonin (0.2 μmol l−1). The V-type H+-ATPase inhibitor concanamycin A (10 μmol l−1) on the luminal side inhibited acidification and decreased Vte. On the hemolymph side the carbonic anhydrase (CA) inhibitor acetazolamide (1 mmol l−1) almost abolished Vte, but had no effect on acidification. Similarly, hemolymph-side DIDS (0.1 mmol l−1), DPC (0.5 mmol l−1), amiloride (1 mmol l−1) and ouabain (2.5 mmol l−1) significantly reduced Vte, whereas Ba2+ (5 mmol l−1) was without effect. DPC and amiloride also reduced Vte when applied to the luminal side of the epithelium. Unilateral substitution of gluconate for Cl− affected Vte in a way consistent with a greater permeability for Cl− versus Na+. Cl− replacement in the lumen decreased Vte, whereas replacement on the hemolymph side increased it. Bilateral replacement left the control voltage unaffected. Na+ replacement on either side of the tissue reduced Vte to different degrees. Omission of luminal amino acids was followed by a significant decrease in Vte. Except for concanamycin A, none of the above manipulations impaired acidification, indicating that acidification requires only the apical proton pump. However, the chemical source of secreted H+ is still unknown and needs to be investigated.
Keywords: V-ATPase, acid secretion, acid–base balance, mosquito, posterior midgut, transepithelial potential
INTRODUCTION
An interesting and perplexing feature of the mosquito larval midgut is that the luminal compartments exhibit one of the highest pH values generated by a biological system. The pH of the luminal contents in mosquito larvae increases from near neutrality in the foregut to a value that exceeds 10 in the anterior midgut then drops to 7.5 in the posterior midgut (Dadd, 1975; Ramsay, 1950). These results are consistent with a vigorous active cycling of base between the gut contents and hemolymph during the digestive cycle, similar to that suggested for lepidopteran larvae (Moffett, 1994). The alkaline environment optimal for mosquito digestive enzymes sterilizes food and dissociates the tannin–protein complexes that are ingested in the plant detritus diet of the larvae (see Clements, 1992).
The generation of large pH gradients along short distances in the absence of morphological barriers is almost certainly the result of region-specific ion transport systems energized by ion-motive ATPases. The relative locations of V-type H+-ATPase and Na+/K+-ATPase in the midgut have been described in larval Aedes aegypti (Patrick et al., 2006) and Anopheles gambiae (Okech et al., 2008a). V-ATPases are located in the basal membrane of the anterior midgut and in the apical membrane of the posterior midgut. Na+/K+-ATPase is found in the apical membrane of the anterior midgut and in the basal membrane of the posterior midgut. Three different types of carbonic anhydrase (CA) have been found in larval mosquito midgut (Linser et al., 2009): an intracellular CA located in the posterior midgut, a glycosylphosphatidylinositol (GPI)-linked, extracellular, membrane-bound CA located on muscle cells, and a soluble, extracellular CA found in the ectoperitrophic space of anterior and posterior midgut. Na+/H+ exchangers were found with immunohistochemical techniques in larval An. gambiae midgut (Rheault et al., 2007; Okech et al., 2008a), but their involvement in acid–base transport in the different segments of the larval mosquito midgut is still not clear. Cation/amino acid cotransporters were localized and characterized in the different midgut sections (Boudko et al., 2005; Okech et al., 2008b). The overall transport mechanisms responsible for acid–base cycling in the anterior and posterior midgut are so far not entirely clear (see Onken and Moffett, 2009).
The entire literature on pH in insects has been preoccupied with alkalinization (Waterhouse, 1949; Dadd, 1975; Dow, 1984; Onken et al., 2008), whereas the reacidification has hardly been addressed. The only report of physiological experiments with posterior midguts is the finding of a lumen-positive, serotonin-stimulated transepithelial voltage (Vte) by Clark and colleagues (Clark et al., 1999), consistent with cation secretion and/or anion absorption. On the basis of the so-far described transporters, the most plausible hypothesis for the mechanism of reacidification involves primary secretion of H+ by the apical V-ATPase together with basal acid and/or base relevant transporters so far unknown.
