Abstract
Clear cell sarcoma (CCS), a tumor of the tendons and aponeuroses of children and young adults, is uniformly fatal once metastatic, exhibiting profound resistance to radio- and chemotherapy. Implicated in human cancer, the receptor tyrosine kinase c-Met mediates hepatocyte growth factor/scatter factor (HGF) signaling and has been shown to be activated in CCS. We previously demonstrated that EWS-ATF1, the pathognomonic translocation associated with clear cell sarcoma, directly activates MITF. Since c-Met is a direct transcriptional target of the MITF transcription factor in melanocytes and melanoma, we examined c-Met expression in clear cell sarcoma with a goal of identifying “drugable” strategies against EWS-ATF1’s oncogenic activity in CCS. We demonstrated that primary CCS and tumor derived cell lines express c-Met that is activated in an autocrine fashion by HGF expression in some CCS cell lines. c-Met expression is critical for CCS invasion, chemotaxis and survival. To explore whether the HGF:c-Met axis could serve as a therapeutic target, we investigated the effects of pathway modulation using a small-molecule inhibitor of c-Met (SU-11274) or a neutralizing antibody to HGF (AMG 102). The use of either pharmacologic agent significantly reduced CCS growth in culture, and HGF inhibition with AMG 102 significantly suppressed CCS growth in vivo in an autocrine xenograft model of CCS. Collectively, these data identify the HGF:c-Met axis as a potential therapeutic target in CCS.
Introduction
Clear cell sarcoma (CCS) is an aggressive soft tissue sarcoma that typically develops in the tendons and aponeuroses of children and young adults (1, 2). A high rate of local and distant recurrence results in a 5 year overall survival of approximately 50% (3-5). Five year survival decreases to 20% for metastatic disease, consistent with the tumor’s profound resistance to conventional chemotherapy and radiation therapy. Molecularly, CCS is characterized by the t(12;22)(q13;12) translocation which results in fusion of the Ewing’s sarcoma gene EWS with the cAMP regulated transcription factor ATF1, a member of the CREB family (6-9). Gene fusion replaces the kinase dependent regulatory region of ATF1 with the amino terminal domain of EWS. By preserving the DNA binding and heterodimerization domains of ATF1, this chimera yields an oncoprotein capable of deregulating transcription of CRE regulated genes (10). We have previously demonstrated that MITF, the melanocyte master transcription factor, is a direct transcriptional target of EWS-ATF1 (11). EWS-ATF1 mimics the Melanocyte Stimulating Hormone/CREB signaling pathway to directly and aberrantly activate MITF expression.
The MiT family regulates several targets that may be central to oncogenesis. MITF directly activates the c-met gene through a conserved E-box element in the c-met proximal promoter (12). c-met is also a transcriptional target of the ASPSCR1-TFE3 fusion, as predicted by the strong homology between TFE3 and MITF (13).
The receptor tyrosine kinase c-Met normally mediates signaling from hepatocyte growth factor/scatter factor (HGF) typically expressed by stromal and mesenchymal cells. c-Met signaling has been implicated in a wide range of biological activities including proliferation, survival and motility; all of which are frequently dysregulated in cancer. Initially identified as an oncogene when fused to the nuclear pore complex protein TPR in carcinogen treated osteosarcoma cells (14, 15), c-Met has been implicated in the oncogenesis of a wide range of cancers including renal, gastric and small cell lung carcinomas, central nervous system tumors as well as several sarcomas (reviewed in (16, 17), see www.vai.org/met). In these cancers, c-Met may be aberrantly activated by mutation, autocrine or paracrine HGF stimulation or overexpression. Co-expression of HGF and c-Met has been noted in a number of human tumors, including carcinomas and hematopoietic malignancies, in addition to certain sarcomas including CCS (18). Activating c-Met mutations have been demonstrated in familial and sporadic papillary renal cell carcinoma, melanoma as well as small and non-small cell lung cancer (19-24). Mice harboring activating mutations of MET spontaneously develop tumors, predominantly sarcomas (25), and Ink4a/Arf deficient mice expressing HGF develop rhabdomyosarcoma (26).
