Abstract
In songbirds, two sound sources inside the syrinx are used to produce the primary sound. Laterally positioned labia are passively set into vibration, thus interrupting a passing air stream. Together with subsyringeal pressure, the size and tension of the labia determine the spectral characteristics of the primary sound. Very little is known about how the histological composition and morphology of the labia affect their function as sound generators. Here we related the size and microstructure of the labia to their acoustic function in two songbird species with different acoustic characteristics, the white-crowned sparrow and zebra finch. Histological serial sections of the syrinx and different staining techniques were used to identify collagen, elastin and hyaluronan as extracellular matrix components. The distribution and orientation of elastic fibers indicated that the labia in white-crowned sparrows are multi-layered structures, whereas they are more uniformly structured in the zebra finch. Collagen and hyaluronan were evenly distributed in both species. A multi-layered composition could give rise to complex viscoelastic properties of each sound source. We also measured labia size. Variability was found along the dorso-ventral axis in both species. Lateral asymmetry was identified in some individuals but not consistently at the species level. Different size between the left and right sound sources could provide a morphological basis for the acoustic specialization of each sound generator, but only in some individuals. The inconsistency of its presence requires the investigation of alternative explanations, e.g. differences in viscoelastic properties of the labia of the left and right syrinx. Furthermore, we identified attachments of syringeal muscles to the labia as well as to bronchial half rings and suggest a mechanism for their biomechanical function.
Keywords: bioacoustics, extracellular matrix, hyaluronan, labia, lateral asymmetry, layer structure, syrinx, vocal fold
Introduction
Songbirds (Passeriformes) produce their complex vocalizations with two sound sources located inside the syrinx. The syrinx generates a primary sound, the spectral characteristics of which are subsequently shaped by the resonance filter characteristics of the vocal tract (Goller & Cooper, 2004; Suthers & Zollinger, 2004; Riede et al. 2006). Although species-specific syrinx morphology has been studied extensively (Müller, 1847; Setterwall, 1901; Ames, 1971; King, 1989), we have little understanding of how the documented morphological variability relates to the function of the syrinx as a sound source.
The syrinx is located at the caudal end of the trachea and consists of a framework of cartilages (tympanum and bronchial cartilagenous half rings) and soft tissue (muscles, connective tissue, nerves and blood vessels) (Fig. 1A,B). The syrinx constitutes a myoelastic-aerodynamic sound source. Aerodynamic energy is transformed into acoustic energy with the help of active and passive components. Laterally positioned labia are set into a prephonatory position by syringeal muscles (Goller & Larsen, 1997a,b; Larsen & Goller, 1999) (Fig. 1C,D) and are then set into oscillation by passing air flow. Labial oscillation is a passive mechanism in which the vibratory pattern is determined by the subsyringeal pressure driving air through the syrinx and by the shape and tension of the labia.
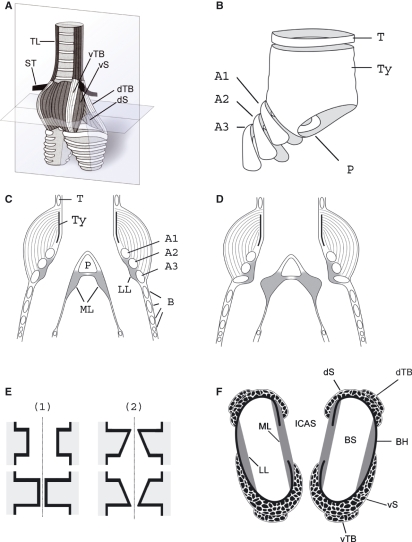
Fig. 1.
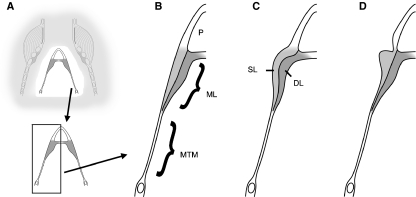
The songbird syrinx. (A) Schematic external ventral view of the excised organ. TL, tracheolateralis muscle; ST, sternotrachealis muscle; dTB and vTB, dorsal and ventral tracheobronchial muscle; dS and vS, dorsal and ventral syringeal muscle. (B) Schematic view of the cartilage framework of the syrinx (modified after Ames, 1971). The drum [or tympanum (Ty)] is located at the caudal end of the trachea. Its shape varies with species. A median dorso-ventral bar [pessulus (P)], spanning the lumen of the trachea, marks the end of the trachea and the beginning of the primary bronchi. The pessulus is one important attachment for the ML. A1–A3, bronchial half rings; T, tracheal ring. (C) Schematic of a frontal section of the syrinx (level indicated by the vertical plate in A). B, bronchial rings. (D) Schematic of the adduction setting of the ML and LL. (E) Simulating the labia oscillation assumes two major movement components: (1) a latero-lateral component and (2) a cranio-caudal component. The latter leads to a repeated change between a convergent and divergent cross-sectional profile. This out-of-phase movement of the upper and lower part of the labia produces larger asymmetries in the forces acting on the labia in the opening and closing phase of the oscillation than the inward and outward movement alone, resulting in a positive pressure on the labia during the opening phase and a net energy input to the labia maintaining a self-sustained oscillation. (F) Schematic horizontal section through the syrinx (level indicated by the horizontal plate in A). BS, bronchial lumen; BH, bronchial half ring.
In this study we investigated the shape, size and microstructure of the labia. The shape and size, together with the tension of the labia, determine their oscillation frequency. Their microstructure affects how they respond to the air stream. This research contributes to the understanding of the functional morphology of the syrinx as a sound source (Larsen & Goller, 2002). The syrinx represents one of the smallest biological sound sources, which is used to produce extremely loud and complex vocalizations – a feature that probably contributed to the evolutionary success of the Passeriformes.
Functional morphology of the syrinx
The syrinx serves two main functions, i.e. control of air flow and labial tension. Active positioning of the labia into and out of the bronchial lumen is used to control air flow during quiet breathing and during sound production. Miskimen (1951) and Chamberlain et al. (1968) suggested that rotational movements of bronchial half rings around the dorso-ventral axis lead to a lumenward movement of the lateral labium (LL) and medial labium (ML) causing a constriction of the bronchial lumen. The endpoints of bronchial half rings arch into the ML, ventrally and dorsally (Fig. 1F), adducting the soft tissue once the half ring is rotated. Active adjustment of the tension of the labia is used for control of the fundamental frequency (F0) of the vocalization but the precise biomechanical mechanism is not understood.