The specific function of the posterior midgut requires it to reacidify the highly alkaline luminal solution in which CO32– is the predominant anion and Na+ and/or K+ are expected to be the dominant cations. In the intact animal, it could be anticipated that proton secretion by the posterior midgut would convert much of the carbonate to bicarbonate/carbonic acid or, in the presence of luminal CA, carbon dioxide may diffuse into the epithelial cells. There it could be converted back to protons and bicarbonate, accelerated by the intracellular CA. Bicarbonate would be expected to be transported to the hemolymph, in order to guarantee transepithelial acid secretion/base absorption. Macroscopic electroneutrality would require that this process be matched by transepithelial cation absorption or anion secretion. In the present study, ion substitution experiments and inhibitors of transporters were used to obtain more information about the transport mechanisms reflected in the Vte generated by the serotonin-responsive cells and the mechanism of acid secretion in the larval posterior midgut of the mosquito A. aegypti.
MATERIALS AND METHODS
Animals
Aedes aegypti L. (Vero Beach strain) eggs were provided by Dr Marc Klowden (University of Idaho, Moscow, ID, USA) from a continuously maintained colony. Eggs were hatched and larvae were maintained in a 1:1 mixture of tap water and deionized water at 26°C and on a 16 h:8 h L:D photoperiod. The water was replaced each morning, and the larvae were fed with ground Tetramin flakes (Tetrawerke, Melle, Germany). Fed 4th instar larvae were used in all experiments.
Solutions and chemicals
The basic NaCl saline used to perfuse the bath (hemolymph side of the epithelium) was based on larval Aedes hemolymph composition (Edwards, 1982a; Edwards, 1982b) and consisted of (in mmol l−1): NaCl, 42.5; KCl, 3.0; MgCl2, 0.6; CaCl2, 5.0; NaHCO3, 5.0; succinic acid, 5.0; malic acid, 5.0; l-proline, 5.0; l-glutamine, 9.1; l-histidine, 8.7; l-arginine, 3.3; dextrose, 10.0; Hepes, 25. The pH was adjusted to 7.0 with NaOH. In Na+-free saline, Na+ was replaced by N-methylglucamine. Instead of 5 mmol l−1 NaHCO3, this saline contained 3 mmol l−1 KHCO3 (no KCl). The pH was adjusted with HCl. In Cl−-free saline, gluconates (Na+, K+, Ca2+) or sulfate (Mg2+) was substituted for the chlorides. The pH was adjusted with NaOH. Amino acid-free saline was prepared by eliminating all the amino acids used and adding d-mannitol to compensate for the difference in osmotic strength. The above components were purchased from Sigma (www.sigmaaldrich.com), Fisher Scientific (www.fishersci.com) or Mallinckrodt (www.mallinckrodt.com). Serotonin (Sigma), ouabain (Sigma) and 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS, Sigma) were directly dissolved in the saline. Amiloride (Sigma) and BaCl2 were added to the saline from an aqueous stock solution. Concanamycin A, diphenylamine-2-carboxylate (DPC) and acetazolamide were from Sigma and were added from stock solutions in dimethylsulfoxide (DMSO, Sigma). The primary solvent alone had no effect on voltage or acidification at the concentrations present in the experiments.
The luminal perfusate for the reacidification experiments was identical to the above saline, but contained 0.04% m-cresol purple (Aldrich, www.sigmaaldrich.com) and reduced buffer (0.25 mmol l−1 Hepes) and was adjusted to pH 10 using NaOH. In some experiments as indicated, bromo-thymol blue was substituted for m-cresol purple and the solution was lightly buffered to pH 7.5 instead of pH 10.
Preparation, mounting, perfusion and measurement of Vte
Methods for the manufacture of perfusion pipettes as well as the preparation, mounting and perfusion of posterior midguts were almost identical to those outlined in detail before for the anterior midgut (Onken et al., 2004a; Onken et al., 2004b) and will be described only briefly here. After the larvae had been killed, the intestinal system was isolated and transferred to the bath of a perfusion chamber. The caeca, the hindgut and the Malphigian tubules were cut off and the anterior midgut was slipped onto an L-shaped perfusion pipette held by a micromanipulator (Brinkmann, www.brinkmann.com) until the tip of the pipette recorded the typical, high, lumen-positive Vte. The preparations were tied in place with a fine human hair. In order to keep the preparation in the focus of the binocular microscope, the open posterior end of the midgut preparation was slipped onto a glass rod manufactured from a glass capillary pipette (20 μl; VWR, www.vwrsp.com) and held by a second micromanipulator. The bath (volume 1 ml) was gravity perfused (rate 15–30 ml h−1) with oxygenated saline. The lumen was perfused with syringe pumps (model ALLADIN 1000; www.wpiinc.com) at a rate of 80 μl h−1.
