Abstract
Developmental biology research has used various avian species as model organisms for studying morphogenesis, with the chick embryo being used by the majority of groups. The focus on the chick embryo led Hamburger and Hamilton to develop their definitive staging series nearly 60 years ago and this series is still the mainstay of all laboratories working with avian embryos. The focus on the chick embryo has somewhat overshadowed the importance of another avian embryo that has proved to be equally powerful, the Japanese quail. Since the late 1960s, chimeras have been produced using chick and quail embryos and this technique has revolutionized the approach taken to the investigation of the cellular and molecular interactions that occur during development. Reviews of the literature demonstrate that many research groups are using the quail embryo in a number of established and new ways, and this species has become a primary animal model in developmental biology. Some staging of quail has been performed but this has been incomplete and variations in descriptions, stages and incubation timings mean that comparisons with the chick are not always easily made. There appears to be general agreement that, at the early stages of embryogenesis, there is little developmental difference between chick and quail embryos, although the basis for this has not been established experimentally. The accelerated ontogeny of quail embryos at mid to late stages of development means that registration with the chick is lost. We have therefore developed a definitive developmental stage series for Japanese quail so that differences are fully characterized, misconceptions or assumptions are avoided, and the results of comparative studies are not distorted.
Keywords: chimera, development, morphogenesis, quail, staging
Introduction
The world of developmental biology research has always extensively utilized different animal models. Various avian species have been particularly useful in developmental studies but it is the chick embryo (Gallus gallus domesticus) that many workers have focused their attention on and it has proved a robust model. The focus on the chick embryo led Hamburger & Hamilton (1951) to produce their definitive staging series nearly 60 years ago; this series is still a fundamental tool used by all laboratories working with avian embryos today. In contrast to earlier descriptions of chick embryo development, which used a simple chronological approach based on hours and days of incubation, Hamburger and Hamilton (1951) described set time-points between which significant development had occurred and this resulted in the creation of an effective 46-stage series. This focus on the chick embryo has somewhat overshadowed the importance of another avian species that has proved equally powerful for developmental studies, the Japanese quail Coturnix coturnix japonica. The discovery by Le Douarin in the late 1960s that chimeras could be made using chick and quail embryos revolutionized the approach taken to studying development and has helped to unlock some of the mysteries underlying various embryonic processes including the cellular and molecular interactions that occur during morphogenesis (review in Le Douarin, 2008).
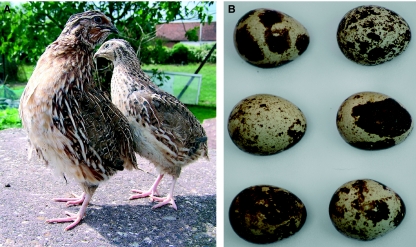
The Japanese quail (Fig. 1A) is a member of the pheasant (Phasianidae) family and is considered to be a separate species from the common quail. In comparison to the chicken egg, Japanese quail eggs are small, measuring about 30 mm in length and weighing approximately 10 g, although as with all avian species there is variation (Fig. 1B). The incubation period of the Japanese quail is approximately 16.5 days. It is thought that the species was developed through domestication of the common quail in China and arrived in Japan in the 11th or 12th century. Originally bred as domestic songbirds, the Japanese quail became popular in the 20th century for meat and egg production. Japanese quail were first reported as a useful research model by Padgett & Ivey (1960) and since then have become a common laboratory species for a range of investigations including developmental, behavioural and environmental investigations. It is important to note that the Japanese quail should not be confused with the larger American bobwhite quail (Colinus virginianus), which is a member of the Odontophoridae family and has a longer developmental ontogeny than the Japanese quail (Reese & Reese, 1962). Although the bobwhite quail is used in some developmental studies and has been staged (Hendrickx & Hanzlik, 1965), it has a more common use in toxicity investigations.
Fig. 1.
Photographs of adult Japanese quail (A) and a clutch of Japanese quail eggs demonstrating variations in shell colouring (B).
Any online search quickly demonstrates how widely the Japanese quail is used today in behavioural, comparative and developmental studies. In developmental terms, quail is still an extremely popular animal model, with studies on neural crest, thyroid, heart, pancreas, spleen, intestinal and craniofacial development having been recently published (Chen et al. 2008; Eames & Schneider, 2008; Hogers et al. 2009; Quinn et al. 2009; Rowan-Hull et al. 2009). Quail are increasingly being used as a comparator organism in cell-based investigations and are still heavily utilized in chick–quail chimeric studies (Binder et al. 2008; Costa-Silva et al. 2009; Grenier et al. 2009;Liem & Aoyama, 2009). More recently, quail–duck chimeras have become a new model of choice of some groups (Lwigale & Schneider, 2008). Despite the extensive exploitation of the Japanese quail in developmental studies, particularly in the use of the chimera system, there has not been a widely disseminated definitive staging study of the quail equivalent to that of Hamburger & Hamilton (1951) for the chick. Several groups have looked at the issue of staging based on external and internal features (Padgett & Ivey, 1960; Zacchei, 1961; Graham & Meier, 1975; Nakane & Tsudzuki, 1999; Sellier et al. 2006) but in most cases have focused on wide developmental time-points, which is a particular problem when looking at early stages of development. Other studies have not made specific comparisons to chick development, despite the fact that quail is often being used as a comparative model to chick and of course is one partner of the chick–quail chimera system. With Japanese quail having an accelerated incubation period this is a notable issue.