In this study, we explored the expression and function of c-Met in CCS and find that c-Met expression requires EWS-ATF1 expression. Motility and viability of CCS are dependent upon signaling by the HGF:c-Met axis. Inhibition of the HGF:c-Met axis may constitute a novel biologically directed therapy for these highly metastatic and treatment refractory cancers.
Materials and methods
Cell culture
Human CCS cell lines DTC-1 (10), SU-CCS-1 (27) and CCS292 cells (11) were cultured in RPMI with 15% fetal bovine serum with penicillin and streptomycin. Detection of EWS-ATF1 expression confirmed the CCS identity of these cells. HEK293 and HT1080 cells were cultured in RPMI or MEM-Alpha with non-essential amino acids with 10% FBS with penicillin and streptomycin, respectively. pLKO.1 expressing c-Met shRNA (Sigma) was used to prepare VSV-G pseudotyped lentivirus by transfection of HEK293 cells with Transit-LT1 (Mirus) as described (28). CCS cells were virally transduced as described (11). ATF1-directed ON-TARGETplus siRNA or control non-targeting pool (Dharmacon) were transfected using RNAiMAX (Invitrogen). Cells were treated with a fully human monoclonal anti-HGF antibody (AMG 102, Amgen). SU11274 (Calbiochem) was dissolved in DMSO and applied to the cells at the concentrations indicated. Control (vehicle) treated cells were treated with DMSO only. Viability and proliferation were determined by direct cell counting or WST1 assay (Roche). For invasion assays, 5 × 104 cells were plated in serum free media in the upper well of an invasion chamber (Matrigel, BD). Normal growth media or CCS292-conditioned media were placed in the lower chamber. After 24-48 hours, membranes were removed, treated with 1% paraformaldehyde followed by 0.1% Triton X-100 and stained with rhodamine-conjugated phalloidin or DAPI. Membranes were imaged on a Zeiss Axiovert 200 and photographed with a Zeiss AxioCam using OpenLab Imaging software.
Immunoblotting
c-Met expression (C-12, Santa Cruz Biotechnology) and phosphorylation (3126, Cell Signaling Technology) and MAPK pathway activity (7180 or 5302 Cell Signaling Technology) and ATF1 expression (25C10G, Santa Cruz Biotechnology) were monitored by immunoblots as described (12). HGF secretion was assessed by ELISA (R&D Systems).
Xenograft studies
1 × 106 CCS292 cells were injected subcutaneously into the flanks of 40 4-6 week old male NCR nude mice (Charles River). Mice were housed in sterilized cages on a 12-h light/dark cycle and fed ad libitum. Groups of 10 mice were treated with 1 mg of AMG 102 or isotype matched control antibody (human IgG2) injected intraperitoneally in 100 μL phosphate buffered saline twice per week. Tumor volumes were measured twice per week with digital calipers. Statistical differences were assayed by repeated measures ANOVA followed by Scheffe post hoc test. Studies were performed under DFCI Animal Care and Use Committee protocol 02-030.
Results
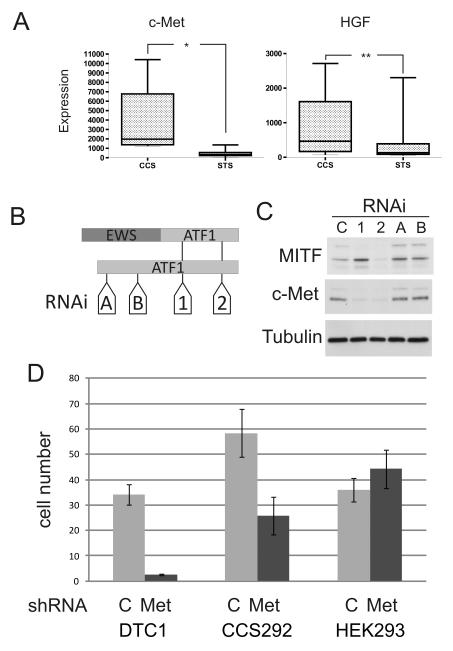
To evaluate if c-Met signaling may play a role in CCS, we analyzed available RNA microarray data derived from primary human CCS (n = 4), a CCS-derived cell line (n = 1) and other soft tissue sarcomas (STS, n = 47) (18). As a group, mean expression of both c-Met and HGF was significantly higher in CCS as compared to other soft tissue sarcomas (Fig. 1A, p < 0.0001 and p < 0.0225 respectively), although higher HGF expression is particularly notable in certain CCS samples. Immunohistochemical evidence of c-Met expression in primary human CCS has been previously reported (29, 30). We examined CCS-derived cell lines and found that c-Met was expressed and phosphorylated on tyrosine residues in the kinase domain (1234/1235) in two of the three lines during normal growth (data not shown, but see Fig. 3A).