Syringeal muscles enable the gating function of the syrinx (Chamberlain et al. 1968; Goller & Suthers, 1995, 1996a). The m. syringealis dorsalis and m. tracheobronchialis dorsalis are the main adductors, whereas the m. tracheobronchialis ventralis abduct the LL (Goller & Suthers, 1995, 1996a). F0 control is largely accompanied by an increase in activity of the m. syringealis ventralis but the activity of all syringeal muscles increases somewhat with F0 (Goller & Suthers, 1996b). The increased general muscle activation at high frequencies may provide structural stability to the syrinx but may also affect labial tension.
The labia consist of the extracellular matrix (ECM) sandwiched between two layers of epithelium (Setterwall, 1901; Haecker, 1900; Warner, 1972; Frank et al. 2006). The process of energy transfer at the labia involves tissue movement, which presumably comprises two major components. The first is a medial-to-lateral component, where the labia oscillate in and out of the bronchial lumen during the process of sound production (Goller & Larsen, 1997a) (Fig. 1E-1). The second is a wave-like movement of the tissue in a cranial-to-caudal direction, causing an asymmetric geometry (Fig. 1E-2). Direct evidence for this second, wave-like component is not available in birds (Fee et al. 1998; Mindlin & Laje, 2005; Elemans et al. 2008) because its demonstration in vivo is extremely difficult.
Both components of movement were demonstrated, however, in another myoelastic-aerodynamic sound source, the mammalian vocal fold (Hirano et al. 1991; Matsushita, 1975; Titze, 2006). For the human vocal fold, it was shown that the second wave-like movement of tissue, producing a changing convergent and divergent geometry, is possible because multiple layers comprise the vocal fold. The three main layers of the human as well as other mammalian vocal folds are a muscle (thyroarytenoid muscle), lamina propria and covering epithelium. The human lamina propria is further differentiated into a superficial, medial and deep layer, making a total of five layers (Hirano et al. 1982). Each layer has its specific viscoelastic properties and therefore responds to elongation with different effective stiffnesses. As a consequence, the development of tension is differentiated, making it difficult to predict how many of the layers are involved in oscillation. This potential for engaging different proportions of the vocal folds into vibration facilitates the production of a wide range of F0 (Titze, 1988).
Similar detail on the functional morphology of the vibrating tissues is lacking for songbirds but is necessary for a thorough understanding of sound production and as a critical input parameter for syringeal models (Mindlin & Laje, 2005). The goal of this study was to investigate two species with different acoustic structures of song and to test the prediction that morphological differences are correlated with acoustic structure. First, we present data on the major ECM components and their distribution and orientation inside the ECM. Second, we measured the size of the labia to determine the largest ‘effective mass’ of the vibrating structure, which is a key factor determining vocal output. Third, we document the insertion of syringeal muscles on the LL and ML to investigate the mechanisms of direct control of labial tension.
Materials and methods
Tissue collection and processing
We investigated the syrinx in males of two species from two different songbird families, six zebra finches (Taeniopygia guttata; Estrildidae) (ZF) and five white-crowned sparrows (Zonotrichia leucophrys oriantha; Emberizidae) (WCS). The 11 birds were killed with a chloropent overdose (150 mg/kg). The syrinx was dissected in a ventral preparation of the thorax. The trachea was cut at 3 mm above the tracheal bifurcation. The syrinx with short segments of the trachea and primary bronchi was carefully dissected out, applying minimal tension in order to avoid rupture of tissue. The isolated tissue was stored in 10% neutral buffered formalin for 2 weeks before further processing. The tissue was then embedded in paraffin and 5-μm cross-sections of the complete syrinx were made.
Staining
Adjacent sections were exposed to one of the following stains: hemotoxylin and eosin for a general histological evaluation; elastica van Gieson to identify elastic fibers; trichrome to demonstrate collagen fibers; and alcian blue (pH 2.5) to determine mucopolysaccharides and glycosaminoglycans. We also performed a digestion procedure with bovine testicular hyaluronidase (2 h at 37 °C) in combination with a subsequent alcian blue stain to increase specificity for various acid mucosubstances in the alcian blue stain. Alcian blue positivity is destroyed following prior incubation with hyaluronidase, if hyaluronan is a major component of the mucosubstances. All stains were performed in conjunction with control stains (artery for elastica van Gieson; liver for trichrome and umbilical cord for alcian blue) on human tissue.
Micrographs were taken with a digital camera (AxioCam HRc, Carl Zeiss, Germany) combined with an Axioplan Zeiss microscope (Axioplan, Carl Zeiss) and computer software (Axiovision 40, v. 4.6.3.0., Carl Zeiss).
Stains were interpreted subjectively and the results were validated using consensus judgment. The initial interpretation was performed by T.R. and F.G. A third person (a colleague with knowledge of syrinx anatomy but blind to the current data) was instructed to rate four results, each in two examples from each species: (i) collagen content (on a scale from 1 to 5 in relation to the reference stain); (ii) elastin content (on a scale from 1 to 5 in relation to the reference stain); (iii) alcian blue stain before and after hyaluronidase digestion (on a scale from 1 to 5 in relation to the reference stain); and (iv) presence of any layer structure. This blind test confirmed all of the results reported here.
Morphometry
The labium size (cross-sectional area and cranio-caudal length) and length of the medial tympaniform membrane (MTM) were measured using Image J (version 1.41o; NIH open source). A curvilinear outline of the labia was drawn to estimate the cross-sectional area. A ‘segmented line’ was drawn along the centerline of the labium starting at the pessulus and ending at the beginning of the MTM and a second segmented line was drawn along the centerline of the MTM, to estimate the cranio-caudal length of the ML and MTM. The area measurements (ML and LL) and both length measurements (ML and MTM) were made at 10 points equally distributed over the dorso-ventral length of the labia, starting dorsally at the point where the pessulus and ML part from the dorso-lateral tracheal wall, and ending ventrally where the pessulus and ML fuse again. All measurements were made in reference to a known distance measured at identical magnification.
The measurements (area and length) were tested for differences along the dorso-ventral axis (10 section levels) using a one-way analysis of variance (anova). Measurements (area and length) were also tested for differences between the left and right syrinx using a one-way anova. This analysis was performed on the ratios of the left and right side measurements.
Force transmission and propagation to the lateral and medial labium
The tension in the labia is presumably the major regulator of the F0 (Mindlin & Laje, 2005). Direct and indirect attachments of muscles to the labia were identified and described. The purpose of this description was to provide a basis for future experimental studies investigating how tension is regulated in the labia.
