Abstract
In the pregnant mouse endometrium, collagen fibrillogenesis is characterized by the presence of very thick collagen fibrils which are topographically located exclusively within the decidualized stroma. This dynamic biological process is in part regulated by the small leucine-rich proteoglycans decorin and biglycan. In the present study we utilized wild-type (Dcn+/+) and decorin-deficient (Dcn−/−) time-pregnant mice to investigate the evolution of non-decidualized and decidualized collagen matrix in the uterine wall of these animals. Ultrastructural and morphometric analyses revealed that the organization of collagen fibrils in the pregnant endometrium of both non-decidualized and decidualized stroma showed a great variability of shape and size, regardless of the genotype. However, the decidualized endometrium from Dcn−/− mice contained fibrils with larger diameter and more irregular contours as compared to the wild-type littermates. In the Dcn−/− animals, the proportion of thin (10–50 nm) fibrils was also higher as compared to Dcn+/+ animals. On day 7 of pregnancy, biglycan was similarly localized in the decidualized endometrium in both genotypes. Lumican immunostaining was intense both in decidualized and non-decidualized stroma from Dcn−/− animals. The present results support previous findings suggesting that decorin participates in uterine collagen fibrillogenesis. In addition, we suggest that the absence of decorin disturbs the process of lateral assembly of thin fibrils, resulting in very thick collagen fibrils with irregular profiles. Our data further suggest that decorin, biglycan and lumican might play an interactive role in collagen fibrillogenesis in the mouse endometrium, a process modulated according to the stage of pregnancy.
Introduction
Decidualization of the uterine stroma is a key event that occurs in early pregnancy in rodents and humans. Previous studies have identified important changes in the composition and structure of extracellular matrix (ECM) during decidualization in both humans and rodents (Aplin, 1996; Abrahamsohn et al. 2002; San Martin et al. 2003a,b, 2004; Teodoro et al. 2003). These changes include alterations in collagen fibril distribution, structure and thickness (Alberto-Rincon et al. 1989; Carbone et al. 2006). In the mouse, during the peri-implantation stage from day 5 to day 8 of pregnancy, decidualizing stromal cells grow, compressing the intercellular spaces so that the volume fraction of ECM is reduced. At the same time, unusually thick collagen fibrils of up to 520 nm diameter appear exclusively in the decidualizing areas of the endometrial stroma (Alberto-Rincon et al. 1989; Carbone et al. 2006). Previous studies using radioautographic analysis have demonstrated the presence of 3H-Pro on the surface of thick collagen fibrils, indicating that they are produced by mature decidual cells (Oliveira et al. 1991). Collagen fibrillogenesis is a complex process that involves several processing steps including association with other extracellular molecules including the proteoglycans (PG). It has been demonstrated that PG in the ECM bind to specific sites on the surface of collagen, exerting a strong influence on its aggregation into fibrils (Ruggeri & Benazzo, 1984; Iozzo, 1997, 1999). Previous studies on the small leucine-rich proteoglycan distribution in the uterus showed that biglycan, decorin and lumican, but not fibromodulin, are present in the endometrial stroma of pregnant mouse (San Martin et al. 2003a,b;). In addition, those studies showed that decorin is detected in the non-decidualized uterine stroma but is absent from decidualized endometrium (San Martin et al. 2003a,b;) Moreover, the same group of authors has showed by immuno-electron microscopy that biglycan and not decorin is associated with the thick collagen fibrils in the mouse endometrium (San Martin & Zorn, 2003). Small leucine-rich proteoglycans (SLRPs) belong to a family of secreted proteoglycans that includes decorin, biglycan, fibromodulin, lumican, epiphican and keratocan (Iozzo & Murdoch, 1996; Iozzo, 1999; Ameye & Young, 2002). There are now five proposed classes of SLRPs with 17 genes encoding clusters of SLRPs, suggesting high conservation and tandem duplication during evolution (Schaefer & Iozzo, 2008). Decorin has been postulated to regulate collagen type I fibril diameter and to be located on the surface of such fibrils, at the D band in the gap region (Pringle & Dodd, 1990). Decorin is known to bind to collagen types I, II, III, V, VI, XII and XIV (Oldberg et al. 1989; Bidanset et al. 1992; Font et al. 1993, 1996; Hedbom & Heinegärd, 1993; Schönherr et al. 1995a,b; Thieszen & Rosenquist, 1995; Wiberg et al. 2001), and fibronectin (Schmidt et al. 1987). Decorin also binds to transforming growth factor β (TGF-β) inhibiting its activity in some situations (Hildebrand et al. 1994). Moreover, decorin has been implicated in the control of cell differentiation and proliferation in a cell-specific manner (Santra et al. 1995; De Luca et al. 1996; Iozzo et al. 1999a,b; Xanus et al. 2001). Access to mice deleted at the decorin gene locus offers a unique opportunity to better understand the role of this proteoglycan in tissue biology. It has been demonstrated that targeted ablation of the decorin gene causes a skin fragility phenotype due to abnormal collagen fibril morphogenesis within the dermis and tail tendon (Danielson et al. 1997).