Padgett & Ivey (1960) were among the first to describe the development of C. c. japonica in detail and, although their work did standardize staging to a degree, it only looked at 24 h time-points and did not directly compare each time-point with specific Hamburger and Hamilton (HH) stages. Between 50 and 55 h of incubation the chick embryo can pass through as many as five different HH stages, hence the number of time-points assessed is important. Zacchei (1961) staged the quail embryo in a similar way to the HH chick staging system; however, they too did not directly compare each time-point with a specific HH stage of chick development. In the light of the later development of the chick–quail chimeric system, this would now seem to be an oversight. Graham & Meier (1975) produced a report that concentrated on collecting very accurate measurements of the anatomical features of quail embryos during a specific period of development. This method allows accurate staging of embryos removed from the egg, although the approach used is difficult to apply to measurements taken in ovo. An important observation to come from the work of Graham & Meier (1975) was the high correlation between developmental age and third toe length, and this has now become an important parameter.
More recently, Sellier et al. (2006) reported an excellent study looking at the first 72 h of development of Japanese quail, chick, turkey, duck, goose and guinea fowl. This in-depth study led the authors to the conclusion that HH staging is a useful tool for staging avian embryos but should be used with some caution for Japanese quail, given the faster ontogeny. An online quail developmental atlas has also been produced and is freely accessible to all workers (Ruffins et al. 2007). This resource provides exciting insights into the intricate and complex internal development of the quail embryo. Ruffins et al. (2007) used cutting-edge imaging technologies that allow us to look into an area of development that has previously been hidden without the invasive use of dissection. This is an excellent tool for understanding internal development, which is the main focus of the atlas, but as the embryos were only harvested every 24 h it cannot readily be used for accurate staging of the embryos. Other workers have also started to use non-invasive methods of following avian development (Hogers et al. 2009).
The fact that so many research groups are using the quail embryo in a number of established and new ways, and the availability of new resources such as a quail atlas, suggests that the quail embryo is enjoying a surge in terms of its use as a primary animal model in developmental biology. In view of this and in response to comments from colleagues, we felt that the production of a definitive staging series for Japanese quail was not only timely but necessary. There appears to be general agreement that, during the early stages of embryogenesis, there is little developmental difference between chick and quail embryos, with the staging of both being based largely on somite number. The basis for this assumption, however, has not been referenced in experimental studies and was therefore a primary objective of this study. In view of a perceived early developmental registration with the chick, the accelerated ontogeny of the quail embryo (approximately 16–17 days compared with 20–21 days) suggests that there are pronounced differences at later stages of development. These differences need to be characterized and reported so that any misconceptions or assumptions can be avoided, and so that results of comparative studies are not distorted.
In addition to focusing on those developmental features highlighted by Hamburger and Hamilton (1951) in their staging series, we also demonstrate generalized feather pigmentation patterns at specific stages. There has been advancement in our knowledge of feather morphogenesis over recent years and we are beginning to understand some of the spatio-temporal patterning events and mechanisms underlying the patterning process. This work has particular impact on the stem cell and reparative fields as feathers demonstrate excellent regenerative abilities and a common precursor cell population is able to produce a diversity of feather morphologies and pigmentation patterns. Much of this work has utilized chick–quail or quail–duck chimeras and therefore it is important that the ‘normal’ patterns of feather development and pigmentation be recorded (Richardson et al. 1990; Eames & Schneider, 2005; Yue et al. 2005).
Materials and methods
Fertile Japanese quail eggs (C. c. japonica) were obtained from Rose Dean Farm (Cambridgeshire, UK) and incubated for set periods of time at 38 °C in humidified (70%) incubators (Gallenkamp). Prior to incubation the eggs were stored at room temperature (21°C) away from direct sunlight and used within 3 days of delivery. The age of the embryo was defined as the time period following onset of incubation up to the time of observation. In the case of early embryos, approximately 1 mL of thin albumin was removed from the eggs before windowing and staining embryos with a minimal amount of 0.5% neutral red (Sigma, Poole, UK) in Hank’s balanced salt solution (HBSS) to aid in subsequent visualization. Neutral red enables features such as developing somites to be analysed and also creates contrast for subsequent photographic imaging. Embryos at 8 days of development or older were killed (cervical dislocation), rinsed in HBSS and immediately fixed in 5% neutral buffered formalin and left in fixative for at least 7 days before analysis and photography. This approach was used for all embryos to minimize shrinkage. A variety of features such as limb bud length, beak length and length of the third toe were measured at appropriate stages using a standardized approach (based on criteria used by Graham & Meier, 1975) and this information was combined with an analysis of wing pigmentation patterns to determine the stage of each embryo. Embryos were staged every hour for the first 72 h and then every 12 h from 3 days until hatching. Embryos designated at stages 4–35 were photographed using a Camedia digital camera (Olympus, Southend-on-sea, UK) attached to an SZX12 microscope (Olympus). For stages 36–45, embryos were photographed using a tripod-mounted Lumix digital camera (Panasonic, Bracknell, UK) in order to achieve a full view of the embryo. Limbs (wing and leg from stages 19–38) were either carefully removed from equivalently staged embryos and photographed (as described) or repositioned with enhanced lighting and prepared in a graphics editing program (Adobe Photoshop, Adobe System Inc. San Jose, CA, USA). For all ex-ovo imaging, embryos were positioned using insect pins in Sylgard (Dow Corning)-lined glass dishes containing fresh HBSS.