The perfusion pipette contained a piece of polyethylene tubing (Intramedic PE 10; VWR) and was closed with a syringe needle. The syringe needle and the tubing were connected to two different pumps, allowing fast changes between the two different luminal perfusion solutions. The Vte was measured at a pH value of 7 in the bath and luminal perfusate as previously described (Onken et al., 2004a; Onken et al., 2004b). Only those preparations that showed the typical marked increase of Vte after application of serotonin (0.2 mmol l−1) (see Clark et al., 1999) were used for data collection.
Reacidification
Reacidification of the luminal perfusate from a pH of 10 was monitored after perfusion stop through the color changes of the pH indicator m-cresol purple. Color changes were documented with a digital camera. All photographs were identically edited (cropped, adjustment of lighting) with iPhoto Express (Ulead Systems, www.ulead.com). The Vte was not monitored during the reacidification experiments. However, in some preliminary experiments we noticed that Vte was hardly affected by changes in luminal pH.
Statistics
All data are presented as means ± s.e.m. Differences between groups were tested with Student's paired t-test. In those cases where controls were followed by two experimental results (e.g. Vte after application of a drug to the bath and Vte after bilateral application), one-way analysis of variance (ANOVA) with Tukey's post-hoc test was performed. Significance was assumed at P<0.05; in the figures, significant differences from controls are indicated by asterisks.
RESULTS
Serotonin stimulates Vte and reacidification
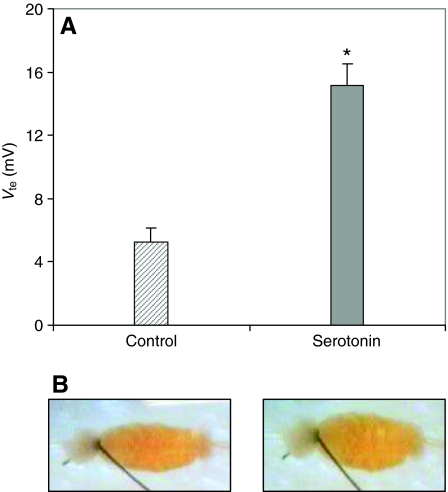
The posterior midgut preparations showed an initial lumen-positive voltage of 42±5.1 mV (range 16–83 mV, N=20). This Vte decreased rapidly as described previously (Clark et al., 1999) and after 10–15 min the voltage stabilized at 5±1 mV (range 1–13 mV, N=20). Addition of serotonin (0.2 μmol l−1) to the bath saline increased the voltage to a mean of 15±3 mV (range 5–27 mV, N=20) (see Fig. 1A).
Fig. 1.
(A) Mean lumen-positive transepithelial voltage (Vte; ±s.e.m.) of posterior midguts in the absence (hatched bar) and presence (gray bar) of serotonin (0.2 μmol l−1). *P<0.05 (B) Photographs of a preparation of the posterior midgut of larval (4th instar) Aedes aegypti, after 5 min of perfusion stop, in the absence (left) and in the presence (right) of serotonin (0.2 μmol l−1).
In 5 experiments, the effect of serotonin on reacidification from pH 10 was also evaluated. In the presence of m-cresol purple, color changes from purple to yellow during acidification. This color change was slow in the absence of serotonin. A marked change could only be observed 3–5 min after perfusion stop. The change was clearly faster and more pronounced in the presence of serotonin. Color changes were observed to be most rapid at the most posterior portion of the posterior midgut and then continued to spread anteriorly. Fig. 1B shows photographs of the tissue during a representative experiment with and without serotonin.
Luminal concanamycin A inhibits Vte and reacidification
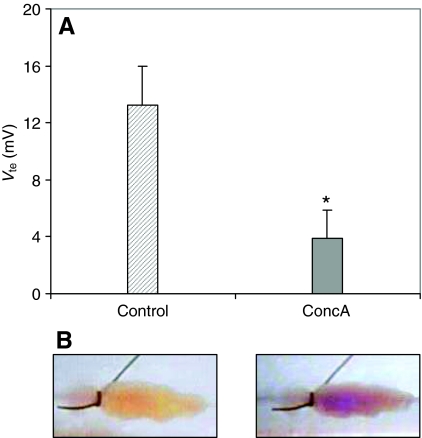
Concanamycin A (10 μmol l−1), a specific inhibitor of V-ATPases (Dröse and Altendorf, 1997), reduced Vte from 13±3 mV to 4±2 mV (N=5, P<0.05) within 15–20 min of application to the luminal perfusate (see Fig. 2A). In reacidification assays (N=5) color changes from purple to yellow were never observed after application of luminal concanamycin A. Fig. 2B shows reacidification before and after addition of the drug to the luminal saline.
Fig. 2.