Fig. 1.
Clear cell sarcoma cells are dependent on c-Met expression. A. c-Met and HGF expression in clear cell sarcoma (CCS) compared to other soft tissue sarcomas (STS). Microarray expression data derived from (18). * = p < 0.0001, ** = p < 0.0225 (t-test). B. Schematic representation of EWS-ATF1 and ATF1 messages with ATF1-directed siRNAs (A, B, 1 and 2). C. CCS292 cells were transfected with control (C, non-targeting pool) or ATF1-directed siRNAs (A, B, 1 and 2). Cellular extracts were immunoblotted for MITF or c-Met. D. DTC1, CCS292 or HEK293 (control) cells were transduced with lentiviruses expressing either control (C) or MET-directed shRNA and selected with puromycin for three to four days.
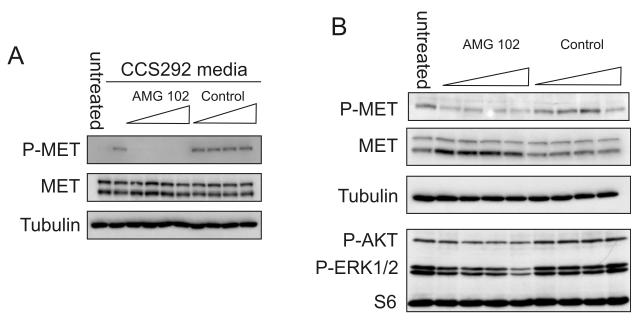
Fig. 3.
AMG 102 inhibits CCS produced HGF. A Serum starved 501mel cells were incubated with conditioned media from CCS292 cells that had been pretreated with AMG 102 or an isotype matched control antibody (2, 4, 8, or 16 μg/mL) for 30 min at RT. After 30 min, extracts of cells were made and immunoblotted for c-Met and phospho-c-Met. Tubulin serves as a loading control. B. AMG 102 or isotype matched control antibody was added to logarithmically proliferating CCS292 cells grown in full serum. After 3 hours, cell extracts were immunoblotted for c-Met and phospho-c-Met by immunoblotting. Tubulin serves as loading control. Cells were assayed for phospho-AKT and p44/42 ERK1/2 by immunoblotting. S6 serves as loading control.
To test for direct regulation of c-Met by MITF in CCS cells, we knocked down MITF expression using lentivirally delivered shRNA and direct siRNA transfection. Despite decreased MITF expression, c-Met levels were unchanged (data not shown). We then examined the effect of EWS-ATF1 knock down using a series of ATF1 siRNAs (Fig. 1B). siRNAs that recognize the region of ATF1 preserved in the EWS-ATF1 fusion nearly completely eliminated c-Met expression in CCS292 cells whereas those that target exclusively wild-type ATF1 had no effect on c-Met levels (Fig. 1C). All siRNAs greatly decreased ATF1 expression (Supp. Fig. 1A).
To test the importance of c-Met signaling in CCS, we examined cell viability after inhibiting c-Met expression. Lentivirally expressed c-Met-directed shRNA was transduced into CCS cells (Sup. Fig 1B). c-Met-directed shRNA greatly decreased DTC-1 or CCS292 viability whereas infection of control HEK293 cells had no effect on viability (Fig. 1D).