Results
A total of nearly 400 embryos were used in the development of this quail staging series, with an emphasis on larger numbers of repeats for embryos studied at the earlier incubation ages where developmental differences between each hour time-point are more pronounced. Preliminary investigations demonstrated that slight variations in temperature and humidity levels, including the use of different incubators, could result in staging variations greater than those observed using a single unaltered incubator. Published studies working with Japanese quail have used a range of incubation temperatures, making it difficult to make direct comparisons between the results from different laboratories (e.g. Zacchei, 1961; Graham & Meier, 1975; Lilja et al. 2001; Grenier et al. 2009). Therefore, for the development of this staging series, all eggs were incubated using a highly standardized approach. Unlike previous studies, we did not detect high levels of embryo mortality at the early and late stages of development, and fertility was very high at approximately 95% (Sittman et al. 1966; Sato et al. 1971). The use of a standardized collection, transportation, storage and incubation procedure may have helped to ensure the high levels of viability and consistency of staging that we observed.
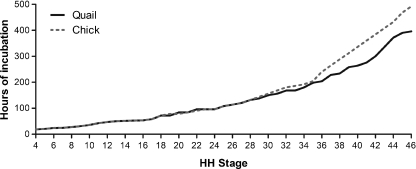
The results of this study are presented in a variety of ways in order to maximize the use of the staging series by different groups of workers. Table 1 shows the developmental rate of the Japanese quail established during this study, which is presented in terms of hours of incubation related to HH stages defined for the chick embryo. As comparators, hours of incubation have also been included for the chick (from Hamburger & Hamilton, 1951) as well as the bobwhite quail (Hendrickx & Hanzlik, 1965). For completeness, and to demonstrate the need for the new and revised series, results from one of the recent Japanese quail partial staging studies have been incorporated (Sellier et al. 2006). The results clearly confirm that there are only minimal differences in the rate of quail embryonic development when compared with chick embryos up to HH stage 28. From stage 29 onwards, the quail embryo develops at an accelerated rate reaching HH stage 46 approximately 100 h earlier than the chick (Fig. 2).
Table 1.
Comparison of the developmental stages of Japanese quail, chick and bobwhite quail embryos.
| HH stage | Japanese quail | Chick (Hamburger & Hamilton, 1951) | Bobwhite quail (Hendrickx & Hanzlik, 1965) | Japanese quail (Sellier et al. 2006) |
|---|---|---|---|---|
| 4 | 18–19 h | 18–19 h | 18 h | |
| 5 | 19–22 h | 19–22 h | ||
| 6 | 23–25 h | 23–25 h | 24 h | |
| 7 | 23–26 h | 23–26 h | ||
| 8 | 26–29 h | 26–29 h | ||
| 9 | 29–33 h | 29–33 h | 30 h | |
| 10 | 33–38 h | 33–38 h | ||
| 11 | 40–45 h | 40–45 h | ||
| 12 | 45–49 h | 45–49 h | 36 h | |
| 13 | 48–52 h | 48–52 h | 42 h | |
| 14 | 50–53 h | 50–53 h | 48 h | |
| 15 | 50–55 h | 50–55 h | 54 h | |
| 16 | 51–56 h | 51–56 h | ||
| 17 | 52–64 h | 52–64 h | 60–66 h | |
| 18 | 72 h | 72 h | ||
| 19 | 3 days | 3–3.5 days | 72 h | |
| 20 | 3.5 days | 3–3.5 days | ||
| 21 | 3.5 days | 3.5 days | ||
| 22 | 4 days | 3.5–4 days | ||
| 23 | 4 days | 4 days | ||
| 24 | 4 days | 4 days | 5 days | |
| 25 | 4.5 days | 4.5 days | 6 days | |
| 26 | 4.5–5 days | 4.5–5 days | 7 days | |
| 27 | 5 days | 5 days | 8 days | |
| 28 | 5.5 days | 5.5 days | 9 days | |
| 29 | 5.5–6 days | 6 days | 10 days | |
| 30 | 6–6.5 days | 6.5 days | 11 days | |
| 31 | 6.5 days | 7 days | 12 days | |
| 32 | 7 days | 7.5 days | 13 days | |
| 33 | 7 days | 7.5–8 days | 14 days | |
| 34 | 7.5 days | 8 days | 15 days | |
| 35 | 8–8.5 days | 8–9 days | 16 days | |
| 36 | 8–9 days | 10 days | 17 days | |
| 37 | 9.5 days | 11 days | 18 days | |
| 38 | 9.5–10 days | 12 days | 19 days | |
| 39 | 10.5–11 days | 13 days | 20 days | |
| 40 | 11 days | 14 days | 21–22 days | |
| 41 | 11.5 days | 15 days | 23 days (hatching) | |
| 42 | 12–13 days | 16 days | ||
| 43 | 14 days | 17 days | ||
| 44 | 15–16 days | 18 days | ||
| 45 | 16–16.5 days | 19–20 days | ||
| 46 | 16.5 days (hatching) | 20–21 days (hatching) |
All stages are presented as time of incubation.
