SUMMARY
Immunity to Mycobacterium tuberculosis in humans and in mice requires interferon gamma (IFNγ). While IFNγ has been studied extensively for its effects on macrophages in tuberculosis, we determined that protective immunity to tuberculosis also requires IFNγ responsive nonhematopoietic cells. Bone marrow chimeric mice with IFNγ-unresponsive lung epithelial and endothelial cells exhibited earlier mortality and higher bacterial burdens than control mice, underexpressed indoleamine-2,3-dioxygenase (Ido) in lung endothelium and epithelium and overexpressed IL-17 with massive neutrophilic inflammation in the lungs. We also found that the products of IDO catabolism of tryptophan selectively inhibit IL-17 production by Th17 cells, by inhibiting the action of IL-23. These results reveal a previously-unsuspected role for IFNγ responsiveness in nonhematopoietic cells in regulation of immunity to M. tuberculosis, and reveal a novel mechanism for IDO inhibition of Th17 responses.
INTRODUCTION
Interferon gamma (IFNγ) is essential to restrict progressive, fatal infection with Mycobacterium tuberculosis. Patients that are either deficient in IFNγ (Fieschi et al., 2003) or are incapable of responding to IFNγ due to mutations in IFNγR1 or IFNγR2 suffer severe tuberculosis (Jouanguy et al., 1997), as well as infections with less virulent species of mycobacteria (Filipe-Santos et al., 2006). Likewise, IFNγ-deficient mice infected with M. tuberculosis die rapidly with high bacterial burdens in the lungs (Cooper et al., 1993; Flynn et al., 1993), implying that IFNγ contributes to the restriction of bacterial growth. Efforts to understand the actions of IFNγ that contribute to restriction and/or killing of M. tuberculosis have been confined to studies of macrophages, since these cells are known to harbor the bacteria in vivo, and since IFNγ was originally characterized as a macrophage-activating factor (Nathan, 1983). Subsequent studies have revealed essential roles for IFNγ-responsive genes such as Nos2 (MacMicking et al., 1997) and Lrg-47 (MacMicking et al., 2003), which provide non-overlapping functions that restrict M. tuberculosis in cultured macrophages and in mice. In addition, IFNγ treatment of cultured macrophages has been shown to promote acidification of mycobacterial phagosomes (Schaible et al., 1998) and autophagy, which can result in limited intracellular killing of M. tuberculosis (Gutierrez et al., 2004). While these studies have advanced our knowledge of the roles of IFNγ in the control of tuberculosis by immune cells, a comprehensive understanding of the contribution of IFNγ to immunity to M. tuberculosis is still lacking.
Since M. tuberculosis infection of cultured macrophages inhibits IFNγ induction of selected genes (Banaiee et al., 2006; Nagabhushanam et al., 2003; Ting et al., 1999), and since IFNγR and the molecules of the JAK/STAT pathway, required for IFNγ signaling, are expressed in diverse cell types, including nonhematopoietic cells such as epithelial and endothelial cells and fibroblasts (Schroder et al., 2004), we hypothesized that control of M. tuberculosis also requires responses to IFNγ by nonhematopoietic cells. To test this hypothesis, we used IFNγR1-deficient and wild type mice to prepare bone marrow chimeric mice whose hematopoietic and nonhematopoietic cells were selectively incapable of responding to IFNγ. We confirmed that early control of M. tuberculosis infection requires IFNγ responsive hematopoietic cells; we found that long-term control of tuberculosis also requires IFNγ-responsive nonhematopoietic cells. In the absence of IFNγR on nonhematopoietic cells, mice succumb to M. tuberculosis infection, with severe inflammation in the lungs. Expression of IFNγ-responsive genes and immunohistochemical analyses revealed that IFNγR-deficient nonhematopoietic cells under-express indoleamine-2,3-dioxygenase in lung epithelium and endothelium during chronic tuberculosis, and this was accompanied by over-expression of IL-17 and massive recruitment of neutrophils to the lungs. These results extend the previously-published observation that control of an intracellular pathogen requires IFNγ-responsive hematopoietic and nonhematopoietic cells when the pathogen invades both cell types (e.g., Toxoplasma gondii) (Yap and Sher, 1999). We thus showed that IFNγ-responsive nonhematopoietic cells are also required for control of M. tuberculosis, which, unlike T. gondii, resides predominantly, if not exclusively, in hematopoietic cells such as macrophages, dendritic cells, and neutrophils (Wolf et al., 2007).