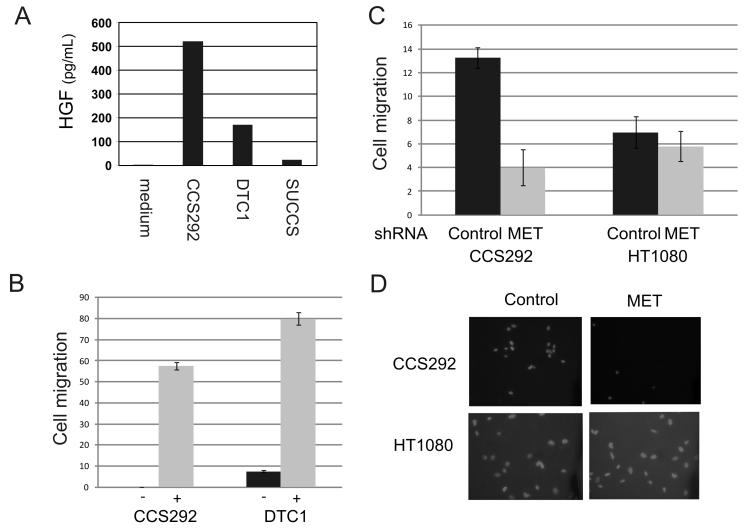
We then explored potential mechanisms for c-Met activation. Since activating c-Met mutations have been identified in several cancers, we fully sequenced c-met exons encoding the juxtamembrane domain through the tyrosine kinase domain. No activating mutations were detected in any of the three CCS cell lines tested. We next tested whether c-Met activation could be mediated through an autocrine mechanism. HGF expression was assayed by ELISA of conditioned media derived from CCS cell lines. CCS292 and DTC-1, but not SU-CCS-1, cells secrete HGF into the media (Fig. 2A).
Fig. 2.
Clear cell sarcoma cells express biologically active HGF. A. Secretion of HGF was quantified by ELISA of conditioned media derived from CCS cell lines CCS292, DTC1 and SU-CCS-1 cultured for 18 hours, as well as fresh full-serum growth medium (medium). B. 5 × 104 CCS292 and DTC1 cells were placed in the upper well of a Matrigel-coated invasion chamber in serum free media with either full serum growth media (−) or CCS-conditioned media (+) in the lower well. After two days, cells were fixed, cells in the upper surface of the membrane were removed and the remaining cells were stained with rhodamine-conjugated phalloidin. At least four representative medium power fields were counted and averaged. C. Two days after transducing CCS292 or fibrosarcoma HT1080 (control) cells with lentivirus encoding MET-directed shRNA, 5 × 104 cells were placed in Matrigel transwells. After two additional days, cells were fixed and stained with DAPI. Representative photomicrographs are shown (D).
HGF is expressed as a single chain propeptide that requires proteolytic cleavage to generate an active α/β heterodimer (31-33). To test whether HGF produced by the CCS cells is biologically active, we treated HGF-responsive melanoma cells (501mel) with conditioned media from CCS cells as well as recombinant HGF. Culture medium derived from CCS292 robustly activated c-Met in 501mel melanoma cells (Supp. Fig. 1B and Fig. 3A). Weaker MET phosphorylation was noted in 501mel cells after exposure to DTC-1 medium (as compared to CCS292) and likely reflects the lower levels of HGF produced by DTC-1 (Supp. Fig. 1B).
Since c-MET has been implicated in cellular motility and metastasis, we examined CCS cells for their ability to invade and if c-Met may mediate this process. CCS cells (CCS292 and DTC1) cultured in Matrigel invasion wells demonstrated a small degree of invasion in the presence of fresh serum containing growth media (Fig. 2B). However, invasion and migration was greatly enhanced when CCS292 conditioned media (which contains HGF) was placed below the membrane (Fig. 2B). Inhibition of MET expression significantly reduced chemotaxis (Fig. 2C, D).
The simultaneous expression of c-Met and HGF by CCS292 cells and the basal level of phospho-c-Met suggest that c-Met may be activated by an autocrine pathway. The recent identification of a fully human monoclonal anti-HGF antibody (AMG 102) (34), offered an opportunity to study the effect of HGF inhibition on CCS. To demonstrate the activity of AMG 102 on CCS-derived HGF, 501mel cells were treated with CCS conditioned media that had been pretreated with AMG 102. At all concentrations tested, AMG 102 completely blocked c-Met activation (Fig. 3A). This result confirms that c-Met activation in this melanoma cell line is mediated exclusively by HGF and not by another secreted factor in the conditioned medium.