Fig. 2.
Graph showing the average developmental rate (hours of incubation) for Japanese quail and chick embryos, related to HH stages.
The following section describes in detail the developmental features of quail embryos at set time-points. To make the staging series as useful as possible in a comparative context, we constructed a 46-stage series, despite the accelerated ontogeny of the quail. At the early stages of development (4–28), the new quail stage series is identical to the HH stage chick series as the rate of development of both species is indistinguishable. At mid-stages (29–35), the descriptions of morphological changes at each stage are still comparable between the two series but the incubation times for the quail are reduced. At the later stages of development (36–46), the HH stage series is no longer comparable to the quail series in terms of either incubation times or morphological descriptions and therefore the quail series is completely distinct for these stages. We continued to use distinct changes in development to create set time-points for the quail stage series embryos. Because the nature of the overall developmental progression is still similar to the chick this resulted in a series of 46 stages in total.
Stages 4–35
Analysis of quail embryos at 18 h to 5.5 days of incubation revealed no significant anatomical differences between chick and quail embryos, and stages were equivalent in terms of the number of hours per day of incubation for each species. Therefore, stage 4–28 quail embryos can be compared directly with chick embryos and embryos should be incubated for identical periods. After 5.5 days of incubation the rate of quail ontogeny began to increase so that, although HH stage descriptions are still valid for each defined quail stage, the incubation times at each quail stage are reduced in comparison to the chick. This is true up until 8.5 days of development or stage 35. Because quail embryos reach each stage faster than chick embryos during this period, attributing equivalent stages to both species based on incubation times is no longer possible. Readers are referred to the original report of Hamburger & Hamilton (1951) for accurate descriptions of these stages, although general details are given in Table 2 and images of each stage are provided in Figs 3 and 4.
Table 2.
Summary of some key features of quail development.
| Quail stage | Time of incubation | Key identifying features |
|---|---|---|
| 4 | 18–19 h | Primitive streak is fully elongated |
| 5 | 19–22 h | Notochord is visible |
| 6 | 23–25 h | Head fold is apparent |
| 7 | 23–26 h | One somite evident |
| 8 | 26–29 h | Four somites evident |
| 9 | 29–33 h | Seven somites evident |
| 10 | 33–38 h | 10 somites evident |
| 11 | 40–45 h | 13 somites evident, heart bent to right |
| 12 | 45–49 h | 16 somites evident and optic stalk apparent |
| 13 | 48–52 h | 19 somites evident |
| 14 | 50–53 h | 22 somites evident. First and second branchial arches visible |
| 15 | 50–55 h | 24–27 somites evident. Third branchial arch is defined |
| 16 | 51–56 h | 26–28 somites evident. A thickened ridge defines the first appearance of the wing bud. No evidence of the leg bud at this stage |
| 17 | 52–64 h | 29–32 somites evident. Presumptive leg bud is visible and the wing budshave expanded slightly |
| 18 | 72 h | Allantois is first apparent. Amnion usually closed |
| 19 | 3 days | Maxillary process becomes distinct. Eyes remain unpigmented |
| 20 | 3.5 days | Allantois is more obvious as it has become vesicular. Faint eye pigmentation visible |
| 21 | 3.5 days | Maxillary process exceeds mandibular process in length |
| 22 | 4 days | Eye pigmentation distinct |
| 23 | 4 days | Limb buds are equal in width and length |
| 24 | 4 days | Limb buds greater in length than width |
| 25 | 4.5 days | Elbow and knee joints distinct |
| 26 | 4.5–5 days | Demarcation of toes |
| 27 | 5 days | Presumptive region of beak can be identified |
| 28 | 5.5 days | Beak outgrowth is distinct |
| 29 | 5.5–6 days | Bend at the wing elbow visible. No egg tooth apparent |
| 30 | 6–6.5 days | One to two scleral papillae are visible. Egg tooth is present |
| 31 | 6.5 days | Six scleral papillae evident |
| 32 | 7 days | Six to eight scleral papillae evident. Toes have lengthened and become more obvious |
| 33 | 7 days | 13 scleral papillae evident |
| 34 | 7.5 days | Differential growth of second and third toes. 13–14 sclera papillae |
| 35 | 8–8.5 days | Eyelids begin to overgrow surface of eyeball |
| 36 | 8–9 days | Black and brown pigmentation is first visible. Beak length = 1.2 mm, third toe length = 3.2 mm |
| 37 | 9.5 days | Area of black pigmentation has expanded to include the forehead and crown. Brown pigmentation is now present in the lumbo-sacral region. Beak length = 1.5 mm, third toe length = 4.1 mm |
| 38 | 9.5–10 days | Black pigmentation visible on lateral aspects of the skull. Distinct stripes of brown pigmentation in the lumbo-sacral region. Beak length = 1.5 mm, third toe length = 4.7 mm |
| 39 | 10.5–11 days | Noticeable increase in length of all pigmented feather germs. Pigmentation patterns expanded in the wing and pigmentation first visible microscopically around intertarsal joints. Beak length = 2.0 mm, third toe length = 6.0 mm |
| 40 | 11 days | Pigmented feather germs are present in the periocular region. Pigmentation now evident on the feet. Beak length = 2.0 mm, third toe length = 6.1 mm |
| 41 | 11.5 days | White feather germs are apparent throughout the length of the embryo and prominent around the eye. Beak length = 2.0 mm, third toe length = 6.1 mm |
| 42 | 12–13 days | Pigmentation visible on toes. Beak length = 2.3 mm, third toe length = 8.6 mm |
| 43 | 14 days | Beak length = 2.6 mm, third toe length = 9.4 mm |
| 44 | 15–16 days | Beak length = 3.0 mm, third toe length = 10.8 mm |
| 45 | 16–16.5 days | Beak length = 3.5 mm, third toe length = 11.9 mm |
| 46 | 16.5 days | Hatching |
Development is defined by time of incubation and attributed to specific numbered stages. The early quail stages (stages 4–28) correspond directly with HH stages for the chick and therefore the descriptions and times of incubation are identical. For quail stages 29–35, the descriptions for both chick and quail are directly comparable but the incubation times at each quail stage are reduced in comparison to the chick. For quail stages 36–46 neither the descriptions used in the HH stage series nor the incubation times are aligned. We have used pigmentation patterns plus beak and toe measurements to define these late quail stages.