(A) Mean Vte (±s.e.m.) of serotonin-stimulated posterior midguts in the absence (hatched bar) and presence (gray bar) of concanamycin A (ConcA; 10 μmol l−1). *P<0.05. (B) Photographs of a preparation of the posterior midgut of larval (4th instar) A. aegypti, after 5 min of perfusion stop in the absence (left) and in the presence (right) of concanamycin A (10 μmol l−1).
Influence of inhibitors of anionic pathways
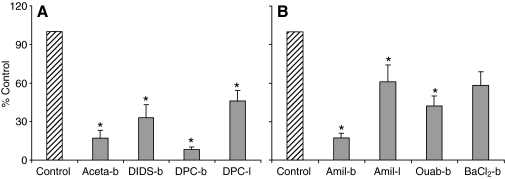
Acetazolamide (1 mmol l−1), a CA inhibitor (Maren, 1967), almost abolished Vte when applied to the hemolymph-side bath. In these experiments (N=5), the drug decreased Vte from 19±3 mV to 3±2 mV (P<0.05, see Fig. 3A).
Fig. 3.
Effects of various inhibitors on Vte, normalized to percentage of control value. (A) Effect of anionic inhibitors: bath-side acetazolamide (Aceta-b; control value=19±3 mV), bath-side DIDS (control value=15±4 mV), bath side DPC (DPC-b; control value=19±2 mV) and luminal side DPC (DPC-l; control value=8±1 mV). (B) Effect of cationic inhibitors: bath-side amiloride (Amil-b; control value=21±5 mV), luminal side amiloride (Amil-l; control value=9±2 mV), bath-side ouabain (Ouab-b; control value=8±3 mV) and bath-side barium (BaCl2-b; control value=10±3 mV). *P<0.05.
In the next group of experiments, we used DIDS and DPC, broad-spectrum inhibitors of anion transporters (see Culliford et al., 2003; Reddy and Quinton, 2002). When DIDS (0.1 mmol l−1) was added to the bathing solution, the Vte was significantly inhibited by approximately 70% from 15±4 mV to 5±2 mV (N=5, P<0.05, see Fig. 3A). DPC (0.5 mmol l−1), when applied first in the bathing medium, decreased Vte by approximately 90% from 19±2 mV to 2±0.4 mV (P<0.05, see Fig. 3A). When DPC was applied to the lumen first, a significant decrease of about 50% was observed (from 8±1 mV to 4±0.4 mV, N=5, P<0.05, see Fig. 3A).
In reacidification assays at a luminal starting pH of 10, the above drugs did not affect the capacity of the tissues to acidify the luminal pH.
Influence of inhibitors of cationic pathways
Addition of ouabain (2.5 mmol l−1), a specific inhibitor of the Na+/K+-ATPase (Skou, 1965), to the bath resulted in a significant reduction of Vte (from 8±3 mV to 3±2 mV, N=6, P<0.05; see Fig. 3B).
The effect of amiloride at a concentration of 1 mmol l−1 was studied on both sides of the tissue. Amiloride is an inhibitor of epithelial sodium channels and various Na+-dependent ion exchangers (see Garty and Benos, 1988) as well as insect K+/2H+ exchange (Wieczorek et al., 1991). When applied to the hemolymph side the drug significantly decreased Vte by almost 90% (from 21±5 mV to 2±1 mV, N=5, P<0.05, see Fig. 3B). When added to the luminal perfusate, amiloride had a less pronounced but still statistically significant effect on the voltage (from 9±2 mV to 6±2 mV, N=5, P<0.05, see Fig. 3B).
Barium chloride (5 mmol l−1), a well-known blocker of K+ channels (VanDriessche and Zeiske, 1985), did not significantly affect Vte (N=5, P>0.05, see Fig. 3B).
In reacidification assays at a luminal starting pH of 10, the above drugs did not affect the capacity of the tissues to acidify the luminal pH.
Influence of substitution experiments
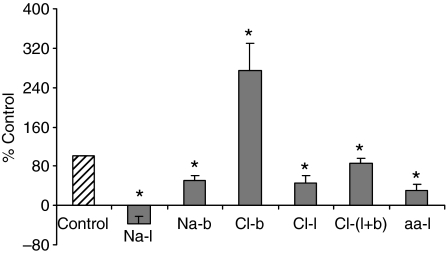
When Cl−-free saline was used on the luminal side, Vte decreased from 26±5 mV to 12±4 mV (N=5). Subsequent use of Cl−-free saline on both sides of the epithelium caused Vte to return to control values (from 12±4 mV to 21±5 mV, Fig. 4). When the hemolymph-side bathing solution was changed to Cl−-free saline, Vte increased significantly by approximately the same magnitude from 9±2 mV to 20±2 mV (N=6, P<0.05, see Fig. 4). The effects of Cl−-free saline were reversible in all cases upon return to normal Cl− levels.