We then tested the effect of HGF inhibition on CCS by treating CCS292 cells with increasing concentrations of AMG 102. In contrast to an isotype-matched control antibody (IgG2), AMG 102 resulted in a marked, albeit incomplete, decrease in activated c-Met (Fig. 3B). Decreased phospho-c-Met was accompanied by an increase in total c-Met, possibly reflecting a diminished rate of receptor turnover in the absence of continuous, autocrine ligand stimulation. We also examined whether AMG 102-mediated c-Met inhibition affected intracellular signaling in CCS292 cells. Both AKT and MAPK signaling were inhibited by AMG 102 treatment in a dose dependent fashion (Fig. 3B).
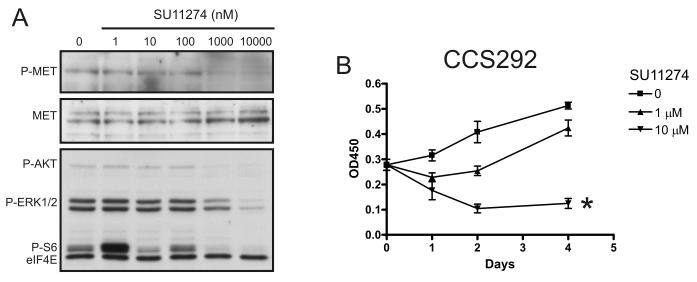
Small molecule inhibitors of c-Met provide an alternative strategy to modulate c-Met. SU11274 is an inhibitor of c-Met (35-37) with activity in both ligand dependent and independent models. Treatment with SU11274 at concentrations reported to inhibit c-Met resulted in a dose-dependent decrease in phospho-c-Met (Fig. 4A). The inhibition of phospho-c-Met was associated with decreased downstream MAPK and AKT phosphorylation (Fig. 4A). We then examined cell proliferation and survival after SU11274 treatment. 1 μM SU11274 transiently decreased cell proliferation (Fig. 4B). However, 10 μM treatment resulted in a sustained decrease in cell proliferation and decreased cell viability. The data using either an inhibitor of HGF (AMG 102) or the c-Met kinase inhibitor (SU11274) suggest that c-Met plays a vital role in a subset of CCS and that its activity plays a dominant role in stimulation of two pathways (PI3K and MAPK) central to cell proliferation and survival.
Fig. 4.
Small molecule inhibition of c-Met. A. CCS292 cells were treated with SU11274 for 6 hours. Extracts were analyzed for phospho-c-Met and total c-Met as well as phospho-AKT, phospho-ERK, phospho-S6. eIF4E serves as loading control. B. CCS292 cells were cultured in the absence or presence of 1 or 10 μM of SU11274. Cell viability was assayed spectrophotometrically with WST-1 (OD450-OD750) * = p = 0.03 (1 way ANOVA).