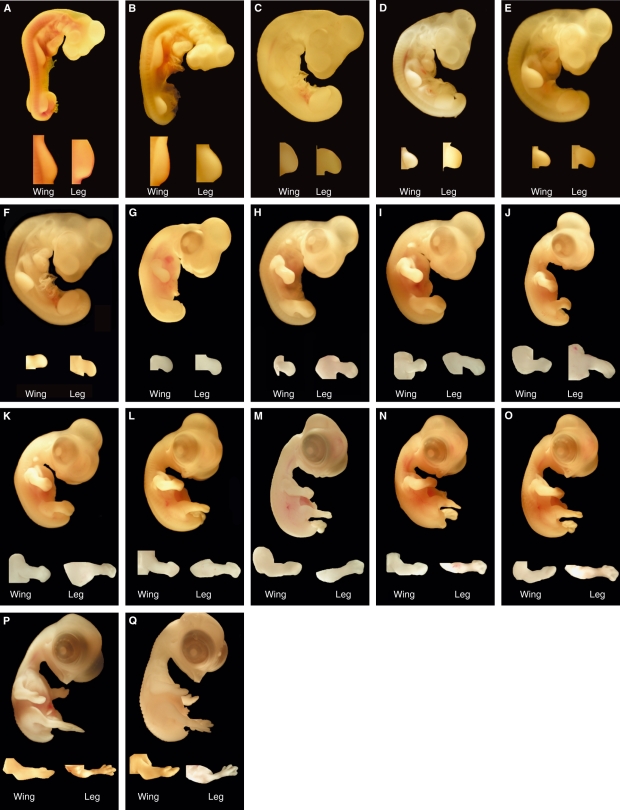
Fig. 3.
In-ovo photographs representing quail embryos at stages 4–18, respectively (A–O). Embryos were unfixed, stained with neutral red solution and with vitelline membranes removed.
Fig. 4.
Photographs representing quail embryos, including limb detail, at stages 19–35, respectively (A–Q).
Stages 36–46
Quail embryos at 8.5–16.5 days of incubation were also staged based on changes in morphology (Figs 5 to 14). Analysis revealed that, due to an even more rapid ontogeny of quail embryos from 8.5 days, there were significant anatomical differences between chick and quail embryos, particularly in relation to beak and third toe length, at each stage. Therefore, the HH staging series is not useful for determining the stage of quail embryos in terms of either incubation time or morphological description. General details of the developmental features including beak and toe lengths are provided in Table 2 and further details about pigmentation patterns, beak and third toe lengths are also given in the figure legends for each stage (Figs 5 to 14).
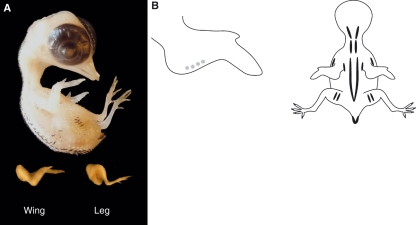
Fig. 5.
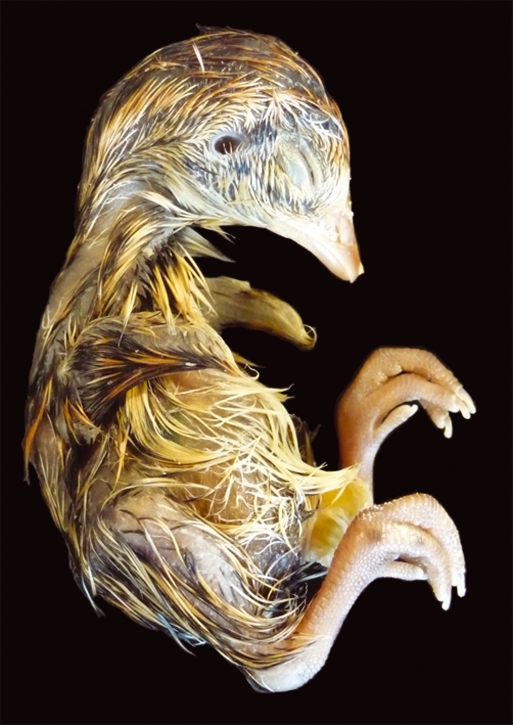
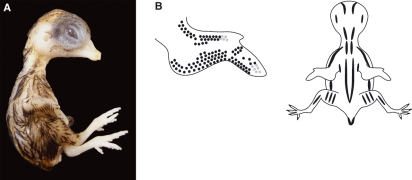
(A) Stage 36 (8–9 days) quail embryo, including limb detail. Quail embryos were killed, rinsed and fixed in 5% neutral buffered formalin before photography. (B) Schematic representation of the average pigmentation pattern observed at stage 36 on the wing and the dorsal surface of the embryo. Black and brown pigment is apparent in feather buds. Black pigmentation is present either side of the spine (tapering off in the region of the pelvis), overlying the scapula and ulna, and on the lateral aspects of the thigh and at the edges of the tail. There is a single line of black feathers in the region overlying the coracoids. Golden-brown pigmentation is also present but less extensive, with the most notable pigmentation being in the region of the tail. The beak length (mean length from the anterior angle of the nostril to the tip of the upper bill) is 1.2 mm and the third toe length (mean length from the middle of the metatarsal joint to the end of the most extreme digital pad on the third toe) is 3.2 mm.