Results
Histology
Collagen
In both species, blue stains were very faint in the ML and LL, indicating a relatively low content of collagen fibers. The collagen fibers were not evenly distributed. In some specimens they were more concentrated toward the bronchial lumen (Fig. 2E,G) but there seemed to be no consistent pattern.
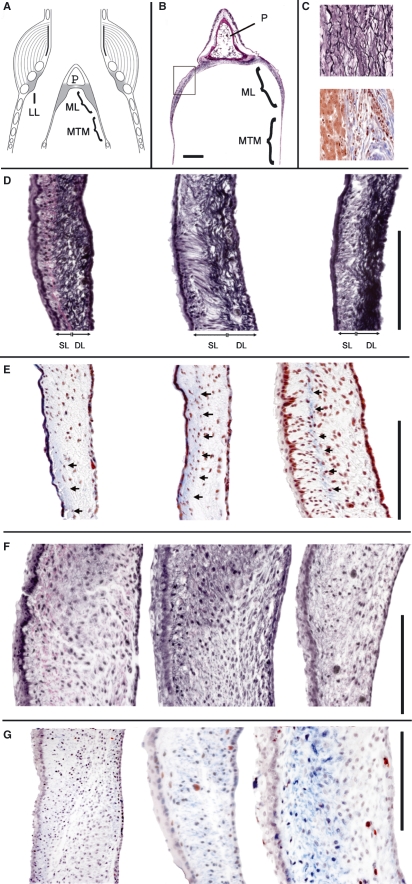
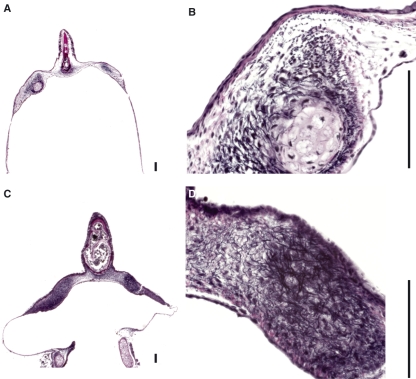
Fig. 2.
Elastica van Gieson (EVG) and trichrome (TRI) stain. (A) Schematic of the syrinx frontal section. (B) Section of the pessulus, ML and MTM. The square indicates the location of the higher magnification images in D–G. (C) Control stains for EVG (upper square) and TRI (lower square). In D–G, the tracheal lumen is to the left and the air sac lumen is to the right. (D) EVG stain in three specimens of the WCS. Note that most of the elastic fibers in the WCS are concentrated near the air sac side of the labium. (E) Masson’s TRI stain in three specimens of the WCS. The arrows indicate a local concentration of blue-stained collagen fibers. (F) EVG stain in three specimens of the ZF. (G) Masson’s TRI stain in three specimens of the ZF. P, pessulus; SL, superficial layer; DL, deep layer. Bar: 100 μm.
Elastic fibers
In the WCS, the ML showed a clear two-layered structure of the ECM. Elastic fiber concentration was high on the side facing the interclavicular air sac (ICAS) (hereafter ‘deep layer’) and elastic fibers were loosely distributed in the subepithelial connective tissue on the side facing the tracheal lumen (hereafter ‘superficial layer’ because this layer was closer to the passing air flow) (Fig. 2D). The majority of the elastic fibers in the deep layer were oriented cranio-caudally. In the superficial layer, the elastic fibers were oriented cranio-caudally as well as dorso-ventrally.
In the ZF the pattern of elastic fiber distribution was different from that in the WCS. Elastic fibers were less abundant and their orientation was cranio-caudal as well as dorso-ventral (Fig. 2F). No layered structure of the ECM could be identified.
Hyaluronan
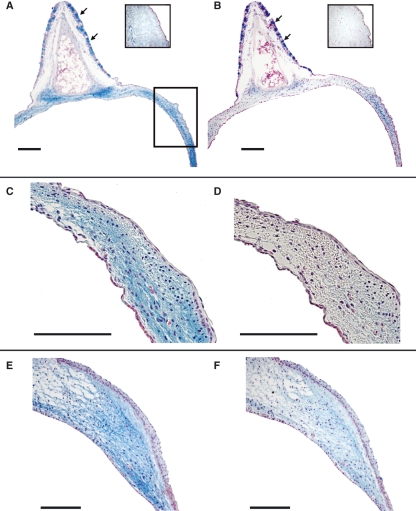
Alcian blue stains indicated the presence of mucopolysaccharides and glycosaminoglycans in the LL and ML (Fig. 3A). The removal of hyaluronan by hyaluronidase digestion with subsequent alcian blue stain indicated that much of the positive stain in the labium can be attributed to hyaluronan (Fig. 3B,D,F). Goblet cells, located in the ciliated respiratory epithelium of the ‘semilunar membrane’, remained intensely positively stained after hyaluronidase treatment. Although the ECM of the ML was mainly made of hyaluronan, which was digested away by hyaluronidase, it was a composition of various mucopolysaccharides and glycosaminoglycans in the goblet cells. This pattern was found in both species, although the content of hyaluronan seemed lower in the ZF (compare Fig. 3D,F). The distribution of mucopolysaccharides and glycosaminoglycans was homogeneous in the LL and the ML of both species.
Fig. 3.
ML. (A,C,E) Alcian blue stain. (B,D,F) Hyaluronidase digestion and Alcian blue stain, same specimens as in A,C,E, respectively. (A–D) WCS. (E and F) ZF. The insets in A and B are control stains for Alcian blue stain and hyaluronidase digestion and Alcian blue stain. The square in A indicates the location of the higher magnification images in C–F. Goblet cells, located in the ciliated epithelium of the semilunar membrane, are indicated by arrows. Bars: 100 μm.
Morphometry
The LL was embedded between the second and third bronchial half rings. Its ECM erected into the bronchial lumen and was delimited by bronchial epithelium.
In the ML, epithelial tissue surrounded the ECM. Respiratory epithelium faced toward the bronchial lumen and simple (stratified) epithelium of the air sac consisting of squamous cells formed the border to the ICAS. The ML transformed caudally into the MTM. These two structures can be distinguished by the presence of ECM in the ML and its absence in the MTM.