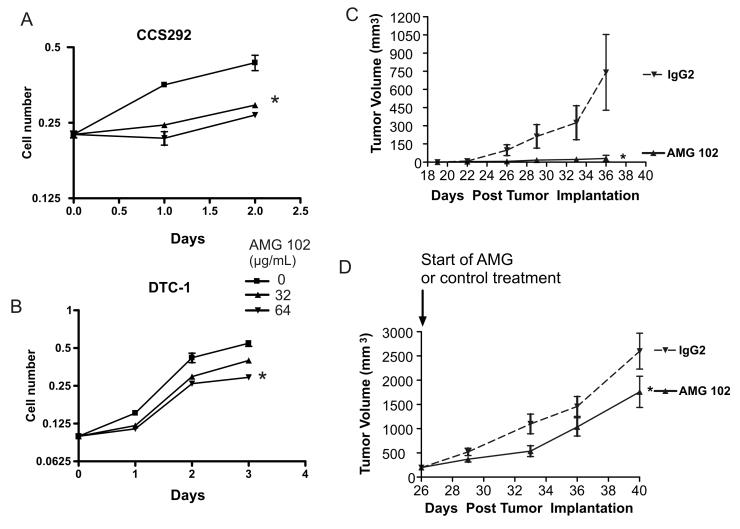
Since HGF -stimulated c-Met activation seems to be a central activator of both survival and proliferation pathways in CCS, we examined the effect of HGF inhibition on tumor cell proliferation in culture and in vivo. We cultured CCS cell lines in the presence of the selective HGF inhibitor, AMG 102. A significant decrease in proliferation was noted in two CCS lines (Fig. 5A, B). CCS292 cells, which express the most HGF, demonstrated the most significant difference with weaker anti-proliferative effects in DTC1. The difference in effect on proliferation correlates with HGF expression (Fig. 2A). For CCS292, the most appreciable inhibition occurred during the first few days of treatment with AMG 102. We then examined the effect of HGF:c-Met inhibition on the progression of CCS tumors in mice. Immunocompromised mice were implanted with CCS292 cells. The effect of AMG 102 treatment was tested employing both established tumors and a minimal disease setting. In the minimal disease setting, treatment with AMG 102 was initiated immediately following tumor cell implantation, whereas in the established tumor model, tumors of approximately 250 mm3 were allowed to develop prior to initiating AMG 102 treatment. Mice were treated twice per week by IP injection of AMG 102 or isotype matched control antibody, and tumor size was measured. Treatment with AMG 102 resulted in significantly decreased growth in both tumor models. In the established tumor model, as a group, tumors in AMG 102 treated mice were 32% smaller (p < 0.005), whereas in the minimal disease setting, much more striking tumor growth suppression was observed (Fig. 5C, D).
Fig. 5.
AMG 102 inhibits CCS proliferation in vitro and in vivo. CCS292 (A) and DTC-1 (B) were cultured in serum-containing growth medium in the absence or presence of 32 or 64 μg/mL of AMG 102. Cell proliferation was assayed by WST-1 assay. Error bars indicate SEM. * = p < 0.01 untreated to 64 μg/mL AMG 102 (t-test). 106 CCS292 cells were subcutaneously injected into one flank site of 40 NCR nude mice. Each treatment group included 10 mice. C. AMG 102 or isotype matched control antibody (IgG2) was administered twice per week beginning at the time of implantation. (* = p < 0.002) D. Mice began treatment with AMG 102 or isotype matched control antibody once the tumors achieved a size of approximately 250 mm3. (* = p < 0.05) Tumor volume was measured twice per week. Error bars indicate SEM.
Discussion
The search for biologically directed therapies for cancer depends on the identification of critical cellular targets in specific tumor types and/or patients. The receptor tyrosine kinase c-Met has been implicated in a growing number of diverse cancers and was shown to be a transcriptional target of the MITF transcription factor in melanocytes (12). We found that a subset of CCS highly expresses the receptor tyrosine kinase c-Met and some of these co-express its ligand HGF. We showed that survival/proliferation as well as invasion and chemotaxis are dependent on c-Met signaling in cellular models of CCS. We found that EWS-ATF1, the product of the pathognomonic translocation associated with CCS, is required for c-Met expression. However, since MITF is also a transcriptional target of EWS-ATF1 target, we cannot exclude the possibility that in conjunction with other putative pathways activated by EWS-ATF1, aberrant MITF expression contributes to c-Met expression. c-Met is activated by autocrine expression of HGF in some of these tumor cell lines. Significant expression of HGF has also been demonstrated in primary CCS tumors, although it is unclear whether HGF was expressed by tumor or stromal cells. The HGF:c-Met axis appears to be a principal activator of intracellular signaling through both MAPK and AKT pathways. Given the unique importance of c-Met as a potential therapeutic target, we demonstrated that CCS is a malignancy with susceptibility to c-Met or HGF inhibition. In the autocrine setting, represented by CCS292, blocking c-Met or HGF function decreased intracellular signaling suggesting that c-Met is the primary regulator of MAPK signaling, even in cells grown in full serum. In vivo, HGF inhibition significantly decreased tumor development and growth in both established and minimal disease settings of CCS.