RESULTS
Immune control of tuberculosis requires IFNγ-responsive nonhematopoietic cells
To test the hypothesis that IFNγ contributes to immune control of tuberculosis by effects on cells other than macrophages, we examined the consequences of the absence of IFNγ responsiveness in hematopoietic versus nonhematopoietic cells on survival and pathology after a low-dose aerosol infection of mice with M. tuberculosis.
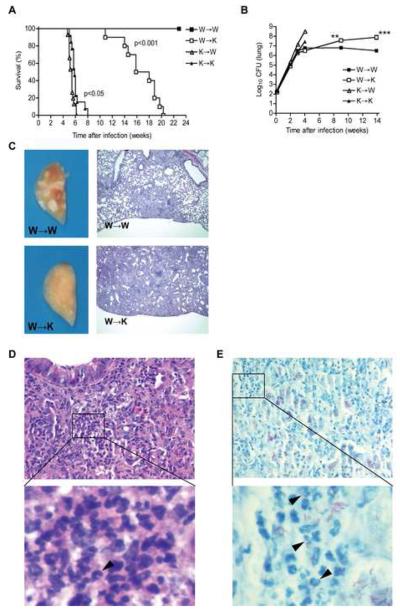
We found that chimeric mice reconstituted with IFNγR1−/− bone marrow cells died soon after infection, regardless of the presence of IFNγR on nonhematopoietic cells (Fig. 1A). Remarkably, wild type mice reconstituted with IFNγR1−/− bone marrow (K→W mice) died significantly earlier than IFNγR1−/− mice reconstituted with IFNγR1−/− bone marrow (K→K mice) (median survival = 5.4 ± 0.2 weeks vs. 5.9 ± 0.3 weeks, p<0.05; fig. 1A). The earlier death of K→W mice was accompanied by higher bacterial burdens in the lungs than in K→K mice as measured 3 weeks (6.4 ± 0.1 vs. 7.2 ± 0.1 log 10; p=0.0018; Fig. 1B and Suppl. Fig. 2A) and 4 weeks post-infection (8.5 ± 0.1 vs. 7.4 ± 0.2 log 10; p=0.0014; Fig. 1B and Suppl. Fig. 2A). The gross lung pathology of K→W mice was also the most severe at that time (Suppl. Fig. 3A) and, although the lesions appeared less extensive at the histological level than in K→K mice, they were more multifocal (Suppl. Fig. 3B). These results provide evidence that when hematopoietic cells, including macrophages, are incapable of responding to IFNγ, the response of nonhematopoietic cells to IFNγ is actually detrimental, as manifest by poorer control of bacterial growth in the lungs. Our observations in K→K and K→W mice also substantiate the long held belief that macrophages need to respond to IFNγ in order to control infection with M. tuberculosis.
Figure 1. Control of long-term M. tuberculosis infection is impaired in the absence of IFNγR1 on nonhematopoietic cells.
(A) Survival of chimeric mice after infection with M. tuberculosis: W→W (n=5), W→K (n=15), K→W (n=15), K→K (n=10). Data are representative of three independent experiments. Groups were compared using a log rank test.
(B) Bacterial load in the lungs of infected chimeric mice as evaluated by plating serial dilutions of lung homogenates on 7H11 agar. Data are representative of three independent experiments and expressed as the mean (± S.E.) of 4 mice per time point and per group. Groups were compared using unpaired Student's t test with a 95% confidence interval. **p<0.01, ***p<0.005. Bacterial loads in the lungs of the respective groups of chimeric mice 3 and 4 weeks post-infection are presented in Supplementary Figure 2A.
(C) Lung gross pathology and histopathology in W→W and W→K mice 14 weeks post-infection with M. tuberculosis. Lung left lobes were fixed in paraformaldehyde for a minimum of 7 days. Histopathology was analyzed by hematoxylin and eosin (H&E) staining of paraformaldehyde-fixed paraffin-embedded 5 mm tissue sections 14 weeks after aerosol infection with M. tuberculosis. Original magnification, 40X.
(D) Neutrophils (arrowhead) infiltrating the lungs of W→K mice as evidenced by H&E staining 14 weeks post-infection. Original magnification, x 40. Insert magnification, x1000. (E) Kinyoun's acid-fast staining with brilliant green counterstaining showing neutrophils infected with M. tuberculosis (arrowheads) in the lungs of W→K mice 14 weeks post-infection. Original magnification, x 40. Insert magnification, x1000.