Fig. 14.
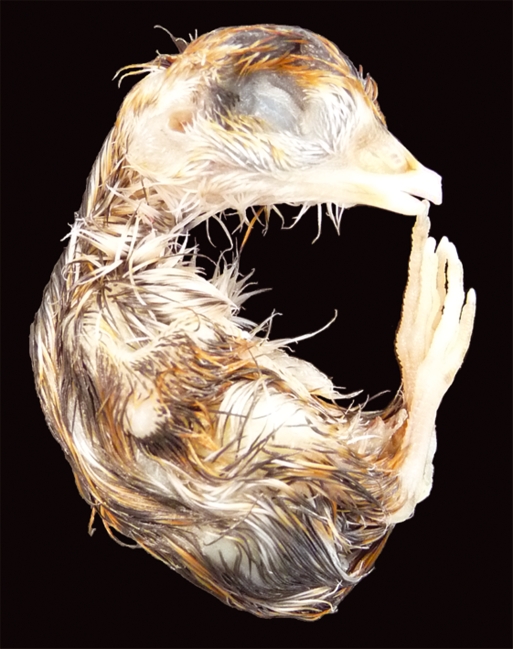
Stage 45 (16–16.5 days) quail embryo. Prehatching stage for the quail embryo with the yolk almost completely internalized, causing the abdomen to swell. Average beak length is 3.5 mm and third toe length is 11.9 mm. Stage 46 (16.5 days) is the hatching stage for the quail embryo.
Fig. 6.
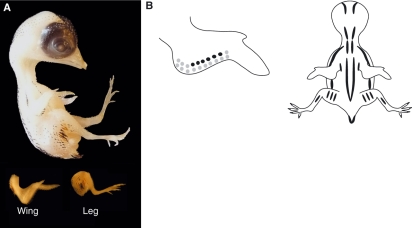
(A) Stage 37 (9.5 days) quail embryo, including limb detail. (B) Schematic representation of the average pigmentation pattern seen at stage 37 on the wing and the dorsal surface of the embryo. Pigmentation is visible on the head, with black pigmentation present on the forehead and crown. Pigmentation continues to increase either side of the spine over the regions of the scapula and ulna, on the thigh and at the edge of the tail. A single distinct line of black feathers in the region of the coracoids is now prominent, with a second and sometimes third line beginning to appear either side of the first row. Golden-brown pigmentation also increases most notably in the regions of the scapula, ulna, thigh and the edge of the tail. Additional golden-brown pigmentation is apparent in the lumbosacral region when viewed microscopically. The average beak length is 1.5 mm and the third toe length is 4.1 mm.
Fig. 7.
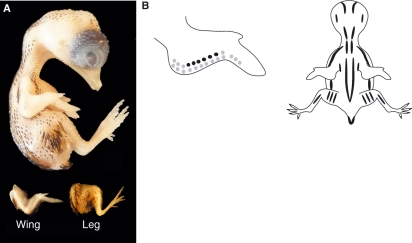
(A) Stage 38 (9.5–10 days) quail embryo, including limb detail. (B) Schematic representation of the average pigmentation pattern seen at stage 38. Black pigmentation is more distinct on the forehead, crown, adjacent to the external auditory meatus, and along the dorsal neck. Pigmentation continues on either side of the spine, over the scapula, ulna, thigh and tail. Several rows of black feathers are visible in the region of the coracoids. Golden-brown pigmentation is now evident in most regions containing black pigmentation and there is a distinct line of golden- brown pigmentation running parallel to the spine. In all regions, mean feather germ lengths have increased. No pigmentation is yet present on the feet. The mean beak length is 1.5 mm and third toe length is 4.7 mm.
Fig. 8.
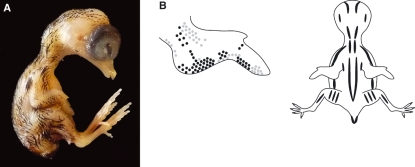
(A) Stage 39 (10.5–11 days) quail embryo. (B) Schematic representation of the average pigmentation pattern seen at stage 39. Little change in the black pigmentation pattern is observed other than increased pigment appearing on the wing, neck and thorax. Faint pigmentation is also visible for the first time around the intertarsal joint. The length of pigmented feather germs is increased. The mean beak length is 2.0 mm and third toe length is 6.0 mm.