Although decorin-null animals are fertile, there is no available detailed evaluation regarding reproductive efficiency or possible changes in the uterine microenvironment of these animals. In light of previous observations on collagen remodeling (Zorn et al. 1986; Alberto-Rincon et al. 1989) and differential localization of various SLRPs in the mouse uterus during decidualization (San Martin et al. 2003a,b, 2004), we felt that it would be important to establish the role of decorin on collagen fibrillogenesis in the pregnant mouse endometrium. To this end, we used ultrastructural and morphometric analyses to determine the morphology and organization of collagen fibrils in the decidualized and nondecidualized endometrium on days 3, 5 and 7 of pregnancy in Dcn−/− mice vis-à-vis wild-type animals. Our results show that decorin plays a key role during physiological collagen fibril formation in decidualized stroma and that this process might be affected by other SLRPs such as lumican.
Material and methods
Animals
Gene-targeted mice deficient in decorin (Dcn−/−) and wild-type control mice (Dcn+/+) were maintained on a 129SvXB1/Swiss mixed background (Danielson et al. 1997). The genotype of the animals was determined by PCR analysis as previously described (Danielson et al. 1997). National and international principles of laboratory animal handling established by the University of Manchester Ethical Committee and by the Ethics Committee for Animal Research of the Institute of Biomedical Sciences of the University of São Paulo, Brazil, were followed.
Tissue collection
Pregnant animals were obtained by mating virgin nulliparous homozygous females with heterozygous males of the same colony. Female mice, aged 2–3 months, and males of the same colony were used. The animals were exposed to a 12 h light/12 h darkness schedule at 22 °C, with food and water available. Females were left to mate and were examined for the presence of a vaginal plug each morning. The time on which a vaginal plug was found was designated as the first day of pregnancy (dop). Pregnant animals on day 3 (n = 4), day 5 (n = 3), and day 7 (n = 5) were used in this study, with at least three animals being used on each day of pregnancy. The animals were killed by cervical dislocation and the uteri were removed and dissected free of fat. Sections of dorsal skin from wild-type mice and knockout animals were used as controls.
Uterine horns on days 3, 5 and 7 of pregnancy were collected, cut into small transverse segments and immediately immersed in a solution of 2.0% glutaraldehyde, 2.0% paraformaldehyde, 2.5 mm calcium chloride, 0.1 m sodium cacodylate, pH 7.3 for 24 h at 4 °C. The tissues were then postfixed for 2 h at room temperature in 1.0% OsO4 in the same buffer, dehydrated and embedded in Spurr resin. Semi-serial sections of the implantation sites (days 5 and 7) were made until the embryos were reached. Sections (0.5 μm-thick) were stained with 1.0% toluidine blue in 1.0% aqueous sodium borate for light microscopic examination. To confirm the pregnancy of the animals on day 3, oviduct and ovaries were cut serially until the non-implanted embryos were reached. The region of the antimesometrial decidua was chosen for ultrastructural analysis. At least five samples from each animal were examined. Thin sections were stained with uranyl acetate and lead citrate, and observed with a JEOL 1010 transmission electron microscope. Electron micrographs were taken from the antimesometrial stroma on day 3 of pregnancy from mature decidua, and from nondecidualized stroma on days 5 and 7 of pregnancy.
Immunoperoxidase staining
Samples were fixed in 4% paraformaldehyde, 0.1 m sodium cacodylate, pH 7.3 for 24 h at 4 °C and embedded in Paraplast (Oxford, St. Louis, MO). Samples were sectioned at 5 μm and mounted on Silane (Sigma, USA) pre-coated slides. Sections were deparaffinized, hydrated and treated with 3% H2O2 in PBS for 30 min to block endogenous peroxidase activity. All steps were performed in a humidified chamber, and care was taken to avoid drying out of sections; each step was followed by washing with phosphate-buffered saline (PBS). For each antibody, nonspecific reactions were blocked by incubating the sections in a solution of Pierce SuperBlock® Blocking Buffer (Pierce, Rockford, IL) for 1 h at room temperature. Additional incubation was performed using a solution of Pierce SuperBlock® Blocking Buffer containing 1.5% normal donkey serum (NDS) (The Jackson Lab, Bar Harbor, ME), and 2% PBS/BSA for 1 h at room temperature.
Sections were incubated in rabbit polyclonal antibodies raised against murine decorin (LF-113) and biglycan (LF-106) (Fisher et al. 1995). These antibodies were provided by Dr. Larry Fisher, National Institute of Dental and Cranial Research, NIH, Bethesda, USA. The antibodies were used at 1 : 1000 in 1% TBS/bovine serum albumin (BSA) containing 1.5% NDS, and 10% Pierce Blocking buffer for 18 h at 4 °C. Anti-lumican was kindly provided by Dr. Shukti Chakravarti, Johns Hopkins University (Baltimore, MD), and used at 1 : 250 in 1% TBS/BSA containing 1.5% NDS, and 10% Pierce Blocking buffer for 18 h at 4 °C. The sections were then washed thoroughly with PBS followed by incubation for 1 h at room temperature with peroxidase-conjugated goat anti-rabbit IgG (KPL, Silver Spring, MD) diluted 1 : 500 in 1% TBS/BSA containing 1.5% NDS, and 10% Pierce blocking buffer. Peroxidase was visualized using 0.03% (w/v) 3, 3′-diaminobenzidine (Sigma) in PBS with 0.03% (v/v) H2O2. To achieve standardization of the immunoreactions, for each antibody, the samples from Dcn+/+ and Dcn−/− were simultaneously incubated with diaminotetraacetic acid (DAB) and the reaction was immediately interrupted with PBS after a period of 10 min.