Fig. 4.
Effect of ion substitution experiments on normalized Vte. The effects of Na+ replacement in the lumen (Na-l; control value=29±2 mV) and in the bath (Na-b; control value=10±3 mV), Cl− replacement in the bath (Cl-b; control value=9±2 mV), lumen (Cl-l; control value=26±5 mV) and both bath and lumen (Cl-l+b; control value=12±4 mV), and amino acid replacement in the lumen side (aa-l; control value=16±3 mV) on Vte are shown here. *P<0.05.
When Na+-free saline was applied to the luminal side, Vte was completely abolished (from 29±2 mV to –9±6 mV, P<0.05, N=6, see Fig. 4). Na+-free saline in the hemolymph-side bath caused Vte to decrease by 50–60% (from 10±3 mV to 5±2 mV, N=5, P<0.05, see Fig. 4).
Amino acid-free saline with d-mannitol on the luminal side of the epithelium caused a statistically significant decrease of Vte by approximately 50%. On an average, Vte dropped from 16±3 mV to 8±1 mV (N=6, P<0.05, see Fig. 4). A simultaneous experiment with no d-mannitol also caused a significant decrease in Vte.
In reacidification assays at a luminal starting pH of 10, the above substitutions did not affect the capacity of the tissues to acidify the luminal pH.
DISCUSSION
Methodological aspects
These studies utilized the same open-ended luminal perfusion technique as in our previous studies of the anterior midgut (Onken et al., 2004; Onken et al., 2008). This approach depends on the assumption that transmural electrical resistance is lower than the sum of luminal core resistance and the resistance of the leak pathway presented by the open end of the gut. The validity of this assumption was supported by preliminary experiments in which electrical isolation of the luminal perfusate was attempted by superfusing the open end of the perfused gut with a stream of non-conductive solution (saturated sucrose); this procedure did not result in a measurable increase in Vte. Even if this assumption was incorrect, relative changes of Vte are still useful for pharmacological analysis of the mechanisms responsible for generating the Vte, even if the values are diminished. The Vte under control conditions varied significantly between different experimental series (see Results). This phenomenon was also observed in studies of the anterior midgut (Onken et al., 2004) and possible reasons for this were discussed in that report.
In most of the acidification experiments, the luminal perfusate was weakly buffered at pH 10. Thus it is possible for acidification to occur passively by diffusion of bath acid into the lumen. However, it is unlikely that passive movement of acid or base between lumen and bath can be a significant factor in the results, for in concanamycin A-treated tissues, no color change was noted over time periods as long as 20 min. Furthermore, in experiments in which the luminal perfusate was weakly buffered at pH 7.5, the tissues generated acidification to pH values below 6.5 as indicated by the color change of bromo-thymol blue (pK=6.5). These results give us confidence that reacidification is an active process, and that neither transmural ionic leakage nor bulk flow through the cut end of the gut makes a significant contribution to the drop in luminal pH that occurs during perfusion stop.
Luminal acidification
As in the anterior midgut (Onken et al., 2004a; Onken et al., 2008), serotonin stimulates both Vte and acid–base transport (Fig. 1), even though the direction of acid–base flow is opposite in the two tissues. Luminal alkalization is driven by a basal V-ATPase in the anterior midgut, whereas the posterior midgut uses an apical V-ATPase for luminal acidification (Fig. 2). This finding is consistent with immunohistochemical results for the location of the V-ATPase in larval A. aegypti (Zhuang et al., 1999; Patrick et al., 2006) and An. gambiae (Okech et al., 2008a).