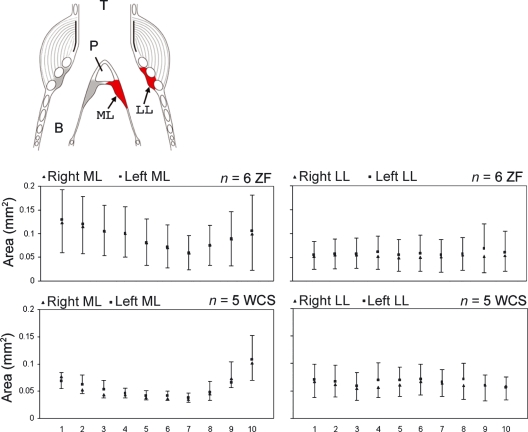
The length measurements (Fig. 4) as well as cross-sectional areas (Fig. 5) changed along the dorso-ventral axis in both species. The section level explained a significant proportion of the variance of ML area (ZF: F9,100 = 5.33, P < 0.001; WCS: F9,80 = 15.7, P < 0.001) and ML length (ZF: F9,100 = 7.96, P < 0.001; WCS: F9,80 = 18.2, P < 0.001) in both species, and MTM length in the WCS (ZF: F9,100 = 0.56, P = 0.82; WCS: F9,80 = 12.87, P < 0.001). Variability of the LL area was not different between section levels (ZF: F9,100 = 0.14, P = 0.99; WCS: F9,80 = 0.32, P = 0.96). This indicated that the shape of the ML but not the LL changed along the dorso-ventral axis.
Fig. 4.
Cranio-caudal length of the ML and MTM. Bars indicate means and error bars indicate SD. Gray bar, ML; open bar, MTM; T, trachea; B, bronchus; P, pessulus.
Fig. 5.
Cross-sectional area measurements of the ML and LL. Squares (left side) and triangles (right side) indicate means and error bars indicate SD. T, trachea; B, bronchus; P, pessulus.
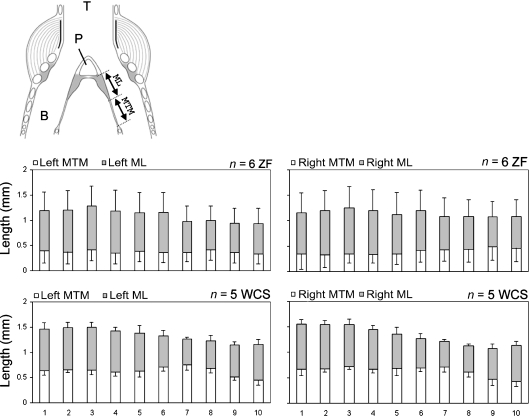
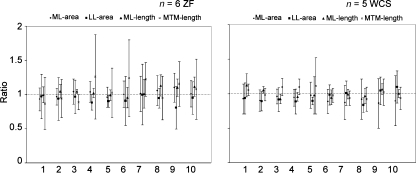
Size differences between the left and right syrinx were not consistent. The variability cannot be explained by systematic differences between the left and right syrinx on a species level (Fig. 6). However, lateral asymmetry was apparent in some individuals. Separate pairwise t-tests for each of the four measurements demonstrated significant differences between all 10 measurements of the left and right syrinx, many of them with the left side being larger (Table 1).
Fig. 6.
Ratios between the right and left syrinx for four measurements (ML area, LL area, ML length and MTM length; Mean ± SD). Horizontal dotted lines indicates a ratio of 1.
Table 1.
Individual pairwise t-tests for four measurements
| ML area | LL area | ML length | MTM length | |
|---|---|---|---|---|
| WCS | ||||
| 9 | T = −6.5 | T = −3.6 | T = −2.7 | T = −2.3 |
| P < 0.001 | P < 0.01 | P< 0.05 | P < 0.05 | |
| 15 | T = −3.1 | T = −1.4 | T = −0.03 | T = −0.21 |
| P < 0.05 | P = 0.19 | P = 0.97 | P = 0.83 | |
| 16 | T = −2.6 | T = 0.38 | T = −0.15 | T = −2.6 |
| P < 0.05 | P = 0.7 | P = 0.88 | P < 0.05 | |
| 11 | T = 2.5 | T = −0.74 | T = 1.39 | T = −0.73 |
| P< 0.05 | P = 0.47 | P = 0.19 | P = 0.47 | |
| 12 | T = 1.9 | T = −4.9 | T = −1.04 | T = 1.03 |
| P = 0.08 | P < 0.001 | P = 0.32 | P = 0.32 | |
| ZF | ||||
| 5 | T = −3.54 | T = −3.4 | T = −2.83 | T = −1.5 |
| P< 0.01 | P < 0.01 | P < 0.05 | P = 0.16 | |
| 18 | T = 1.48 | T = −4.2 | T = 2.16 | T = −1.82 |
| P = 0.17 | P < 0.01 | P = 0.058 | P = 0.10 | |
| 20 | T = 1.23 | T = −4.96 | T = 0.29 | T = 5.39 |
| P = 0.24 | P< 0.001 | P = 0.77 | P < 0.001 | |
| 23 | T = −3.59 | T = −0.77 | T = 0.64 | T = −0.06 |
| P< 0.01 | P = 0.46 | P = 0.53 | P = 0.95 | |
| 31 | T = −0.32 | T = 0.70 | T = 2.57 | T = −0.38 |
| P = 0.75 | P = 0.49 | P ≤ 0.05 | P = 0.71 | |
| 21 | T = 0.87 | T = 4.18 | T = 5.95 | T = −0.18 |
| P = 0.4 | P < 0.01 | P < 0.001 | P = 0.86 | |
The measurements of five WCSs and six ZFs were taken at 10 points along the dorso-ventral axis. The 10 measurements from each side of the syrinx were paired and tested for similarity. Negative T values indicate that the measurement on the left side was larger. Significant results (significance level P = 0.05) are indicated in boldface. Numbers in the left column indicate bird identification.
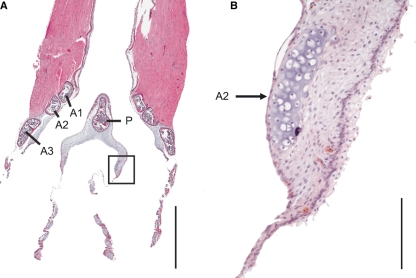
The ventral and dorsal endpoints of the second and third bronchial half rings arched into the ML. Their cross-sections appeared at some point in the ML adding to the total cross-sectional area (Fig. 7). In both species this point was in the ventral third along the dorso-ventral axis (level 7 or 8, Fig. 7).
Fig. 7.