The sections were counterstained with Mayer’s hematoxylin. The specificity of immunolabeling was tested by omitting the primary antibody. The samples were examined with a Nikon Eclipse E600 microscope. Images were captured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Quantitative analysis
For quantitative analysis, the greater diameter of cross-sectioned collagen fibrils was manually measured on electron micrographs with final magnification of ×50 000, and histograms were obtained. The data presented are the percentage of collagen fibrils over 50 nm in diameter and the median values from different days of pregnancy. Statistical analysis tested the difference in proportions of collagen fibrils over 50 nm between the two groups. The Chi-squared test was used for the statistical analysis using Minitab 15 statistical software (Minitab Inc., State College, PA).
Results
Collagen fibril ultrastructure
Pre-decidualization: day 3
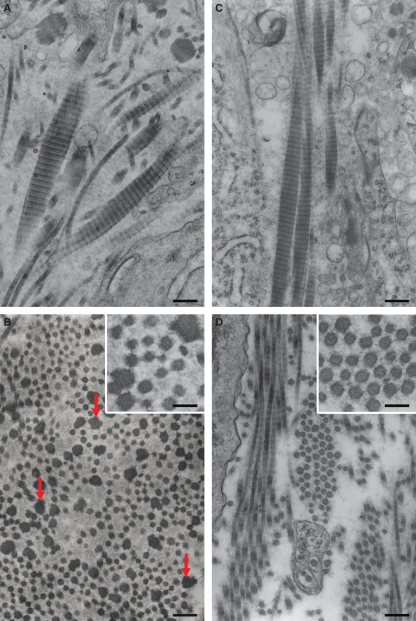
On day 3 of pregnancy, the endometrial stroma of decorin-deficient mice showed a great variability in the shape and size of collagen fibrils (Fig. 1A) as compared to the wild-type animals (Fig. 1C). However, no alteration was observed in the morphology or periodicity of the cross-banding of the collagen fibrils. In the Dcn−/− animals, collagen bundles contained a mixture of thin and thick fibrils. Cross-sections showed that the thin collagen fibrils had a rounded and smooth surface, whereas fibrils larger than 40–50 nm presented an irregular and rough profile (Fig. 1A, insert). In contrast, Dcn+/+ mice showed quite uniform fibril outlines that were circular in cross-section (Fig. 1C, insert).
Fig. 1.
Representative image of a bundle of collagen showing cross-sectioned collagen fibrils: (A) day 3, Dcn−/− endometrium showing thin collagen fibrils with a rounded and smooth surface intermingled with thick fibrils (arrow). (B) Day 5, Dcn−/− decidua showing thicker and irregular collagen fibrils (arrows). (C) Day 3, Dcn+/+ endometrium showing a bundle of collagen fibrils with uniform outlines. (D) Day 5, Dcn+/+ decidua showing a bundle of collagen fibrils with heterogeneous diameters. Insets: high magnification showing the outline of collagen fibrils. Scale bars, 250 nm. Insets, 125 nm.
Post-decidualization: days 5–7
On day 5 of pregnancy, in both genotypes, collagen fibrils of heterogeneous diameters and with very irregular profiles were present in the narrow extracellular spaces between mature decidual cells (Fig. 1B,D). As discussed below, however, decorin-null animals contained a significantly higher proportion of fibrils with larger diameters.
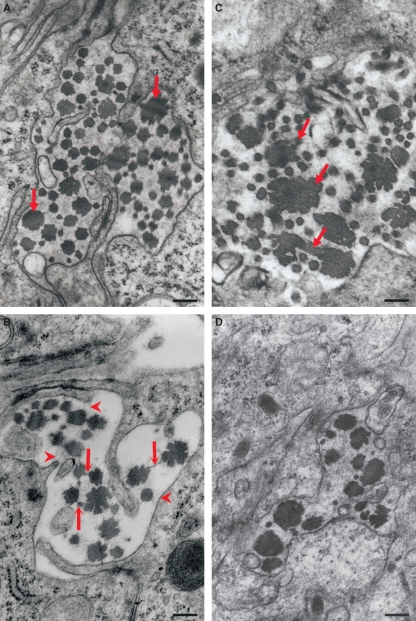
By day 7, the phenotypic appearance of collagen fibrils in the mature decidua from Dcn−/− mice continue to progress, reaching very irregular and large fibrils (Fig. 2A). The thin collagen fibrils exhibited mostly a rounded and smooth surface. In contrast, the thick ones had very irregular outlines in cross-section, varying from a rosette-like appearance to a trefoil shape with deep indentations and protuberances (Fig. 2A–C). Interestingly, thin filaments were observed connecting collagen fibrils (Fig. 2B) with each other and with the surface of the decidual cells (Fig. 2B). In addition, some thick collagen fibrils (> 200 nm) appeared to be interconnected, forming aberrant patterns (Fig. 2C). In the wild-type animals, although the bundles of collagen fibrils were also heterogeneous, they were formed mostly by thick collagen fibrils (Fig. 2D). In longitudinal section, however, the periodicity of the cross-banding of collagen fibrils was typical of collagen in both genotypes (Fig. 3A–D).
Fig. 2.
Representative image of the decidualized endometrium on day 7 of pregnancy. (A) Dcn−/−, showing the coexistence in the collagen bundle of thicker and irregular collagen fibrils (arrows) and much thinner fibrils (arrowheads). (B) Dcn−/− showing thin filaments connecting the collagen fibrils to each other (arrows) as well as to the decidual cell surface (arrowhead). (C) Note in Dcn−/− that some thick collagen fibrils appeared to be connecting to another, forming an aberrant pattern (arrow). (D) Decidualized endometrium from and Dcn+/+ animal. Scale bars, 250 nm (A,B,D) and 125 nm (C).
