Abstract
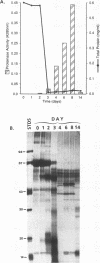
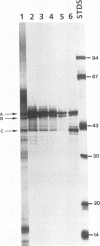
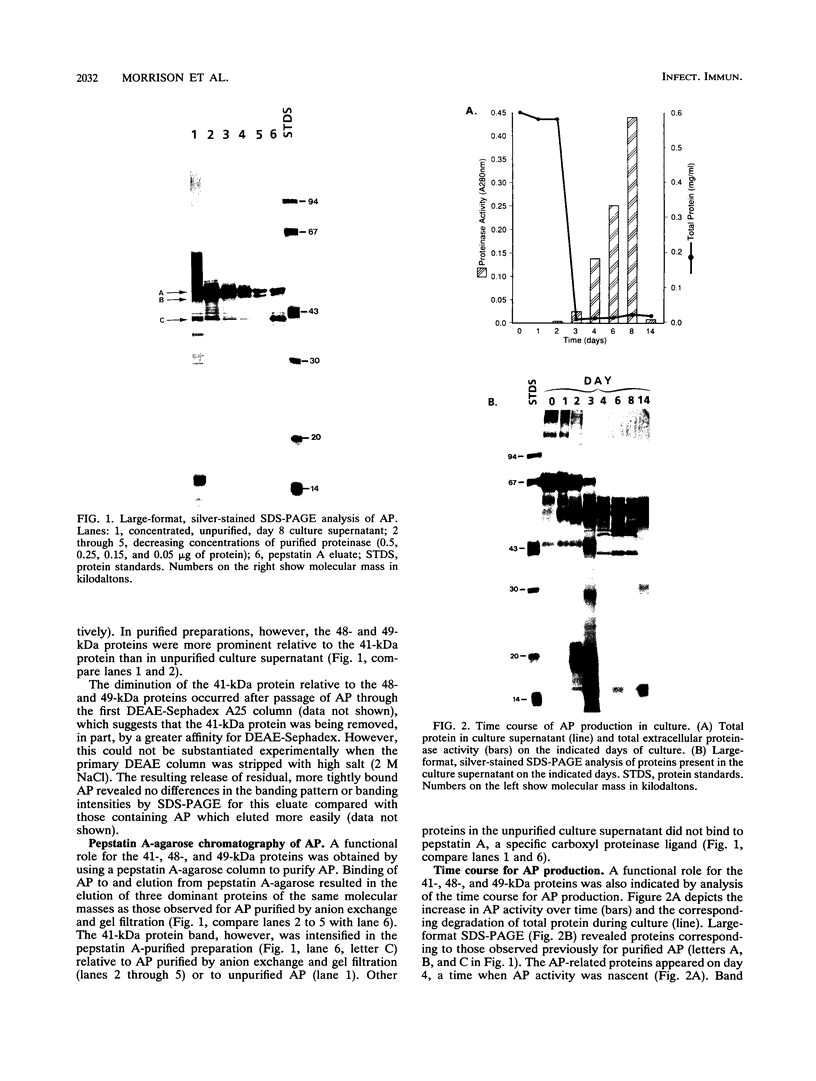
Three dominant proteins (41, 48, and 49 kDa) were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in purified preparations of the extracellular aspartyl proteinase (AP) of Candida albicans. All three proteins bound to the specific carboxyl proteinase ligand, pepstatin A, and were associated with maximum AP activity. The N-terminal amino acid sequence for the 48- and 49-kDa proteins matched that reported by others for AP, whereas the sequence for the 41-kDa protein was unique and was not homologous to any known protein. Time course studies demonstrated the simultaneous presence of all three proteins, supporting evidence that the 41- and 48-kDa proteins were not breakdown products of AP. Previous studies did not detect carbohydrate in SDS-polyacrylamide gels of purified AP preparations stained with periodic acid and silver, making glycosylation an unlikely explanation for the observed differences in the molecular masses of the proteins. Some monoclonal antibodies directed against the 49-kDa protein reacted with the 41- and 48-kDa proteins, indicating cross-reactive epitopes. Other monoclonal antibodies, however, reacted only with the 49-kDa protein. We conclude that three pepstatin A-binding proteins occur in purified AP preparations: two have the same amino acid N terminus as that reported for AP, whereas the third has a unique sequence. All three proteins should be considered when undertaking studies to determine the role of AP in candidal pathogenesis or when preparing specific antibodies for antigen capture assays.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A., Ganesan K., Datta A. Induction of secretory acid proteinase in Candida albicans. J Gen Microbiol. 1991 Oct;137(10):2455–2461. doi: 10.1099/00221287-137-10-2455. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crandall M., Edwards J. E., Jr Segregation of proteinase-negative mutants from heterozygous Candida albicans. J Gen Microbiol. 1987 Oct;133(10):2817–2824. doi: 10.1099/00221287-133-10-2817. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan K., Banerjee A., Datta A. Molecular cloning of the secretory acid proteinase gene from Candida albicans and its use as a species-specific probe. Infect Immun. 1991 Sep;59(9):2972–2977. doi: 10.1128/iai.59.9.2972-2977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Lowe P. A., Lyons A., Thomas P. G., Eaton M. A., Millican T. A., Patel T. P., Bose C. C., Carey N. H., Doel M. T. Molecular cloning and nucleotide sequence of cDNA coding for calf preprochymosin. Nucleic Acids Res. 1982 Apr 10;10(7):2177–2187. doi: 10.1093/nar/10.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Homma M., Kanbe T., Chibana H., Tanaka K. Detection of intracellular forms of secretory aspartic proteinase in Candida albicans. J Gen Microbiol. 1992 Mar;138(3):627–633. doi: 10.1099/00221287-138-3-627. [DOI] [PubMed] [Google Scholar]
- Hube B., Turver C. J., Odds F. C., Eiffert H., Boulnois G. J., Köchel H., Rüchel R. Sequence of the Candida albicans gene encoding the secretory aspartate proteinase. J Med Vet Mycol. 1991;29(2):129–132. [PubMed] [Google Scholar]
- Jarvis W. R., Martone W. J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992 Apr;29 (Suppl A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M., Peterkin V., Reiss E., Morrison C. J. Effect of growth conditions on the extracellular production of the aspartic proteinase by Candida albicans. Adv Exp Med Biol. 1991;306:265–267. doi: 10.1007/978-1-4684-6012-4_31. [DOI] [PubMed] [Google Scholar]