Frontal section from a ventral aspect of a ZF syrinx. The bronchial half ring arches into the ML ventrally and dorsally (see also Fig. 1F). The square in A indicates the area of magnification in B. A1, A2 and A3, first, second and third bronchial half ring, P, pessulus. Bars: in A: 1 mm; in B: 100 μm.
Force transmission and propagation to the lateral and medial labium
We attempted to identify the direction of forces applied to the LL and ML. The syrinx sits inside the ICAS such that ICAS pressure variations relative to bronchial pressure translate to the labia. This force is likely to have a greater effect on the ML than the LL because the latter is attached more firmly to skeletal elements. A pressure difference between the air sac and bronchial lumen will push the ML and MTM out of their resting position, either into or out of the bronchus. Because the ICAS is a single chamber enclosing both syringeal sound sources, such a mechanism for controlling labial position and tension seems to be rather unspecific and limited. It is unlikely that both sides of the syrinx are affected independently. If this mechanism played a major role, a close relationship between the air sac pressure and F0 of the sound should exist but physiological measurements suggested only a poor relationship in oscines (Goller et al. 2006), whereas they were more closely linked in a suboscine (Amador et al. 2008).
Labial tension is mostly regulated by elongation of the labia from their original unstrained length and restoring forces due to elastic recoil. We therefore attempted to elucidate the biomechanics of how syringeal muscles might affect such control. Based on muscle attachment to syringeal cartilages and direct insertion on the labia, both direct and indirect muscular control is likely to occur.
Lateral labium
The major mass of the LL in both species sits medially to the third bronchial half ring. The ECM also extends to the first and second bronchial half rings. In the ZF a few muscle fibers attach directly to the LL. Branches of the ventral syringeal muscle reach between the second and third bronchial half ring. In the WCS most of the connection with muscles appears to be indirect, via cartilage of the first three bronchial half rings. In both species, we found direct as well as indirect attachments of a muscle to the LL.
The muscle attachment to the bronchial half rings suggests that the movement of the cartilaginous half rings effects labial deformation (elongation). An outward bending of the half ring causes elongation of the labium, whereas elastic recoil of the half ring and labium would restore its original length. In addition to a rotation of the cartilage, as suggested by Miskimen (1951) and Chamberlain et al. (1968), we hypothesize that contraction of the ventral syringeal muscle deforms the bronchial half ring.
Medial labium
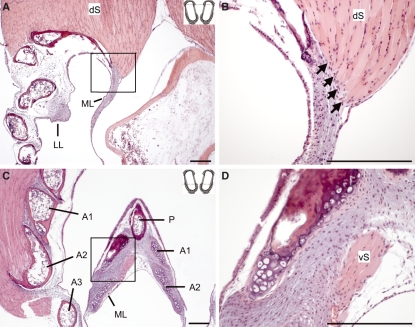
Mid-organ cross-sections (compare Fig. 1A,C) suggest that the ML is cranially attached to the pessulus, a cartilaginous or bony structure. Caudally the ML transforms into the very thin MTMs, which attach to the medial walls of the bronchi. However, sections toward the dorsal aspect of the syrinx show that the dorsal syringeal muscle attaches to part of the ML. Muscle fibers attach medially to the dorsal ends of the bronchial half rings (Fig. 8A,B). Sections through the ventral aspect of the syrinx indicate that the dorsal syringeal muscle also attaches directly to the ML (Fig. 8C,D).
Fig. 8.
Two sections from a WCS syrinx indicating direct muscle attachments on labial tissue. The insets at the top right corners of A and C indicate the sectioning level along the dotted line (compare Fig. 1F). (A) Section through the dorsal part of the syrinx. The square in A indicates the area of magnification in B. Note that some fibers of the dorsal syringeal muscle (dS) attach directly to the ML (indicated by arrows). (C) Section through the ventral part of the syrinx. (D) Magnification of the medial aspect of the ML (as indicated by a square in C). The ventral syringeal muscle (vS) attaches to the ML. A1, A2 and A3, first, second and third bronchial half ring; P, pessulus. Bars: 100 μm.
Tension must be regulated across the dorso-ventral plane to be effective for frequency control during sound production. An outward bending of the bronchial half ring would not only stretch the LL but also the ML in the dorso-ventral plane because the bronchial half ring reaches ventrally and dorsally into the ML. The connection between this endpoint of the half ring and the ECM of the labium seems to be enhanced. In all specimens in both species, we found a concentration of elastic fibers near the boundary between the ECM and cartilage (Fig. 9). This may indicate that the connection between the endpoint of the second bronchial half ring and the ML is subjected to repetitive stress, for example by stretching.
Fig. 9.
The area around the endpoints of the bronchial half rings shows many elastic fibers indicating that the connection between cartilage and soft tissue is enhanced/stabilized, suggestive of a permanent force acting there. (A,B) WCS; (C,D) ZF. P, pessulus. Bars: 100 μm.
Discussion
The results of our analysis provide four new findings. First, the labia in the WCS are multi-layered structures. The ECM consists of two layers that are distinguished by the concentration and orientation of elastic and collagen fibers. Second, hyaluronan is a major component of the mucosubstances of the labia in both the WCS and ZF. Hyaluronan is an important biochemical substance contributing to viscoelastic properties as well as metabolic and immunological features. Third, the size of the labia differs along the dorso-ventral axis. Differences between the left and right side of the syrinx in the WCS and ZF were not consistent at species level. The lateralization of sound production in these two songbird species cannot be explained by a simple size difference between the two sound sources. Fourth, we identified direct muscle connections to the labia. We therefore hypothesize that a combination of a bending mechanism of the second and/or third bronchial half rings mediated by muscle activity as well as direct connections between muscles and the labia are the main biomechanical mechanism of tension control. The functional relevance of each of these four findings will be discussed below.
Layer structure of labia: basis for a differentiated stress response
Based on the concentration and orientation of collagen, and in particular elastic fibers, two distinct layers can be distinguished in the ECM of the ML in the WCS, i.e. a deep and a superficial layer. The two-layered structure provides a basis for a differentiated pattern of tension regulation of the labium. The force that is most likely to be applied to the labium is a stretching along the dorso-ventral axis. Because of the integrity of the labium, such strain must be applied to the two layers simultaneously. If both layers are stretched the same, the resulting stiffness of each layer will be different. We speculate that the higher concentration of elastic fibers in the deep layer of the ECM will lead to a higher stiffness in that layer. The effective compliance of the ML will be an integrated value resulting from the interaction of the viscoelastic properties of all involved layers. It is difficult to predict these properties but they facilitate a high degree of complexity in frequency control.