Processes that contribute to Vte
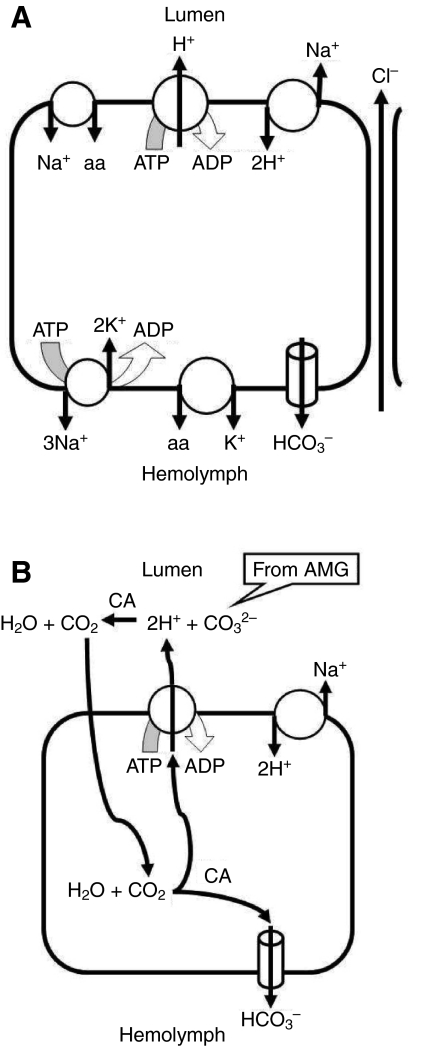
The Vte measured in these studies is the algebraic sum of the cytoplasm-negative transbasal potential and the ordinarily larger, cell-negative transapical potential. Therefore, a smaller lumen-positive Vte can result in principle either from a reduction in the magnitude of the transapical potential or from an increase in the magnitude of the transbasal potential, and vice versa. Fig. 5A summarizes our pharmacological dissection of the cellular processes involved in the determination of Vte and acid secretion by the posterior midgut.
Fig. 5.
(A) Summary of transport processes of the posterior midgut suggested or confirmed by the present studies. (B) Hypothesis for alkali recovery by the posterior midgut driven by the V-ATPase. The soluble extracellular carbonic anhydrase (CA) present in the posterior midgut (Linser et al., 2009) accelerates equilibration of carbonate secreted by the anterior midgut (AMG) with H+ secreted by the posterior midgut. The resulting CO2 diffuses into the posterior midgut cells where it is rehydrated, a process catalyzed by the intracellular CA. The resulting H+ can be resecreted by the apical H+ pump, whereas the HCO3− must leave the cells across the basal membrane. The net effect is to exchange luminal (bi)carbonate for Cl− from the hemolymph.
In the apical membrane of the principal cells, V-ATPase activity generates a large transapical membrane potential that results in a lumen-positive Vte, as supported by the concanamycin A results (Fig. 2A). Possible roles for an apical Na+/2H+ exchanger and Na+-coupled amino acid transporters [see figure 6 in Okech et al. (Okech et al., 2008b)] were considered in these studies. Both of these processes would be expected to result in an inward current that would depolarize the transapical potential and reduce Vte. Our finding of a reduced Vte after omission of luminal amino acids (Fig. 4) certainly supports the presence of amino acid transporters. Also, our finding of a completely abolished Vte after luminal Na+ replacement (Fig. 4) is consistent with both Na+-coupled amino acid transport and a depolarizing Na+/2H+ exchanger (NHA). Such an exchanger has been reported from the apical membrane of posterior midgut of An. gambiae (Rheault et al., 2007). However, the reduced Vte after luminal amiloride (Fig. 3B) seems not to be consistent with the presence of apical cation–proton exchange with a depolarizing stoichiometry. However, it is possible that this result is due to leakage of amiloride from the lumen to the hemolymph side of the tissue, where it has a far more pronounced effect on Vte (see below). Likewise, the effect of luminal DPC (Fig. 3A) can be interpreted most straightforwardly as the result of diffusion of the lipophilic drug to the basal side of the cells, where it exerts a much more profound effect (see below).
The Na+/K+-ATPase is found at the basal membrane of posterior midgut principal cells, as indicated by immunohistochemistry (Patrick et al., 2006; Okech et al., 2008a), consistent with our finding of a reduced Vte after bath application of ouabain (Fig. 3B). However, this transporter should hyperpolarize the basal membrane and tend to reduce Vte; thus, the effect of inhibiting it is secondary to its effect on some other Na+-dependent process, such as the apical Na+/2H+ exchanger.
The dramatic effect of 1 mmol l−1 hemolymph-side amiloride on Vte cannot be explained with confidence on the basis of the present studies. Since 0.1 mmol l−1 amiloride did not yield a similar result, it is unlikely that the effect is due to inhibition of epithelial-type Na+ channels. It can be ruled out that 1 mmol l−1 amiloride collapses the Vte by depriving the apical V-ATPase of protons, since acid secretion continues in its presence. Therefore we must conclude that, whatever its action, 1 mmol l−1 amiloride abolishes Vte by hyperpolarizing the transbasal potential. Since this is a high concentration in comparison with those typically used to inhibit Na+/H+ exchangers, it is possible that a non-specific effect is involved.