Fig. 3.
Decidualized endometrium on day 7 of pregnancy from the Dcn−/− (A) and Dcn+/+ (C) animals showing longitudinal section of a bundle of fibrils with typical periodicity of the collagen cross-banding. Nondecidualized stroma from Dcn−/− (B) and Dcn+/+ (D) animals. In B, observe the presence of thick and very irregular fibrils (arrows) and the coexistence of heterogeneous thin fibrils. Thin filaments are seen to connect the collagen fibrils in both genotypes (Insets in B and D). Scale bars, 250 nm.
Morphometric analyses
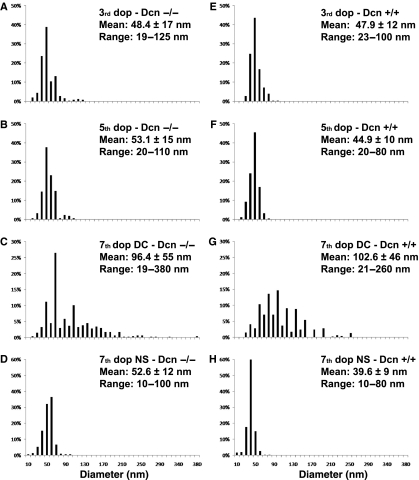
A quantitative morphometric analysis was performed on both genotypes and at various stages of pregnancy. First, the results showed that in the pre-decidualized endometrium, although the percentage of collagen fibrils with diameters > 50 nm did not vary significantly between genotypes (P = 0.558) [being 31.2% in the Dcn−/− (median = 42.5) and 28.8% in the Dcn+/+ animals (median = 46.5)], the Dcn−/− mice exhibited a wider range with fibrils varying from 19 to 125 nm in diameter (Fig. 4A,E). Secondly, in the post-decidualized endometrium at day 5, the percentage of collagen fibrils > 50 nm in diameter was significantly different between genotypes (P = 0.001), being 44% in the Dcn−/− (median = 50.0), and 20% in the Dcn+/+ animals (median = 41.75). In addition, the thicker collagen fibrils found in the Dcn−/− mice were up to 110 nm in diameter, whereas in the wild-type animals the maximum diameter of the collagen fibrils was 80 nm (Fig. 4B,F). Thirdly, at day 7, the collagen fibrils of decorin-null mice had an even wider range of diameters, between 19 and 380 nm, compared with collagen fibrils of the wild-type, which ranged from 21 to 260 nm (Fig. 4C,G). In addition, in Dcn−/−, 50% of the fibrils were 10–80 nm in diameter, whereas in the wild-type animals only 39% of collagen fibrils were 10–80 nm in diameter. The percentage of collagen fibrils over 50 nm in diameter was significantly different between genotypes (P < 0.001), being 91.6% in the Dcn+/+ (median = 100.0) and 84% in the Dcn−/− animals (median = 80.0).
Fig. 4.
Distribution of collagen fibril diameters in the pregnant endometrium from Dcn−/− and Dcn +/+ animals. P< 0.001. DC, mature decidua. NS, nondecidualized stroma.
On day 7, in areas of nondecidualized stroma, fibril diameter remained much smaller than in the decidualized areas in both genotypes (Fig. 4D,H) and bundles of collagen fibrils with very heterogeneous diameters were evident in Dcn−/− animals (Fig. 3B). The percentage of collagen fibrils over 50 nm in diameter was significantly different between genotypes (P = 0.001), being 3% in the Dcn+/+ (median = 40.0) and 45% in the Dcn−/− animals (median = 50.0) (Fig. 4D,H). Interestingly, the collagen fibrils of this region were embedded in a flocculent material with a moderate electron density. In both genotypes, thin filaments were seen to connect the collagen fibrils to each other (Fig. 3B,D).
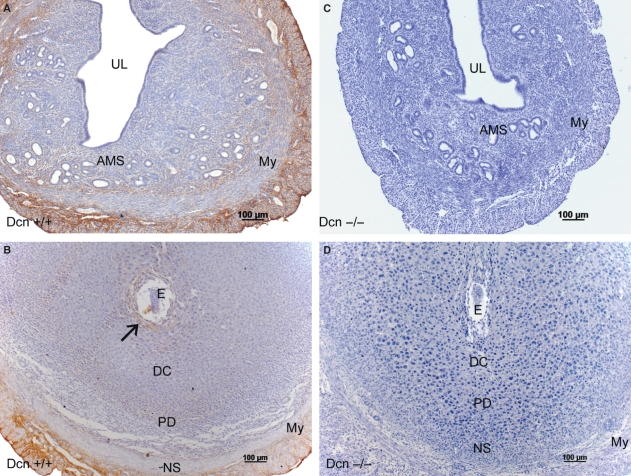
Immunolocalization of decorin
As expected, no immunoreactive decorin was detected in the uterus of Dcn−/− mice (Fig. 5A–B). In wild-type animals, immunoreactivity for decorin was present in uterine tissues (Fig. 5C–D). The deletion of the decorin gene did not disturb the general organization of the decidua.
Fig. 5.
