Abstract
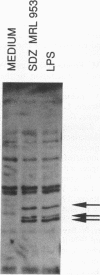
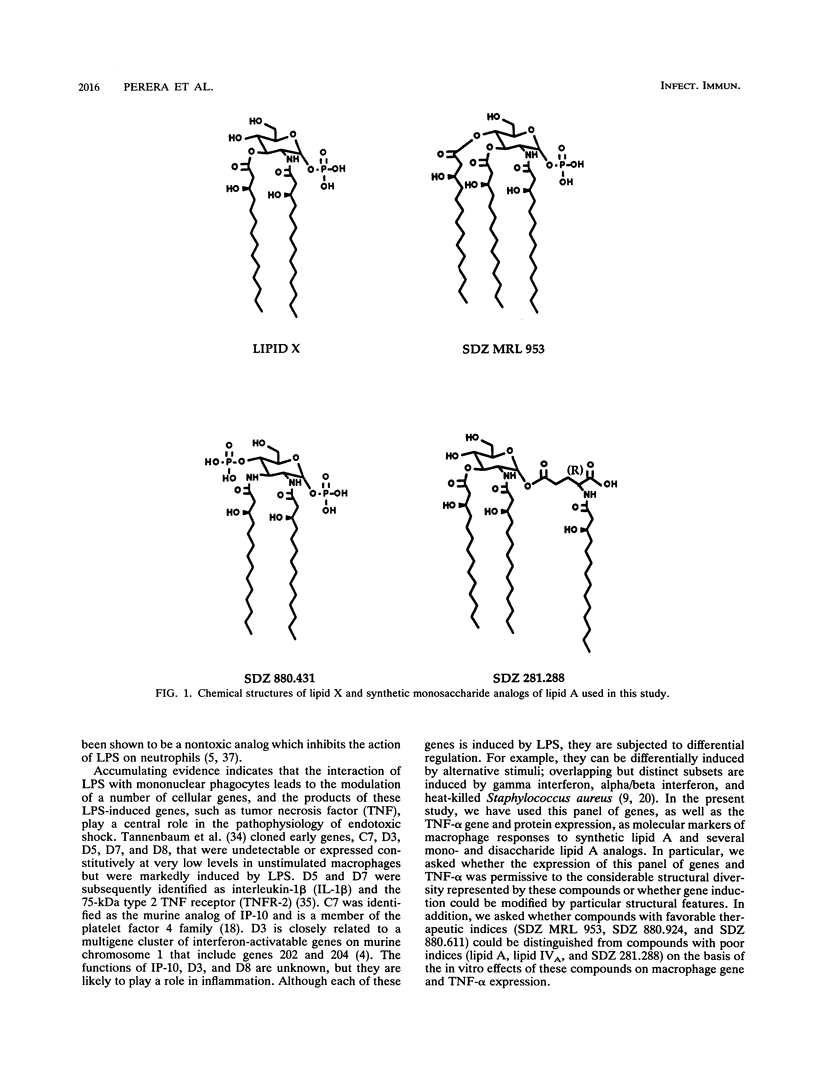
Numerous lipid A analogs have been synthesized in an attempt to dissociate endotoxic activities from beneficial immunomodulatory activities. In the present study, we have evaluated select lipid A analogs in macrophages for their ability to induce a panel of lipopolysaccharide (LPS)-inducible genes to gain insights into the molecular mechanisms which underlie endotoxicity. We evaluated three monosaccharide lipid A analogs: SDZ MRL 953, an agonist with an improved therapeutic margin over endotoxin; SDZ 281.288, a more toxic analog; and SDZ 880.431, an analog with proven LPS-inhibitory activity. In addition, three disaccharide lipid A analogs (i.e., lipid IVA, SDZ 880.611, and SDZ 880.924) that differ in acylation and phosphorylation patterns were also examined and compared with synthetic lipid A. With the exception of SDZ 880.431, each of these structurally diverse analogs was able to induce the complete panel of LPS-inducible genes, specifically genes which encode tumor necrosis factor alpha (TNF-alpha), interleukin-1 beta, 75-kDa type 2 TNF receptor (D7), IP-10, D3, and D8. These results underscore that macrophage stimulation by lipid A analogs is permissive to considerable structural diversity. Structures with favorable therapeutic indices (SDZ MRL 953, SDZ 880.611, and SDZ 880.924) were not different from structures with poor therapeutic indices (lipid A, lipid IVA, and SDZ 281.288) with regard to gene induction. Nonetheless, the nontoxic SDZ MRL 953 was approximately 1,000-fold less potent than synthetic lipid A at inducing TNF-alpha secretion, and perhaps this contributes to the lack of toxicity exhibited by this compound. The ability of compound SDZ 880.431 to inhibit TNF-alpha secretion induced by both SDZ MRL 953 and smooth LPS suggests that the monosaccharide and smooth LPS share a receptor or a portion thereof. A pattern of protein tyrosine phosphorylation similar to that induced by LPS was stimulated by the monosaccharide SDZ MRL 953 and SDZ 281.288 and disaccharides lipid IVA, SDZ 880.924, and SDZ 880.611, providing evidence for a common signalling pathway.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschauer H., Grob A., Hildebrandt J., Schuetze E., Stuetz P. Highly purified lipid X is devoid of immunostimulatory activity. Isolation and characterization of immunostimulating contaminants in a batch of synthetic lipid X. J Biol Chem. 1990 Jun 5;265(16):9159–9164. [PubMed] [Google Scholar]
- Baker P. J., Hraba T., Taylor C. E., Myers K. R., Takayama K., Qureshi N., Stuetz P., Kusumoto S., Hasegawa A. Structural features that influence the ability of lipid A and its analogs to abolish expression of suppressor T cell activity. Infect Immun. 1992 Jul;60(7):2694–2701. doi: 10.1128/iai.60.7.2694-2701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choubey D., Snoddy J., Chaturvedi V., Toniato E., Opdenakker G., Thakur A., Samanta H., Engel D. A., Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989 Oct 15;264(29):17182–17189. [PubMed] [Google Scholar]
- Danner R. L., Van Dervort A. L., Doerfler M. E., Stuetz P., Parrillo J. E. Antiendotoxin activity of lipid A analogues: requirements of the chemical structure. Pharm Res. 1990 Mar;7(3):260–263. doi: 10.1023/a:1015874012484. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Hamilton T. A., Bredon N., Ohmori Y., Tannenbaum C. S. IFN-gamma and IFN-beta independently stimulate the expression of lipopolysaccharide-inducible genes in murine peritoneal macrophages. J Immunol. 1989 Apr 1;142(7):2325–2331. [PubMed] [Google Scholar]