The presence of basal anion transporter(s) is suggested by the effects of DIDS and DPC, both of which result in a decrease in Vte (Fig. 3A) consistent with inhibition of a depolarizing basal process. The inhibitor experiments do not distinguish between bicarbonate channels and anion exchangers or other bicarbonate transporters. However, Cl− replacement experiments (Fig. 4) showed symmetrical effects on Vte of luminal and bath replacement and no significant effect on Vte of bilateral Cl− replacement. Although possibly subject to artifact due to electrode junction potentials, these results argue against an electrogenic Cl− transporter in either membrane, but are consistent with paracellular Cl− permeability that may relax a transcellular cation movement from hemolymph to lumen.
Unlike the typical situation in animal cells, K+ channels seem not to be major contributors to the transbasal potential, because the K+ channel blocker Ba2+ had no significant effect on Vte (Fig. 3B).
The enzyme CA catalyzes the hydration reaction of CO2. As described in the Introduction, three forms of CA have been characterized in larval mosquito midgut (Linser et al., 2009) – an intracellular one, an extracellular membrane-bound one associated with certain gut muscle cells, and a soluble, extracellular one found in the ectoperitrophic space. Of these three, only the intracellular and the muscle-bound forms are present in our experiments, because in isolated, perfused preparations the peritrophic membrane is removed and the soluble extracellular CA is washed away; in addition, since the muscle form is not associated with epithelial cells, it should not be responsible for the effects on Vte. Thus the effect of acetazolamide on Vte (Fig. 3A) must reflect an influence on the cytosolic CA form (Fig. 5B). In principle, this result could be explained by depriving the apical V-ATPase of H+, diminishing H+ secretion, and/or depletion of intracellular HCO3−, diminishing HCO3− transport to the hemolymph.
CONCLUSION
Of all the inhibitors or treatments applied in these studies, only the V-ATPase inhibitor concanamycin A was able to significantly inhibit reacidification. These results show that Vte is not a reliable indicator of acidification, and suggest that sufficient redundancy exists in pathways of proton entry and conjugate ion movement to protect acidification from the effects of blocking any single cellular transport process.
In vivo, the acidification could also involve the soluble extracellular CA present in the posterior midgut (Linser et al., 2009) which could accelerate equilibration of carbonate secreted by the anterior midgut with H+ secreted by the posterior midgut. The resulting CO2 could diffuse into the posterior midgut cells, where it could be rehydrated and the resulting H+ and HCO3− separated, as suggested in Fig. 5B. In this way, the cycle of anterior alkali secretion and posterior alkali recovery would be complete; however, the relative rates of the two processes could be adjusted to maintain the acid–base homeostasis of the whole animal.
This research was supported by the National Institutes of Health (RO1-AI06346301). Deposited in PMC for release after 12 months.
- aa
- amino acid
- CA
- carbonic anhydrase
- DIDS
- 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid
- DMSO
- dimethylsulfoxide
- DPC
- diphenylamine-2-carboxylate
- Vte
- transepithelial potential
REFERENCES
- Boudko D. Y., Kohn A. B., Meleshkevitch E. A., Dasher M. K., Seron T. J., Stevens B. R., Harvey W. R. (2005). Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA 102, 1360-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. M., Koch A., Moffett D. F. (1999). The anterior and posterior `stomach' regions of larval Aedes aegypti midgut: regional specialization of ion transport and stimulation by 5-hydroxytryptamine. J. Exp. Biol. 202, 247-252 [DOI] [PubMed] [Google Scholar]
- Clements A. N. (1992). The Biology Of Mosquitoes London: Chapman & Hall; [Google Scholar]
- Culliford S. J., Ellory J. C., Lang H. J., Englert H., Staines H. M., Wilkins R. J. (2003). Specificity of classical and putative Cl− transport inhibitors on membrane transport pathways in human erythrocytes. Cell. Physiol. Biochem. 13, 181-188 [DOI] [PubMed] [Google Scholar]
- Dadd R. H. (1975). Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J. Insect Physiol. 21, 1847-1853 [DOI] [PubMed] [Google Scholar]