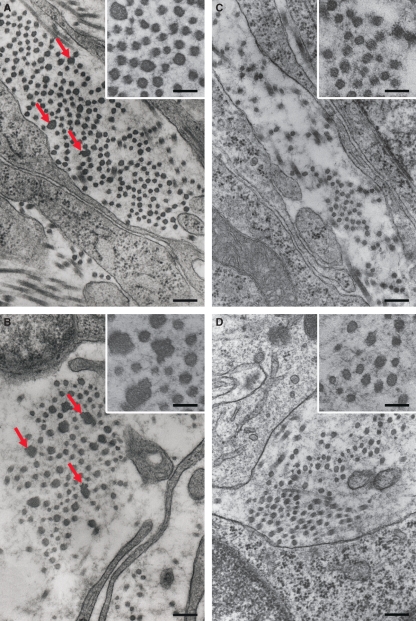
Immunoperoxidase for decorin: Dcn+/+ (A–B); Dcn−/− (C–D) animals. (A) Day 3 of pregnancy showing a weak reaction in the entire antimesometrial stroma (AMS). (B) On day 7 a discrete immunolabeling is present surrounding the implantation crypt (arrow) and minimal immunolabeling in the mature decidua and in the predecidual region. The myometrium and the mesometrium are immunopositive for decorin. (C,D) No immunoreactivity is observed in the uterine tissues of Dcn−/−animals, on day 3 and 7 of pregnancy. My, myometrium; UL, uterine lumen; E, embryo; DC, mature deciduas; PD, predecidua; NS, nondecidualized stroma; M, mesometrium. Mayer’s hematoxylin.
In wild-type animals on day 3, a weak reaction for decorin was restricted to the deep stroma close to the myometrium (Fig. 5C). Decorin was also present in the connective tissue of the myometrium and the mesometrium. On day 7, an immunoreaction for decorin was detected surrounding the implantation crypt (Fig. 5D). Only minimal immunoreactivity for decorin was scattered in the mature decidua and in the predecidual region (Fig. 5D). The myometrium and the mesometrium were both immunopositive for decorin (Fig. 5D). As a positive control we utilized parallel sections of dorsal skin and found diffuse decorin immunoreactivity throughout the dermis, and in the subcutaneous and perimysial connective tissue (not shown).
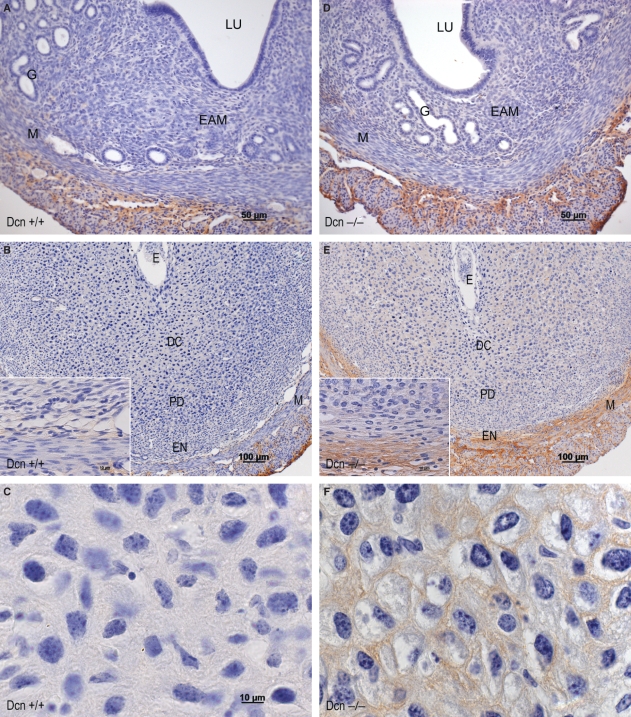
Immunolocalization of biglycan
In wild-type animals on day 3, biglycan was expressed exclusively in the connective tissue between smooth muscle cells of the myometrial external layer (Fig. 6A). In contrast, in Dcn−/− animals, it was detected in the endometrial stroma and glandular epithelium, as well as in the external layer of the myometrium (Fig. 6C). By day 7, immunostaining for biglycan was predominantly detected in the mature decidual region and in the nondecidualized stroma in both genotypes (Fig. 6B,D).
Fig. 6.
Immunoperoxidase for biglycan: Dcn+/+ (A–B); Dcn−/− (C–D) animals. (A) On day 3 immunoreaction is present exclusively in the connective tissue between smooth muscle cells of the myometrial external layer. (B) On day 7, immunoreaction predominates in the mature decidual region and in the nondecidualized. (C) Note that in Dcn−/− on day 3, besides being present in the external layer of the myometrium, the immunoreactivity is also present in the endometrial stroma and glandular epithelium. (D) However, on day 7, the immunostaining for biglycan is similar in both genotypes. UL, uterine lumen; E, embryo; DC, mature decidua; PD, predecidua; NS, nondecidualized stroma; My, myometrium; M, mesometrium. Mayer’s hematoxylin.
Immunolocalization of lumican
On day 3, no expression of lumican was detected in the endometrial stroma, except for a small area of the deep stroma, in either genotype (Fig. 7A,D). In the Dcn−/− animals, however, the reactivity for lumican was increased in the deep stroma (Fig. 7A,D). By day 7, no lumican expression was detected in either decidualized or nondecidualized stroma in wild-type animals (Fig. 7B,C). In contrast, in Dcn−/− animals there was an increase in lumican expression in both the mature decidual region and the nondecidualized endometrium (Fig. 7E,F).
Fig. 7.