We examined the tumors that developed despite anti-HGF antibody treatment and found that c-Met was strongly activated in these tumors (data not shown). This result, taken together with the xenograft minimal disease finding, suggests that the antibody most potently inhibits the survival/proliferation of isolated tumor cells or very small tumors. Once the tumor becomes established, the antibody may be no longer capable of inhibiting autocrine signaling. It is possible that the local availability of antibody is insufficient to block the HGF produced by a growing tumor or that the microenvironment of a larger tumor fosters HGF signaling. However, the minimal disease model may mimic the scenario faced by clinicians with a high risk tumor. After resection of a large primary tumor in the absence of gross metastatic disease, microscopic disease often leads to local or distant recurrences and thus such HGF suppression may exhibit efficacy in the adjuvant setting. Targeting MITF-activated c-Met in melanoma could serve a similar therapeutic role (23).
Although it remains to be determined exactly what fraction of CCS tumors exhibit c-Met activation (by either autocrine or paracrine mechanisms), knock-down data suggest that the importance of c-Met to CCS may sometimes be independent of HGF production (i.e. ligand independent receptor activation)(38). In addition, other strategies could result in c-Met activation. For example, in vivo, activation could be mediated through paracrine mechanisms as seen in other tumor types (39). Our study suggests the potential for therapeutically targeting HGF:c-Met in CCS. Pathological interrogation of c-Met expression and phosphorylation status in human tumors should permit selection of patients most likely to respond to HGF:c-Met directed therapy.
Supplementary Material
Acknowledgments
We would like to thank Karen Rex for data analysis, Vivien Igras and Diane Page for assistance with xenograft studies and David Roadcap and James Bear for assistance with invasion assays. This work was supported by grants from NIH (K08CA100400 to IJD) and the Doris Duke Medical Research Foundation (DEF). DEF is Distinguished Clinical Investigator of the Doris Duke Medical Research Foundation and also gratefully acknowledges support from the National Institutes of Health, Adelson Medical Research Foundation and the Melanoma Research Alliance. IJD gratefully acknowledges support from the V Foundation for Cancer Research, the Rita Allen Foundation and the Corn-Hammond Fund for Pediatric Oncology.
References
- 1.Enzinger FM. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163–74. doi: 10.1002/1097-0142(196509)18:9<1163::aid-cncr2820180916>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7:405–13. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear Cell Sarcoma (Malignant Melanoma) of Soft Parts: A Clinicopathologic Study of 30 Cases. Cancer. 1999;86:969–75. [PubMed] [Google Scholar]
- 4.Kawai A, Hosono A, Nakayama R, et al. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109:109–16. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt JJ, Pritchard DJ, Soule EH. Clear cell sarcoma. A clinicopathologic study of 27 cases. Cancer. 1983;52:1482–8. doi: 10.1002/1097-0142(19831015)52:8<1482::aid-cncr2820520825>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Bridge JA, Sreekantaiah C, Neff JR, Sandberg AA. Cytogenetic findings in clear cell sarcoma of tendons and aponeuroses. Malignant melanoma of soft parts. Cancer Genet Cytogenet. 1991;52:101–6. doi: 10.1016/0165-4608(91)90059-4. [DOI] [PubMed] [Google Scholar]
- 7.Stenman G, Kindblom LG, Angervall L. Reciprocal translocation t(12;22)(q13;q13) in clear-cell sarcoma of tendons and aponeuroses. Genes Chromosomes Cancer. 1992;4:122–7. doi: 10.1002/gcc.2870040204. [DOI] [PubMed] [Google Scholar]
- 8.Reeves BR, Fletcher CD, Gusterson BA. Translocation t(12;22)(q13;q13) is a nonrandom rearrangement in clear cell sarcoma. Cancer Genet Cytogenet. 1992;64:101–3. doi: 10.1016/0165-4608(92)90336-7. [DOI] [PubMed] [Google Scholar]
- 9.Zucman J, Delattre O, Desmaze C, et al. EWS and ATF-1 gene fusion induced by t(12:22) translocation in malignant melanoma of soft parts. Nature Genetics. 1993;4:341–5. doi: 10.1038/ng0893-341. [DOI] [PubMed] [Google Scholar]
- 10.Brown AD, Lopez-Terrada D, Denny C, Lee KAW. Promoters containing ATF-binding sites are de-regulated in cells that express the EWS/ATF1 oncogene. Oncogene. 1995;10:1749–56. [PubMed] [Google Scholar]