A variable stress response of the labia allows for a differentiated response to the passing air stream. For any given strain of the superficial layer, the probability of responding to the passing air flow with deformation will always be higher. If the stiffness of the deep layer is large enough to resist oscillation, it will serve as a base on which the more compliant superficial layer, in combination with the epithelium, moves. This basic concept was originally introduced for the human vocal folds as the ‘body-cover model’ (Hirano, 1972). The idea is that a less stiff cover (epithelium and part of the lamina propria) moves atop a stiffer body (thyroarytenoid muscle and deep part of the lamina propria). Based on a body-cover layered structure of the vocal fold, Titze (1988) derived the analytical relationships that characterize the linear, small-amplitude self-sustained oscillation of the vocal fold. The propagation of a shear wave on the surface of the vocal fold, the mucosal wave (Fig. 1E-2), creates an asymmetric driving pressure that is more positive during vocal fold opening than during vocal fold closing, establishing a key mechanism for the transfer of energy from the glottal air flow to the oscillating tissue.
A specific layered structure of ECM components is a general design feature often found in the morphology of organs that accommodate stress, particularly rhythmically applied stress. For example, the wall of arterial vessels undergoes rhythmical extension, inflation and torsion (e.g. Gasser et al. 2006), which is facilitated by the three-layered composition into the intima (tunica intima), media (tunica media) and adventitia (tunica externa). The media and adventitia are fiber-reinforced materials, and only their intact orientation and distribution ensure their specific mechanical function (Silver et al. 1989).
ECM components
The components of the ECM can be classified into two groups – the fibrous proteins and glycosaminoglycans. The fibrous proteins, which include the collagens and elastins, provide the ECM with structural support, shape, form and the ability to withstand stress. The proteoglycans and glycoproteins surround the fibrous elements and influence a number of tissue and cellular functions including osmosis, cell migration, cell differentiation, molecular transport and molecular concentration.
Collagen and elastin have been described as common components of the ECM in the lateral tympaniform membranes and ML in several bird species (various passerine species, Warner, 1972; Japanese quail, Coturnix japonica, Ellis, 1973a; Meadow lark, Sturnella magna, Ellis, 1973b; house wren, Troglodytes aedon, Ellis & Thome, 1975; mallard, Anas platyrhynchos, Frank et al. 2006). The presence of hyaluronan has not been previously documented but it is a major component of all ECMs (Fraser et al. 1997), including that of mammalian vocal folds (Pawlak et al. 1996; Hahn et al. 2006; Schweinfurth & Thibeault, 2008). Hyaluronan is a non-sulfated ubiquitous connective tissue glycosaminoglycan that is synthesized by hyaluronan synthase enzymes. Three vertebrate genes have been isolated and characterized for these enzymes: HAS1, HAS2 and HAS3 (Spicer & McDonald, 1998). In normal tissues, hyaluronan has a role in homeostasis, matrix stability and tissue hydration (Fraser et al. 1997). It is thought that hyaluronan interacts with fibroblasts and other ECM proteins to mediate the cellular synthesis and degradation of various ECM proteins such as collagen, proteoglycans and fibronectin (Ward et al. 2002; Hirano et al. 2003a,b; Thibeault et al. 2004; David-Raoudi et al. 2008). In tissue that is exposed to extreme mechanical/vibrational stress, which is likely to cause lesions, a good healing potential is beneficial and hyaluronan possibly mediates such an action in the labia.
Hyaluronan also affects the biomechanical properties of soft tissue and, in particular, it can improve shear properties (Caton et al. 2007). In the light of a multi-layered labium structure, shear stress becomes a critical factor. The movement of the two ECM layers against each other would be promoted by high concentrations of hyaluronan. In fact, the efficiency by which a passing air stream can set the tissue into vibration is likely to be positively affected by hyaluronan. Such effects have been demonstrated in the vocal folds with a direct positive relationship between the hyaluronan concentration in the ECM and the phonation threshold pressure (minimum lung pressure to initiate phonation) (Chan et al. 2001; Chan & Titze, 2006).
Morphological laterality
Each side of the syrinx makes an important contribution to a song repertoire. Different species of songbirds use the two sides of their syrinx in different stereotyped ways to generate the species-specific spectral and temporal features of their songs (e.g. Nottebohm, 1971; Suthers, 1990, 1999). The functional specialization of each side is associated with morphological left/right asymmetries of the cartilaginous framework of the syrinx in Anatidae (King, 1989; Miller et al. 2008) and muscle asymmetries in the Japanese quail (Burke et al. 2007). An asymmetry of the songbird labia has been noted but has not been quantified before (e.g. Warner, 1972).
In most investigated songbird species the left sound source produces, on average, a lower F0 than the right side (Suthers & Zollinger, 2004), suggesting some degree of specialization of each source. Excised ZF syrinx preparations indicated that the labia of the left sound source have lower resonance peaks than those of the right side (Fee, 2002). Here, we found no significant differences in the cross-sectional area of the labia or the cranio-caudal length of the labia and MTM. We assume that other morphological features must contribute to the functional difference of the two sound sources, e.g. viscoelastic properties of the oscillating tissue, which contribute to sex and species differences in mammals, are likely candidates (Riede et al. 2009).
F0 regulation by labia length changes and the role of the labia layer structure
In the following we attempt to link two acoustic characteristics of the song repertoire of the ZF and WCS to a morphological difference between the two species. We discuss two acoustic features: the F0 range and the harmonic structure.
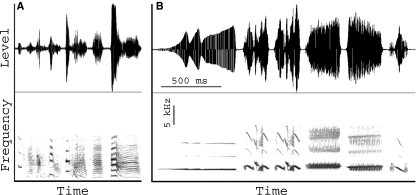
Songs in both species consist of a series of three to eight acoustically different syllables (Fig. 10). The range of F0 overlaps for the two species but the WCS song covers a wider F0 range (ZF: 500–3000 Hz; WCS: 1000–8000 Hz) and F0 modulation is larger. ZFs generate surprisingly low frequencies.
Fig. 10.
Waveforms (upper panel) and spectrograms (lower panel) of songs from a male ZF (A) and a male WCS (B). Units in B apply to both panels. Horizontal lines and numbers in the spectrogram indicate the duration of individual acoustic units (‘syllables’).