Immunoperoxidase for lumican: Dcn+/+ (A–C); Dcn−/− (D–F) animals. (A) On day 3, no immunoreactivity at endometrial stroma, except for a small area in the deep stroma. (B) On day 7, absence of immunoreaction in both decidualized or nondecidualized stroma. (C) High magnification showing absence of immunoreaction in the decidualized region. (D) In the Dcn−/− animals, increasing immunoreactivity in the deep stroma on day 3. (E) On day 7 in Dcn−/− animals, increasing immunoreactivity for lumican in both the mature decidual region and the nondecidualized endometrium. (F) High magnification showing immunoreactivity in the decidualized region. UL, uterine lumen; E, embryo; DC, mature decidua; PD, predecidua; NS, nondecidualized stroma; My, myometrium. Mayer’s hematoxylin.
Discussion
It is well established that remodeling of endometrial ECM is an important event during decidualization in mice, influencing collagen production (Oliveira et al. 1991, 1998) and fibrillogenesis (Alberto-Rincon et al. 1989; Carbone et al. 2006) as well as the biosynthesis of both glycosaminoglycans (Carson et al. 1987; Zorn et al. 1995) and proteoglycans (Greca et al. 1998, 2000; San Martin et al. 2003a,b, 2004). In this context, ECM is expected to play a significant role in embryo implantation and development (Kadler, 2004; Aplin, 2008). Collagen fibrillogenesis is a complex process involving lateral and axial growth, lateral aggregation and regulation of interfibrillar spaces (Linsenmayer et al. 1990; Linsenmayer, 1991; Wight et al. 1991; Birk et al. 1995). Proteoglycan deficiencies produce changes in collagen fibril size, shape, and organization in several tissues. Mice lacking decorin and other proteoglycans such as biglycan, lumican and fibromodulin, all produce abnormal collagen fibrils, characterized by uncontrolled lateral fibril assembly, which in turn results in fibrils with enormous diameters and aberrant profiles (Danielson et al. 1997; Svensson et al. 1999; Chakravarti et al. 2000, 2006).
During decidualization, proteoglycans as well as collagen types I, III, and homotrimeric (α1) 3 type V are likely to be involved in the initial steps of collagen fibrillogenesis (San Martin & Zorn, 2003;Teodoro et al. 2003;Spiess et al. 2007) and recent studies have demonstrated that the thick collagen fibrils of mouse decidua are heterotypic, containing collagen types I, III and V (Spiess et al. 2007). The present study has shown that deletion of the decorin gene results in significant alteration in collagen fibrillogenesis and fibril morphology in both decidualized and nondecidualized endometrium. The main observed consequences of the decorin disruption were: (i) greater heterogeneity of collagen fibril diameters, (ii) increased thickness of fibrils and (iii) a marked irregularity of fibril outlines. Increased thickness of collagen fibrils is seen exclusively in the decidualized region in which decorin is not present (Alberto-Rincon et al. 1989; San Martin & Zorn, 2003). The present data also show an increase in fibril thickness (though not to the very large values seen in decidua) in the nondecidualized endometrium of Dcn−/− animals, substantially confirming the role of decorin in collagen fibrillogenesis in the mouse endometrium. Increased thickness of collagen fibrils has also been observed in the skin and tail tendon from decorin-deficient animals (Danielson et al. 1997).
Accumulation of thin collagen fibrils and an increase in collagen fibril diameter may imply an overall increase in the deposition of collagen, and such an increase in content has, in fact, been demonstrated in the pregnant mouse endometrium (Oliveira et al. 1991; Teodoro et al. 2003). Further study is required to assess whether similar collagen increases occur in the decorin null endometrium.
Previous ultrastructural analysis of serial sections suggests that the increase in diameter and the irregular profile of the decidual collagen fibrils result from lateral aggregation of thin fibrils (Carbone et al. 2006). In addition, it has been shown that decorin is absent at the stage when this lateral aggregation is occurring in early decidua, suggesting that this absence may be responsible for the exceptional fibril thickening observed during mouse decidualization (San Martin & Zorn, 2003). The effects in endometrium of deleting the decorin gene are complex: besides promoting an impressive thickening of collagen fibrils, a higher percentage of thin fibrils is seen, perhaps suggesting a general deregulation, and perhaps also some reduction in the rate of lateral aggregation of thin collagen fibrils into thick ones.
Recent studies have demonstrated that in a variety of tissues, different SRLPs show regulatory roles in collagen fibrillogenesis and control of lateral fibril growth (Reed & Iozzo, 2002, Zhang et al. 2006, 2009; Rühland et al. 2007), as well as biomechanical properties of collagenous matrices (Robinson et al. 2005; Ferdous et al. 2008). The class I SLRP biglycan has been demonstrated to compensate in vivo and in vitro for loss of decorin, regulating and modulating collagen fibril assembly during the development of the cornea, and tendon in decorin-deficient mice (Zhang et al. 2006, 2009). Specifically, at early developmental stages biglycan is upregulated and in in vitro collagen fibrillogenesis assays are capable of functionally compensating for decorin, albeit at higher concentrations (Zhang et al. 2009). Notably, our analysis of biglycan expression has demonstrated its increased expression in the nondecidualized endometrium, coinciding with structural changes in collagen fibrils, such as increase of diameter and the appearance of irregular profiles. However, the expression of biglycan in the decidualized endometrium was similar in both genotypes. In addition, in Dcn−/− animals the expression of lumican was increased in decidualized stroma on day 7, coinciding with the increase of percentage of thin fibrils that reached almost double that in wild-type animals. Thus possible compensation for the absence of decorin may involve these two other SLRPs acting at different stages of tissue differentiation. In fact, there is evidence that lumican regulates collagen fibrils diameter and prevents lateral fibril growth in the adult cornea (Chakravarti et al. 2000, 2006). Despite this, it is not possible from our results to establish a hierarchy for the role of the studied molecules in the control of collagen fibrillogenesis in the endometrial stroma.