- Lott T. J., Page L. S., Boiron P., Benson J., Reiss E. Nucleotide sequence of the Candida albicans aspartyl proteinase gene. Nucleic Acids Res. 1989 Feb 25;17(4):1779–1779. doi: 10.1093/nar/17.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Morrow B., Srikantha T., Soll D. R. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992 Jul;12(7):2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pohl J., Pereira H. A., Martin N. M., Spitznagel J. K. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990 Oct 15;272(1-2):200–204. doi: 10.1016/0014-5793(90)80484-z. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D. Comparative production and rapid purification of Candida acid proteinase from protein-supplemented cultures. Infect Immun. 1990 Feb;58(2):508–514. doi: 10.1128/iai.58.2.508-514.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D., Morrow B. J. Candida albicans acid proteinase: characterization and role in candidiasis. Adv Exp Med Biol. 1991;306:173–183. doi: 10.1007/978-1-4684-6012-4_22. [DOI] [PubMed] [Google Scholar]
- Remold H., Fasold H., Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968 Oct 8;167(2):399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- Richardson M. D. Opportunistic and pathogenic fungi. J Antimicrob Chemother. 1991 Jul;28 (Suppl A):1–11. doi: 10.1093/jac/28.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Ross I. K., De Bernardis F., Emerson G. W., Cassone A., Sullivan P. A. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of a proteinase-deficient mutant. J Gen Microbiol. 1990 Apr;136(4):687–694. doi: 10.1099/00221287-136-4-687. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Böning B. Detection of Candida proteinase by enzyme immunoassay and interaction of the enzyme with alpha-2-macroglobulin. J Immunol Methods. 1983 Jun 24;61(1):107–116. doi: 10.1016/0022-1759(83)90014-5. [DOI] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Tegeler R., Trost M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia. 1982 Sep;20(3):233–244. doi: 10.1080/00362178285380341. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Uhlemann K., Böning B. Secretion of acid proteinases by different species of the genus Candida. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):537–548. [PubMed] [Google Scholar]
- Rüchel R., de Bernardis F., Ray T. L., Sullivan P. A., Cole G. T. Candida acid proteinases. J Med Vet Mycol. 1992;30 (Suppl 1):123–132. [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Ste-Marie L., Sénéchal S., Boushira M., Garzon S., Strykowski H., Pedneault L., de Repentigny L. Production and characterization of monoclonal antibodies to cell wall antigens of Aspergillus fumigatus. Infect Immun. 1990 Jul;58(7):2105–2114. doi: 10.1128/iai.58.7.2105-2114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Buckley H. R. Production and characterization of three monoclonal antibodies to Candida albicans proteins. Infect Immun. 1984 Mar;43(3):1012–1018. doi: 10.1128/iai.43.3.1012-1018.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Zweibel S. M., Buckley H. R. Identification and molecular weight characterization of antigens from Candida albicans that are recognized by human sera. Infect Immun. 1984 Feb;43(2):715–721. doi: 10.1128/iai.43.2.715-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togni G., Sanglard D., Falchetto R., Monod M. Isolation and nucleotide sequence of the extracellular acid protease gene (ACP) from the yeast Candida tropicalis. FEBS Lett. 1991 Jul 29;286(1-2):181–185. doi: 10.1016/0014-5793(91)80969-a. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]