It is generally assumed that F0 is determined by the mass and tension of the labia as well as by the subsyringeal lung (air sac) pressure (Mindlin & Laje, 2005). The ZF syrinx seems better suited for the generation of low frequencies, whereas the WCS syrinx seems specialized for high frequencies. Based on body size differences (ZF: 12–14 g; WCS: 20–22 g), one would expect the larger syrinx in the WCS but the MLs of the ZF syrinx are longer (gray bars in Fig. 4) and larger (Figs 5 and 2D,E vs. 2F,G). A second aspect that suggests an adaptation for high frequencies in the WCS is the layered structure of the labia. This makes development of differentiated tension in the tissue possible. This, in effect, may recruit different amounts of labial tissue into vibration. In a more uniformly structured tissue, like the labium of the ZF, tissue is more likely to vibrate in an all-or-none fashion (Fig. 1C,D). At low strains, these differences between layered and uniform labia might not be so large because stress often increases slowly in soft tissue. The labium is more likely to oscillate uniformly (Fig. 11A,B). However, at higher strains the superficial layer might be selectively involved in oscillation (Fig. 11C) because its compliance remains below that of the deep layer. Therefore, it will respond earlier (at lower lung pressure) to the passing air stream. One prominent acoustic effect of a layered structure is the potential to produce a wider F0 range than a similar sized but uniform structure, as the effective mass that is involved in oscillation can be reduced to the superficial layer. Imaging techniques would be helpful in demonstrating the involvement of variable tissue amounts depending on syringeal settings. In fact, direct imaging of the oscillating labia at low vs. high syringeal muscle activity has already suggested differences in oscillation modes (Fee et al. 1998; Jensen et al. 2007).
Fig. 11.
Schematic frontal section of the syrinx illustrating recruitment of layers into oscillation. The details in B–D are indicated in A (compare Fig. 1C,D). The two layers of the ML can be recruited variably into oscillation. (B) Relaxed position of the ML. (C) The ML is uniformly deformed. (D) The superficial layer is selectively involved in oscillation. P, pessulus; SL, superficial layer; DL, deep layer.
The ZF syllables are characterized by a dense harmonic structure (e.g. syllable 6 in Fig. 10A vs. syllable 1 in Fig. 10B). The intensity of the upper harmonics is often stronger than that of the F0. In contrast, the WCS syllables demonstrate little energy in higher harmonics; the F0 is always the strongest energy band. The syringeal sound generator is a potential source of tonal sounds (beside vocal tract effects and non-linear coupling effects) but this role has been investigated very little (Sitt et al. 2008). The primary source of sound is the time-varying syringeal air flow produced by labial oscillation. Labia design can affect not only the oscillation frequency (as explained above) but also the ratio between the open and closed phase during a single cycle as well as the ratio of the opening to the closing phase during the open phase. Both parameters are critical for the spectral shape of the source signal (Titze, 1988).
The link between labial morphology and their acoustic function remains largely hypothetical at this stage. A combination of in-vivo and in-vitro studies is needed to further investigate this relationship. The understanding of morphological detail will allow us to focus future experimental testing and computational simulation.
Acknowledgments
We thank Kanako Omichi for the artwork in Figs 1 and 11, Dr Ron Meyers for comments on an earlier draft, and Dr Ed King for an introduction to the Axioplan system. Dr Jorge Mendez kindly performed the evaluation of the staining results. This research was supported by NIH grants DC 04390 and DC06876.
References
- Amador A, Goller F, Mindlin GB. Frequency modulation during song in a suboscine does not require vocal muscles. J Neurophysiol. 2008;99:2383–2389. doi: 10.1152/jn.01002.2007. [DOI] [PubMed] [Google Scholar]
- Ames PL. The morphology of the syrinx in passerine birds. New Haven, Connecticut: Peabody Museum of Natural History, Yale University; 1971. [Google Scholar]
- Burke MR, Adkins-Regan E, Wade J. Laterality in syrinx muscle morphology of the Japanese quail (Coturnix japonica) Physiol Behav. 2007;90:682–686. doi: 10.1016/j.physbeh.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton TBS, Thibeault SL, Klemuk S, et al. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- Chamberlain DR, Gross WB, Cornwell GW, et al. Syringeal anatomy in the common crow. Auk. 1968;85:244–252. [Google Scholar]
- Chan R, Titze IR. Dependence of phonation threshold pressure on vocal tract acoustics and vocal fold tissue mechanics. J Acoust Soc Am. 2006;119:2351–2362. doi: 10.1121/1.2173516. [DOI] [PubMed] [Google Scholar]
- Chan R, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- David-Raoudi M, Tranchepain F, Deschrevel B, et al. Differential effects of hyaluronan and its fragments on fibroblasts: Relation to wound healing. Wound Repair Regen. 2008;16:274–287. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Elemans CPH, Zaccarelli R, Herzel H. Biomechanics and control of vocalization in a non-songbird. J R Soc Interface. 2008;5:691–703. doi: 10.1098/rsif.2007.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CJ. Syringeal histology, I. Japanese quail, Coturnix coturnix japonica. Iowa State J Res. 1973a;48:7–46. [Google Scholar]
- Ellis CJ. Syringeal histology, II. Meadowlark (Sturnella magna, S. neglecta) Iowa State J Res. 1973b;48:175–191. [Google Scholar]
- Ellis CJ, Thome P. Syringeal histology, III. House Wren (Troglodytes aedon) Iowa State J Res. 1975;50:1–16. [Google Scholar]
- Fee MS. Measurement of the linear and nonlinear mechanical properties of the oscine syrinx: implications for function. J Comp Physiol A. 2002;188:829–839. doi: 10.1007/s00359-002-0349-z. [DOI] [PubMed] [Google Scholar]
- Fee MS, Shraiman B, Pesaran B, et al. The role of nonlinear dynamics of the syrinx in the vocalizations of a songbird. Nature. 1998;395:67–71. doi: 10.1038/25725. [DOI] [PubMed] [Google Scholar]