Thus, there are tissue-specific changes in regulating the coordinated expression of these two class I SLRPs, presumably mediated by tissue-specific transcription factors signaling pathways and or ovarian hormones (Salgado et al. 2008). Further studies with immunoelectron microscopy are necessary to investigate a possible interaction between biglycan, lumican, and collagen fibrils in endometrium of decorin-null mice. The use of biglycan (Bgn−/0) knockout (Xu et al. 1998), lumican knockout (Lum−/−) or Bgn−/0, and Dcn−/− double knockout animals (Corsi et al. 2002) may add more information to our understanding of the complex steps of collagen fibrillogenesis during decidualization.
Acknowledgments
This work was supported by a grant from Fundação de Amparo à Pesquisa de São Paulo (FAPESP) – 01/09019-9/01/006633-6, CNPq-475581/2006-7. J.C.T.S. was a recipient of a Fellowship from CNPq. We thank Karl Kadler, Sally Bolton and Ben Abell from the Wellcome Trust Centre for Cell-Matrix Research, Faculty of Life Sciences, University of Manchester, for the animal facilities and genotyping. We kindly thank Gaspar Ferreira de Lima, Gerson Batista (in memoriam), and Edson Rocha de Oliveira for their technical assistance. We thank Rosana Prisco for the help with the statistical analysis. We would like also to thank Drs Larry Fisher, National Institute of Dental and Cranial Research, NIH, Bethesda, MD, USA, and Shukti Chakravarti, Johns Hopkins University, Baltimore, MD, USA, for their generous gift of antibodies.
References
- Abrahamsohn PA, Zorn TMT, Oliveira S. Decidua in rodents. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. 1st edn. London: Taylor and Francis; 2002. pp. 279–293. [Google Scholar]
- Alberto-Rincon MC, Zorn TMT, Abrahamsohn PA. Diameter increase of collagen fibrils of the mouse endometrium during decidualization. Am J Anat. 1989;186:417–429. doi: 10.1002/aja.1001860411. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107–116. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Aplin JD. Cell biology of human implantation. Placenta. 1996;17:269–275. doi: 10.1016/s0143-4004(96)90050-8. [DOI] [PubMed] [Google Scholar]
- Aplin JD. Endometrial extracellular matrix. In: Aplin JD, Fazleabas A, Glasser SR, Giudice LC, editors. The Endometrium. 2nd edn. London: Informa press; 2008. pp. 364–378. [Google Scholar]
- Bidanset DJ, Guidry C, Rosenberg LC, et al. Binding of the proteoglycan decorin to collagen type IV. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix developmental. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Carbone K, Pinto NM, Abrahamsohn PA, et al. Arrangement and fine structure of collagen fibrils in the decidualized mouse endometrium. Microsc Res Tech. 2006;69:36–45. doi: 10.1002/jemt.20265. [DOI] [PubMed] [Google Scholar]
- Carson DC, Dutt A, Tang JP. Glycoconjugate synthesis during early pregnancy: hyaluronate and function. Dev Biol. 1987;120:228–235. doi: 10.1016/0012-1606(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Petroll WM, Hassel JR, et al. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthal Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Zhang G, Chervoneva I, et al. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006;235:2493–2506. doi: 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and others connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Santra M, Baldi A, et al. Decorin-induced growth suppression is associated with upregulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- Ferdous Z, Lazaro LD, Iozzo RV, et al. Influence of cyclic strain and decorin deficiency on 3D cellularized collagen matrices. Biomaterials. 2008;29:2740–2748. doi: 10.1016/j.biomaterials.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Stubbs JT, Young MF. Antisera and cDNA probes to human and certain bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–65. [PubMed] [Google Scholar]
- Font B, Aubert-Foucher E, Goldschimidt D, et al. Binding of collagen XIV with dermatan sulfate side chain of decorin. J Biol Chem. 1993;268:25015–25018. [PubMed] [Google Scholar]
- Font B, Eichenberger D, Rosenberg LM, et al. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996;15:341–348. doi: 10.1016/s0945-053x(96)90137-7. [DOI] [PubMed] [Google Scholar]
- Greca CP, Abrahamsohn PA, Zorn TMT. Ultrastructural cytochemical study of proteoglycans in the endometrium of pregnant mice using cationic dyes. Tissue Cell. 1998;30:304–311. doi: 10.1016/s0040-8166(98)80043-8. [DOI] [PubMed] [Google Scholar]
- Greca CP, Nader HB, Abrahamsohn PA, et al. Ultrastructural cytochemical characterization of collagen-associated proteoglycans in endometrium of mice. Anat Rec. 2000;259:413–423. doi: 10.1002/1097-0185(20000801)259:4<413::AID-AR50>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Heinegärd D. Binding of fibromodulin and decorin to separate sites on fibrilar collagens. J Biol Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans: functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, et al. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999a;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Chakravani F, Perrotti D, et al. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci U S A. 1999b;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler K. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res C Embryo Today. 2004;72:1–11. doi: 10.1002/bdrc.20002. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF. Collagen. In: Hay ED, editor. Cell Biology of Extracellular Matrix. 2nd edn. New York: Plenum; 1991. pp. 7–44. [Google Scholar]