- Homma J. Y., Matsuura M., Kanegasaki S., Kawakubo Y., Kojima Y., Shibukawa N., Kumazawa Y., Yamamoto A., Tanamoto K., Yasuda T. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985 Aug;98(2):395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Takahashi I., Ogawa T., Tsujimoto M., Shimauchi H., Ikeda T., Okamura H., Tamura T., Harada K. Immunobiological activities of synthetic lipid A analogs with low endotoxicity. Infect Immun. 1986 Dec;54(3):673–682. doi: 10.1128/iai.54.3.673-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Hildebrandt J., Schütze E., Rosenwirth B., Proctor R. A., Liehl E., Stütz P. Immunostimulatory, but not antiendotoxin, activity of lipid X is due to small amounts of contaminating N,O-acylated disaccharide-1-phosphate: in vitro and in vivo reevaluation of the biological activity of synthetic lipid X. Infect Immun. 1991 Jul;59(7):2351–2358. doi: 10.1128/iai.59.7.2351-2358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Schütze E., Hildebrandt J., Aschauer H., Liehl E., Macher I., Stütz P. SDZ MRL 953, a novel immunostimulatory monosaccharidic lipid A analog with an improved therapeutic window in experimental sepsis. Antimicrob Agents Chemother. 1991 Mar;35(3):500–505. doi: 10.1128/aac.35.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey C. L., Brandes M. E., Perera P. Y., Vogel S. N. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992 Oct 1;149(7):2459–2465. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T. A. A macrophage LPS-inducible early gene encodes the murine homologue of IP-10. Biochem Biophys Res Commun. 1990 May 16;168(3):1261–1267. doi: 10.1016/0006-291x(90)91164-n. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Strassman G., Hamilton T. A. cAMP differentially regulates expression of mRNA encoding IL-1 alpha and IL-1 beta in murine peritoneal macrophages. J Immunol. 1990 Nov 15;145(10):3333–3339. [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Textor J. A. Activation and inhibition of Limulus amebocyte lysate coagulation by chemically defined substructures of lipid A. Infect Immun. 1985 Aug;49(2):286–290. doi: 10.1128/iai.49.2.286-290.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Will J. A., Burhop K. E., Raetz C. R. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986 Jun;52(3):905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers T. J., Macher I., Chung J., Kugler E. The production of tumor necrosis factor by mouse bone marrow-derived macrophages in response to bacterial lipopolysaccharide and a chemically synthesized monosaccharide precursor. J Immunol. 1987 May 1;138(9):2935–2940. [PubMed] [Google Scholar]
- Takada H., Kotani S. Structural requirements of lipid A for endotoxicity and other biological activities. Crit Rev Microbiol. 1989;16(6):477–523. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C. S., Koerner T. J., Jansen M. M., Hamilton T. A. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988 May 15;140(10):3640–3645. [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dervort A. L., Doerfler M. E., Stuetz P., Danner R. L. Antagonism of lipopolysaccharide-induced priming of human neutrophils by lipid A analogs. J Immunol. 1992 Jul 1;149(1):359–366. [PubMed] [Google Scholar]
- WEIL M. H., SHUBIN H., BIDDLE M. SHOCK CAUSED BY GRAM-NEGATIVE MICROORGANISMS. ANALYSIS OF 169 CASES. Ann Intern Med. 1964 Mar;60:384–400. doi: 10.7326/0003-4819-60-3-384. [DOI] [PubMed] [Google Scholar]
- Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]