Fig. 9.
(A) Stage 40 (11 days) quail embryo. (B) Schematic representation of the average pigmentation pattern seen at stage 40. Pigmented feather germs are now prominent within the periocular region, particularly ventral to the eye. Increased pigmentation is also visible around the external auditory meatus. Faint pigmentation is evident on the feet around the intertarsal and metatarsal joints. No white feathers can be identified at this stage. The beak length is unchanged at 2.0 mm and third toe length is 6.1 mm.
Fig. 10.
Stage 41 (11.5 days) quail embryo. The length of the feather germs is increased over the whole embryo. White feather germs are apparent throughout the length of the embryo, the most noticeable being ventral to the eye and towards the beak. Pigmentation on the feet is more prominent, particularly around the metatarsal joint. Specific pigmentation patterns are not shown from stage 41 onwards as there is increased variation between embryos. The beak and third toe lengths are unchanged at 2.0 and 6.1 mm, respectively.
Fig. 11.
Stage 42 (12–13 days) quail embryo. Pigmentation on the feet is increasingly prominent and for the first time apparent along the ventral surface of the toes. There is an increased density of feather germs and each feather is considerably longer and produces a ‘glossy’ look. General patterns and features alter little after stage 42 although the embryos continue to increase in size. The overall length of the bird is variable and therefore the beak and third toe length should be used for accurate staging. The mean beak length is 2.3 mm and third toe length is 8.6 mm.
Fig. 12.
Stage 43 (14 days) quail embryo. Mean beak length increases to 2.6 mm and third toe length to 9.4 mm.
Fig. 13.
Stage 44 (15–16 days) quail embryo. Mean beak length is 3.0 mm and third toe length is 10.8 mm.
Discussion
The Japanese quail remains one of the favoured animal models in developmental biology and is being used to investigate a variety of developmental systems. Quail are most notably used as part of the chick–quail chimeric methodology and more recently as part of other chimeric approaches (Le Douarin, 2008; Lwigale & Schneider, 2008). In view of the continued popularity of the Japanese quail as an experimental animal we have developed a definitive staging system for quail as a key laboratory aid for those using this organism in their investigations. Although development is a continuum, this stage series, like that of Hamburger & Hamilton (1951), focuses on defining snap-shots of morphogenesis during the incubation period. The use of a standardized collection, transportation, storage and incubation procedure has ensured that, in addition to high levels of viability, we have been able to define a reproducible staging series. Variations in developmental timings between embryos have been reported in the past (Padgett & Ivey, 1960) and have been attributed to variations in the latency period (the time taken for the embryo to warm to the incubation temperature), physical factors associated with the incubation and egg variation. All workers should be aware of these so that they can try to standardize procedures where possible to ensure that a consistent approach is taken.
The creation of this staging series has focused on analysing Japanese quail embryos at specific time-points during the incubation period and making comparisons with chick development. By noting the appearance of various developmental features after specific periods of incubation (hours and days) it has been possible to attribute quail development to HH stages for early gestation. The results of this study demonstrate that there are only minimal differences identified in the rate of quail embryonic development when compared with chick embryos up to 5.5 days of incubation. Therefore, up to this period, chick and quail embryos can be directly compared using either incubation times or descriptive details and the stages used are identical (stages 4–28). After 5.5 days of incubation there is a slight increase in the developmental rate of the quail embryos and hence attributing equivalent stages to both chick and quail embryos based on incubation times is no longer possible. The overall descriptions for each stage (stages 29–35) are still similar, however, so comparisons are still possible. From 8.5 days of incubation the rate of development for the quail accelerates in comparison to the rate for the chick, so there are significant anatomical differences between chick and quail embryos at these time-points. Therefore, at these later stages of development (stages 36–46) the HH stage series is no longer comparable to the quail series in terms of either incubation times or morphological descriptions, making the quail series completely distinct for these stages. We continued to use distinct changes in development to create set time-points for the quail stage series embryos. Because the nature of the overall developmental process is still similar to the chick, this resulted in a series of 46 stages in total. Like the HH stage series, we used details of beak and third toe lengths to help determine many of the quail stages. The reported beak and third toe lengths of Graham & Meier (1975) differ from our results and it is possible that this is due to slight differences in the methods of measuring. When measuring the third toe we decided not to measure from the very tip (i.e. including the length of nail in the measurement) as this part of the toe was subject to differing curvatures and increased variations, and so could not be measured as accurately as the distance from the metatarsal joint to the most extreme digital pad, which could be gently flattened for accurate measurement.
One obvious difference between chick and Japanese quail embryos is the appearance of pigmentation patterns within the feather germs. From quail stage 36, black and golden-brown pigmentation starts to appear in certain regions of the embryo. To aid workers we have defined the patterns of pigmentation from stage 36 until stage 40, after which pigmentation patterns become less consistent between individuals. It is hoped that the demonstration of pigmentation patterns may be particularly helpful to those investigating the spatio-temporal patterning mechanisms underlying the patterning process in feather morphogenesis.
In conclusion, we have developed a definitive staging system for the Japanese quail. We have recorded the appearance of specific developmental features of quail embryos and attributed these to defined incubation times. Although quail embryos can be directly compared with chick embryos at early time-points, a distinct quail staging series is required for later developmental periods. We therefore hope that this new series will act as a key laboratory aid for those using the Japanese quail in their developmental investigations.