- 11.Davis IJ, Kim JJ, Ozsolak F, et al. Oncogenic MITF dysregulation in clear cell sarcoma: Defining the MiT family of human cancers. Cancer Cell. 2006;9:473–84. doi: 10.1016/j.ccr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 12.McGill GG, Haq R, Nishimura EK, Fisher DE. c-met expression is regulated by mitf in the melanocyte lineage. J Biol Chem. 2006;281:10365–73. doi: 10.1074/jbc.M513094200. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda M, Davis IJ, Argani P, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–29. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 14.Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell PJ, Cooper CS. The human tpr gene encodes a protein of 2094 amino acids that has extensive coiled-coil regions and an acidic C-terminal domain. Oncogene. 1992;7:2329–33. [PubMed] [Google Scholar]
- 16.Knudsen BS, Vande Woude G. Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev. 2008;18:87–96. doi: 10.1016/j.gde.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Migliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer. 2008;44:641–51. doi: 10.1016/j.ejca.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Segal NH, Pavlidis P, Noble WS, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol. 2003;21:1775–81. doi: 10.1200/JCO.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 19.Puri N, Ahmed S, Janamanchi V, et al. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res. 2007;13:2246–53. doi: 10.1158/1078-0432.CCR-06-0776. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 21.Jeffers M, Schmidt L, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A. 1997;94:11445–50. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–88. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 23.Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–81. [PubMed] [Google Scholar]
- 24.Salvi A, Marchina E, Benetti A, Grigolato P, De Petro G, Barlati S. Germline and somatic c-met mutations in multifocal/bilateral and sporadic papillary renal carcinomas of selected patients. Int J Oncol. 2008;33:271–6. [PubMed] [Google Scholar]
- 25.Graveel C, Su Y, Koeman J, et al. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci U S A. 2004;101:17198–203. doi: 10.1073/pnas.0407651101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp R, Recio JA, Jhappan C, et al. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med. 2002;8:1276–80. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 27.Epstein AL, Martin AO, Kempson R. Use of a newly established human cell line (SU-CCS-1) to demonstrate the relationship of clear cell sarcoma to malignant melanoma. Cancer Research. 1984;44:1265–74. [PubMed] [Google Scholar]
- 28.Du J, Widlund HR, Horstmann MA, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–76. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Wallenius V, Hisaoka M, Helou K, et al. Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors. Am J Pathol. 2000;156:821–9. doi: 10.1016/S0002-9440(10)64950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hisaoka M, Ishida T, Kuo TT, et al. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32:452–60. doi: 10.1097/PAS.0b013e31814b18fb. [DOI] [PubMed] [Google Scholar]
- 31.Naldini L, Tamagnone L, Vigna E, et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. Embo J. 1992;11:4825–33. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann G, Naldini L, Weidner KM, et al. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci U S A. 1992;89:11574–8. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lokker NA, Mark MR, Luis EA, et al. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. Embo J. 1992;11:2503–10. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess T, Coxon A, Meyer S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66:1721–9. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Le P, Liang C, et al. Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol Cancer Ther. 2003;2:1085–92. [PubMed] [Google Scholar]
- 36.Sattler M, Pride YB, Ma P, et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63:5462–9. [PubMed] [Google Scholar]
- 37.Berthou S, Aebersold DM, Schmidt LS, et al. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants. Oncogene. 2004;23:5387–93. doi: 10.1038/sj.onc.1207691. [DOI] [PubMed] [Google Scholar]
- 38.Bergstrom JD, Hermansson A, de Stahl T Diaz, Heldin NE. Non-autocrine, constitutive activation of Met in human anaplastic thyroid carcinoma cells in culture. Br J Cancer. 1999;80:650–6. doi: 10.1038/sj.bjc.6690406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.