- Dow J. A. T. (1984). Extremely high pH in biological systems: a model for carbonate transport. Am. J. Physiol. 246, R633-R636 [DOI] [PubMed] [Google Scholar]
- Dröse S., Altendorf K. (1997). Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200, 1-8 [DOI] [PubMed] [Google Scholar]
- Edwards H. A. (1982a). Ion concentration and activity in the haemolymph of Aedes aegypti larvae. J. Exp. Biol. 101, 143-151 [Google Scholar]
- Edwards H. A. (1982b). Free amino acids as regulators of osmotic pressure in aquatic insect larvae. J. Exp. Biol. 101, 153-160 [Google Scholar]
- Garty H., Benos D. J. (1988). Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol. Rev. 68, 309-373 [DOI] [PubMed] [Google Scholar]
- Linser P. J., Smith K. E., Seron T. J., Neira Oviedo M. (2009). Carbonic anhydrases and anion transport in mosquito midgut pH regulation. J. Exp. Biol. 212, 1662-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. (1967). Carbonic anhydrase: chemistry, physiology and inhibition. Physiol. Rev. 47, 595-781 [DOI] [PubMed] [Google Scholar]
- Moffett D. F. (1994). Recycling of K+, acid-base equivalents, and fluid between gut and hemolymph in lepidopteran insect larvae. Physiol. Zool. 67, 68-81 [Google Scholar]
- Okech B. A., Boudko D. Y., Linser P. J., Harvey W. R. (2008a). Cationic pathway of pH regulation in larvae of Anopheles gambiae J. Exp. Biol. 211, 957-968 [DOI] [PubMed] [Google Scholar]
- Okech B. A., Meleshkevitch E. A., Miller M. M., Popova L. B., Harvey W. R., Boudko D. Y. (2008b). Synergy and specificity of two Na+-aromatic amino acid symporters in the model alimentary canal of mosquito larvae. J. Exp. Biol. 211, 1594-1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken H., Moffett D. F. (2009). Revisiting the cellular mechanism of strong luminal alkalinization in the anterior midgut of larval mosquitoes. J. Exp. Biol. 212, 373-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken H., Moffett S. B., Moffett D. F. (2004a). The transepithelial voltage of the isolated anterior stomach of mosquito larvae (Aedes aegypti): pharmacological characterization of the serotonin-stimulated cells. J. Exp. Biol. 207, 1779-1787 [DOI] [PubMed] [Google Scholar]
- Onken H., Moffett S. B., Moffett D. F. (2004b). The anterior stomach of larval mosquitoes (Aedes aegypti): effects of neuropeptides on transepithelial ion transport and muscular motility. J. Exp. Biol. 207, 3731-3739 [DOI] [PubMed] [Google Scholar]
- Onken H., Moffett S. B., Moffett D. F. (2008). Alkalinization in the isolated and perfused anterior midgut of the larval mosquito, Aedes aegypti. J. Insect Sci. 8, 46, 20 pp., available online: insectscience.org/8.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. L., Aimanova K., Sanders H. R., Gill S. S. (2006). P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J. Exp. Biol. 209, 4638-4651 [DOI] [PubMed] [Google Scholar]
- Ramsay J. A. (1950). Osmotic regulation in mosquito larvae. J. Exp. Biol. 27, 145-157 [DOI] [PubMed] [Google Scholar]
- Reddy M. M., Quinton P. M. (2002). Effect of anion transport blockers on CFTR in the human sweat duct. J. Membr. Biol. 189, 15-25 [DOI] [PubMed] [Google Scholar]
- Rheault M. R., Okech B. A., Keen S. B. W., Miller M. M., Meleshkevitch E. A., Linser P. J., Boudko D. Y., Harvey W. R. (2007). Molecular cloning, phylogeny and localization of AgNHA1 the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J. Exp. Biol. 210, 3848-3861 [DOI] [PubMed] [Google Scholar]
- Skou J. C. (1965). Enzymatic basis for active transport of Na+ and K+ across the cell membrane. Physiol. Rev. 45, 596-617 [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. (1985). Ionic channels in epithelial cell membranes. Physiol. Rev. 65, 833-903 [DOI] [PubMed] [Google Scholar]
- Waterhouse D. F. (1949). The hydrogen ion concentration in the alimentary canal of larval and adult Lepidoptera. Aust. J. Sci. Res. B 428, 437 [Google Scholar]
- Wieczorek H., Putzenlechner M., Zeiske W., Klein U. (1991). A vacuolar type proton pump energizes K+/H+-antiport in a plasma membrane. J. Biol. Chem. 266, 15340-15347 [PubMed] [Google Scholar]
- Zhuang Z., Linser P. J., Harvey W. R. (1999). Antibody to H+ V-ATPase subunit E colocalizes with portasomes in alkaline larval midgut of a freshwater mosquito (Aedes aegypti L.). J. Exp. Biol. 202, 2449-2460 [DOI] [PubMed] [Google Scholar]