- Linsenmayer TF, Fitch JM, Birk DE. Heterotypic collagen fibril and stabilizing collagens. Controlling elements in corneal morphogenesis? Ann N Y Acad Sci. 1990;580:143–160. doi: 10.1111/j.1749-6632.1990.tb17926.x. [DOI] [PubMed] [Google Scholar]
- Oldberg Å, Antonsonn P, Lindblom K, et al. A collagen-binding 59-kd protein is structurally related to the small proteoglycans PG-S1 and PG-S2. EMBO J. 1989;8:2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SF, Nagata T, Abrahamsohn PA, et al. Electron micrographic study on the incorporation of 3H-proline by mouse decidual cells. Cell Mol Biol. 1991;37:315–323. [PubMed] [Google Scholar]
- Oliveira SF, Abrahamsohn PA, Zorn TMT. Autoradiography reveals regional metabolic difference in the endometrium of pregnant and non-pregnant mice. Braz J Med Biol Res. 1998;31:307–312. doi: 10.1590/s0100-879x1998000200015. [DOI] [PubMed] [Google Scholar]
- Pringle GA, Dodd CM. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J Histochem Cytochem. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang T-F, Kazam E, et al. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;126:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Ruggeri A, Benazzo F. Collagen proteoglycan interaction. In: Ruggeri A, Motta PM, editors. Ultrastructure of the Connective Tissue Matrix. Boston: Nijhoff Publ; 1984. pp. 113–125. [Google Scholar]
- Rühland C, Schönherr E, Robenek H, et al. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at early stages of fibrillogenesis. FEBS J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- Salgado RM, Favarro RR, Martin SS, et al. The estrous cycle modulates small leucine-rich proteoglycans expression in mouse uterine tissues. Anat Rec. 2008;292:138–153. doi: 10.1002/ar.20797. [DOI] [PubMed] [Google Scholar]
- San Martin S, Zorn TMT. The small proteoglycan biglycan is associated with thick collagen fibrils in the mouse decidua. Cell Mol Biol. 2003;49:673–678. [PubMed] [Google Scholar]
- San Martin S, Soto-Suazo M, Zorn TMT. Distribution of versican and hyaluronan in the mouse uterus during decidualization. Braz J Med Biol Res. 2003a;36:1067–1071. doi: 10.1590/s0100-879x2003000800013. [DOI] [PubMed] [Google Scholar]
- San Martin S, Soto-Suazo M, Oliveira SF, et al. Small leucine-rich proteoglycans (SLRPs) in uterine tissues during pregnancy in mice. Reproduction. 2003b;125:585–595. doi: 10.1530/rep.0.1250585. [DOI] [PubMed] [Google Scholar]
- San Martin S, Soto-Suazo M, Zorn TMT. Perlecan and syndecan-4 in uterine tissues during the early pregnancy in mice. Am J Reprod Immunol. 2004;52:53–59. doi: 10.1111/j.1600-0897.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Santra M, Skorski T, Calabretta B, et al. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. The biology of small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G, Robneck H, Harrach B, et al. Interaction of small dermatan sulfate proteoglycan from fibroblast with fibronectin. J Cell Biol. 1987;104:1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E, Hausser H, Beavan L, et al. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem. 1995a;270:8877–8883. doi: 10.1074/jbc.270.15.8877. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Witsh-Prehm P, Harrach B, et al. Interaction of biglycan with type I collagen. J Biol Chem. 1995b;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Spiess K, Teodoro WR, Zorn TM. Distribution of collagen types I, III, and V in pregnant mouse endometrium. Connect Tissue Res. 2007;48:99–108. doi: 10.1080/03008200601166194. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt F, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Teodoro WR, Witzel SS, Velosa AP, et al. Increase of interstitial collagen in the mouse endometrium during decidualization. Connect Tissue Res. 2003;44:96–103. [PubMed] [Google Scholar]
- Thieszen SL, Rosenquist TH. Expression of collagens and decorin during aortic arch artery development: implications for matrix pattern formation. Matrix Biol. 1995;14:573–582. doi: 10.1016/s0945-053x(05)80006-x. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Hedbom E, Khairullina A, et al. Biglycan and decorin bind close to the N-terminal region of the collagen VI triple helix. J Biol Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- Wight TN, Heinegärd DK, Hascall VC. Proteoglycans: structure and functions. In: Hay ED, editor. Cell Biology of Extracellular Matrix. 2nd edn. New York: Plenum; 1991. pp. 45–70. [Google Scholar]
- Xanus J, Comalada M, Cardo M, et al. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophage and enhances cell survival through induction of p27 (Kip1) and p21 (Walf1) Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher L, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chernova I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, et al. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn TMT, Bevilacqua EM, Abrahamsohn PA. Collagen remodeling during decidualization in the mouse. Cell Tissue Res. 1986;244:443–448. doi: 10.1007/BF00219220. [DOI] [PubMed] [Google Scholar]
- Zorn TM, Pinhal MA, Nader HB, et al. Biosynthesis of glycosaminoglycans in the endometrium during the initial stages of pregnancy of the mouse. Cell Mol Biol. 1995;41:97–106. [PubMed] [Google Scholar]