Acknowledgments
The authors would like to thank Rose Dean Farm, Cambridgeshire for providing the quail eggs and organizing safe transportation to our laboratory.
References
- Binder BJ, Landman KA, Simpson MJ, et al. Modeling proliferative tissue growth: a general approach and an avian case study. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:031912. doi: 10.1103/PhysRevE.78.031912. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sible JC, McNabb FM. Effects of maternal exposure to ammonium perchlorate on thyroid function and the expression of thyroid-responsive genes in Japanese quail embryos. Gen Comp Endocrinol. 2008;159:196–207. doi: 10.1016/j.ygcen.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, da Costa MC, Melo FR, et al. Fibronectin promotes differentiation of neural crest progenitors endowed with smooth muscle cell potential. Exp Cell Res. 2009;315:955–967. doi: 10.1016/j.yexcr.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Eames BF, Schneider RA. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development. 2005;132:1499–1509. doi: 10.1242/dev.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames BF, Schneider RA. The genesis of cartilage size and shape during development and evolution. Development. 2008;135:3947–3958. doi: 10.1242/dev.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Meier GW. Standards of morphological development of the quail, Coturnix coturnix japonica, embryo. Growth. 1975;39:389–400. [PubMed] [Google Scholar]
- Grenier J, Teillet M-A, Grifone R, et al. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE. 2009;4:e4381. doi: 10.1371/journal.pone.0004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger AG, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88 [PubMed] [Google Scholar]
- Hendrickx AG, Hanzlik R. Developmental stages of the bob-white quail embryo (Colinus virginianus) Biol Bull. 1965;129:523–531. doi: 10.2307/1539730. [DOI] [PubMed] [Google Scholar]
- Hogers B, van der Weerd L, Olofsen H, et al. Non-invasive tracking of avian development in vivo by MRI. NMR Biomed. 2009;22:365–373. doi: 10.1002/nbm.1346. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. Developmental patterning deciphered in avian chimeras. Dev Growth Differ. 2008;50(Suppl. 1):S11–S28. doi: 10.1111/j.1440-169X.2008.00989.x. [DOI] [PubMed] [Google Scholar]
- Liem IK, Aoyama H. Body wall morphogenesis: limb-genesis interferes with body wall-genesis via its influence on the abaxial somite derivatives. Mech Dev. 2009;126:198–211. doi: 10.1016/j.mod.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Lilja C, Blom J, Marks HL. A comprehensive study of embryonic development of Japanese quail selected for different patterns of postnatal growth. J Zool. 2001;104:115–122. doi: 10.1078/0944-2006-00016. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Schneider RA. Other chimeras: quail-duck and mouse-chick. Methods Cell Biol. 2008;87:59–74. doi: 10.1016/S0091-679X(08)00203-3. [DOI] [PubMed] [Google Scholar]
- Nakane Y, Tsudzuki M. Development of the skeleton in Japanese quail embryos. Develop Growth Differ. 1999;41:523–534. doi: 10.1046/j.1440-169x.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- Padgett CS, Ivey WD. The normal embryology of the Coturnix quail. Anat Rec. 1960;137:1–11. doi: 10.1002/ar.1091370102. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, Jr, McKernan M, Lavoie ET, et al. Effects of estradiol on the development of the bursa of Fabricius in Japanese quail. J Exp Zool Part A Ecol Genet Physiol. 2009;311:91–95. doi: 10.1002/jez.504. [DOI] [PubMed] [Google Scholar]
- Reese EP, Reese TW. The quail, Coturnix coturnix as a laboratory animal. J Exp Anal Behav. 1962;5:265–270. doi: 10.1901/jeab.1962.5-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MK, Hamburger AG, Wolpert L. Mechanisms of pigment pattern formation in the quail embryo. Development. 1990;109:81–89. [Google Scholar]
- Rowan-Hull AM, Rao R, Robertson SA, et al. ISL-1 is induced in stomach mesenchyme in the presence of pancreatic epithelia. J Pediatr Surg. 2009;44:348–352. doi: 10.1016/j.jpedsurg.2008.10.085. [DOI] [PubMed] [Google Scholar]
- Ruffins SW, Martin M, Keough L, et al. Digital three-dimensional atlas of quail development using high-resolution MRI. Sci World J. 2007;7:592–604. doi: 10.1100/tsw.2007.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Hoshino T, Mizuma Y, et al. A series of normal stages in the early development of the Japanese quail, Coturnix coturnix japonica, embryo. Tohoku J Agr Res. 1971;22:80–95. [Google Scholar]
- Sellier N, Brillard J-P, Dupuy V, et al. Comparative staging of embryo development in chicken, turkey, duck, goose, guinea fowl, and Japanese quail assessed from five hours after fertilization through seventy-two hours of incubation. J Appl Poult Res. 2006;15:219–228. [Google Scholar]
- Sittman K, Abplanalp H, Fraser RA. Inbreeding depression in Japanese quail. Genetics. 1966;54:371–379. doi: 10.1093/genetics/54.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, et al. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchei AM. [The embryonal development of the Japanese quail (Coturnix coturnix japonica T. and S.)] Arch Ital Anat Embryol. 1961;66:36–62. [PubMed] [Google Scholar]