- Frank T, Walter I, Probst A, et al. Histological aspects of the syrinx of the mallard (Anas platyrhynchos) Anat Histol Embryol. 2006;35:396–401. doi: 10.1111/j.1439-0264.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J Royal Soc Interface. 2006;3:15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral motor dynamics of song production in the zebra finch. Ann NY Acad Sci. 2004;1016:130–152. doi: 10.1196/annals.1298.009. [DOI] [PubMed] [Google Scholar]
- Goller F, Larsen ON. A new mechanism of sound generation in songbirds. Proc Natl Acad Sci USA. 1997a;94:14787–14791. doi: 10.1073/pnas.94.26.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen ON. In situ biomechanics of the syrinx and sound generation in pigeons. J Exp Biol. 1997b;200:2165–2176. doi: 10.1242/jeb.200.16.2165. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Implications for lateralization of bird sound from unilateral gating of bilateral motor patterns. Nature. 1995;373:63–66. [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J Neurophysiol. 1996a;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996b;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper BG, Suthers RA. Respiratory dynamics and syllable morphology in songbirds. Acta Zool Sin. 2006;52(Suppl):471–474. [Google Scholar]
- Haecker V. Der Gesang der Voegel, seine anatomischen und biologischen Grundlagen. Jena: Gustav Fischer; 1900. [Google Scholar]
- Hahn MS, Kobler JB, Starcher BC, et al. Quantitative and comparative studies of the vocal fold extracellular matrix. I: Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- Hirano M. The vocal fold during phonation. Igaku no Ayumi. 1972;80:622–627. [Google Scholar]
- Hirano M, Kakita Y, Ohmaru K, et al. Structure and mechanical properties of the vocal fold. In: Lass NJ, editor. Speech and Language: Advances in Basic Research and Practice. New York: Academic Press; 1982. pp. 271–297. Vol. 7, pp. [Google Scholar]
- Hirano M, Yoshida T, Tanaka S. Vibratory behavior of human vocal folds viewed from below. In: Gauffin J, Hammarberg B, editors. Vocal Fold Physiology. San Diego, CA: Singular Publishing Group; 1991. pp. 1–6. [Google Scholar]
- Hirano S, Bless DM, Heisey D, et al. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2003a;112:617–624. doi: 10.1177/000348940311200708. [DOI] [PubMed] [Google Scholar]
- Hirano S, Bless DM, Massey RJ, et al. Morphological and functional changes of human vocal fold fibroblasts with hepatocyte growth factor. Ann Otol Rhinol Laryngol. 2003b;112:1026–1033. doi: 10.1177/000348940311201206. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Cooper BG, Larsen ON, et al. Songbirds use pulse tone register in two voices to generate low-frequency sound. Proc R Soc B. 2007;274:2703–2710. doi: 10.1098/rspb.2007.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AS. Functional anatomy of the syrinx. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 4. London, UK: Academic Press; 1989. pp. 105–192. [Google Scholar]
- Larsen ON, Goller F. Role of syringeal vibrations in bird vocalization. Proc R Soc Lond B. 1999;266:1609–1615. [Google Scholar]
- Larsen ON, Goller F. Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol. 2002;205:25–35. doi: 10.1242/jeb.205.1.25. [DOI] [PubMed] [Google Scholar]
- Matsushita H. The vibratory mode of the vocal folds in the excised larynx. Folia Phoniatr. 1975;27:7–18. doi: 10.1159/000263963. [DOI] [PubMed] [Google Scholar]
- Miller EH, Seneviratne SS, Jones IL, et al. Syringeal anatomy and allometry in murres (Alcidae : Uria) J Ornithol. 2008;149:545–554. [Google Scholar]
- Mindlin GB, Laje R. The Physics of Birdsong. Berlin: Springer; 2005. [Google Scholar]
- Miskimen M. Sound production in passerine birds. Auk. 1951;68:493–504. [Google Scholar]
- Müller J. Berlin: Abhandlungen der Königlichen Akademie der Wissenschaften; 1847. Über die bisher unbekannten typischen Verschiedenheiten der Stimmorgane der Passerinen; pp. 321–391. [Google Scholar]
- Nottebohm F. Neural lateralization of vocal control in a passerine bird. I. Song. J Exp Zool. 1971;177:229–262. doi: 10.1002/jez.1401770210. [DOI] [PubMed] [Google Scholar]
- Pawlak AS, Hammond T, Hammond E, et al. Immunocytochemical study of proteoglycans in vocal folds. Ann Otol Rhinol Laryngol. 1996;105:6–11. doi: 10.1177/000348949610500102. [DOI] [PubMed] [Google Scholar]
- Riede T, Suthers RA, Fletcher N, et al. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc Natl Acad Sci USA. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Lingle S, Hunter E, et al. Cervids with different vocal behavior demonstrate different viscoelastic properties of their vocal folds. J Morphol. 2009 doi: 10.1002/jmor.10774. doi 10.1002/jmor.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfurth JM, Thibeault SL. Does hyaluronic acid distribution in the larynx relate to the newborn’s capacity for crying. Laryngoscope. 2008;118:1692–1699. doi: 10.1097/MLG.0b013e3181782754. [DOI] [PubMed] [Google Scholar]
- Setterwall CG. Studier of ver Syrinx hos Polymyoda Passeres. pp. 1–128. Lund: Akad Afhandl. [Google Scholar]
- Silver FH, Christiansen DL, Buntin CM. Mechanical properties of the aorta: A review. Crit Rev Biomed Eng. 1989;17:323–358. [PubMed] [Google Scholar]
- Sitt JD, Amador A, Goller F, et al. Dynamical origin of spectrally rich vocalizations in birdsong. Phys Rev. 2008;78 doi: 10.1103/PhysRevE.78.011905. article number:011905. [DOI] [PubMed] [Google Scholar]
- Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273:1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA. The motor basis of vocal performance in songbirds. In: Hauser M, Konishi M, editors. The Design of Animal Communication. Cambridge, MA: MIT Press; 1999. pp. 37–62. [Google Scholar]
- Suthers RA, Zollinger SA. Producing song: the vocal apparatus. Ann NY Acad Sci. 2004;1016:109–129. doi: 10.1196/annals.1298.041. [DOI] [PubMed] [Google Scholar]
- Thibeault SL, Rousseau B, Welham NV, et al. Hyaluronan levels in acute vocal fold scar. Laryngoscope. 2004;114:760–764. doi: 10.1097/00005537-200404000-00031. [DOI] [PubMed] [Google Scholar]
- Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- Titze IR. The Myoelastic Aerodynamic Theory of Phonation. Denver, CO: The National Center for Voice and Speech; 2006. [Google Scholar]
- Ward PD, Thibeault SL, Gray SD. Hyaluronic acid: Its role in voice. J Voice. 2002;16:303–309. doi: 10.1016/s0892-1997(02)00101-7. [DOI] [PubMed] [Google Scholar]
- Warner RW. The anatomy of the syrinx in passerine birds. J Zool Lond. 1972;168:381–393. [Google Scholar]