In contrast to the mice whose hematopoietic cells were incapable of responding to IFNγ, mice whose nonhematopoietic cells were IFNγR1−/− (W→K mice) survived the acute phase of the infection (Fig. 1A). They were no less capable of arresting progressive growth of M. tuberculosis in the lungs 4 weeks after infection than were mice with wild type hematopoietic and nonhematopoietic cells (W→W mice) (Fig. 1B). In fact, they had significantly fewer bacteria in their lungs at week 3 and 4 (Suppl. Fig. 2A). Both groups also displayed small pulmonary lesions at that time (Suppl. Fig. 3). However, the W→K mice succumbed earlier than the W→W mice during the chronic phase of infection (median survival = 16.9 ± 1.1 weeks vs. 27 ± 0.6 weeks, p<0.001). All uninfected bone marrow chimeric mice survived for over 30 weeks, indicating that death of the infected W→K mice was due to M. tuberculosis. To assess the cause of death of the W→K mice, we examined their ability to control growth of M. tuberculosis and found that as the infection progressed beyond its fourth week, M. tuberculosis continued to grow in the lungs of W→K mice while the bacterial burden was maintained at a plateau in W→W mice (Fig. 1B). Consequently, the lung bacterial load in W→K mice surpassed that in W→W mice and by 14 weeks, as the mice of this group began to succumb, the number of mycobacteria in the lungs reached 7.9 ± 0.2 log10; 1.4 log10 higher than in W→W mice at the same time (p<0.001). The W→K mice also had significantly higher bacterial loads in the mediastinal lymph node (p<0.01) (Suppl. Fig. 2B) and the spleen (p<0.01) (Suppl. Fig. 2C) on week 14 post-infection. Gross pathologic examination of the lungs showed that W→W mice had numerous focal subpleural lesions, whereas W→K mice displayed no surface lesions (Fig. 1C). Microscopic examination revealed organized cellular aggregates limited to the peripheral areas of the lungs in W→W mice, whereas the entire pulmonary tissue of W→K mice was diffusely affected, with a marked increase in cellularity (Fig. 1C). Dense infiltrates of polymorphonuclear granulocytes, most likely neutrophils, were observed in the lungs of W→K mice (Fig. 1D). In some areas of the lungs, neutrophils and M. tuberculosis could be visualized in close association (Fig. 1E). Together, these observations indicate that the response to IFNγ in nonhematopoietic cells plays an essential role in the control of M. tuberculosis growth in the lungs after the acute phase of the infection.
Cell recruitment to the lungs of IFNγR1 bone marrow chimeras infected with M. tuberculosis
To determine whether the susceptibility of W→K mice was due to defective activation and/or recruitment of immune cells to the site of infection, we examined the cell populations in the lungs during the course of infection.
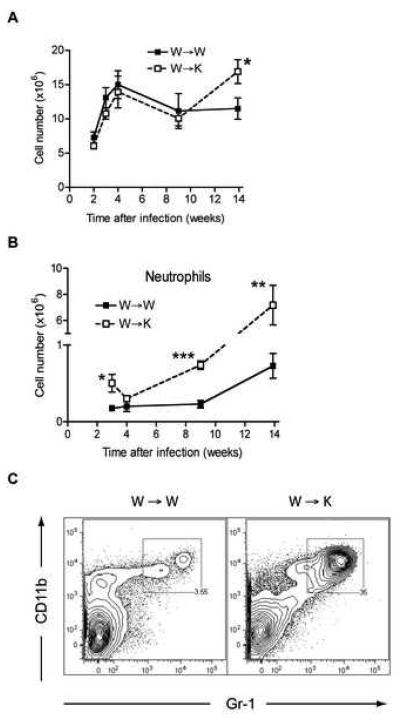
The total number of cells did not differ significantly between W→W and W→K mice for up to 9 weeks of infection with M. tuberculosis (Fig. 2A). During this phase, the number of leukocytes in the lungs increased sharply in both groups, peaked at 4 weeks post-infection then decreased progressively until week 9. After week 9, the total number of cells remained unchanged in the lungs of W→W mice but increased significantly in W→K mice. Analysis of the T cell populations in the lungs of chimeric mice during infection revealed no significant difference in CD4+ or CD8+ T cells between the two groups of mice (Suppl. Fig. 4A). In both groups of mice, the population of CD4+ T cells increased sharply after infection, peaking at 4 weeks then contracting to a plateau during the chronic phase of infection. The number of CD8+ T cells also increased upon M. tuberculosis infection, peaked at 4 weeks but experienced a smaller decline, without any plateau. As we have previously reported (Wolf et al., 2008), CD4+ and CD8+ T cells also increased in number in the mediastinal (lung-draining) lymph node following infection; there was no significant difference in T cell expansion in the lymph node between W→W and W→K mice (data not shown).
Figure 2. Cell populations in the lungs of IFNγR chimeric mice during M. tuberculosis infection.
(A) Total cell number in the lungs and their viability were assessed on single cell suspensions obtained from infected chimeric mice. Viability was higher than 90%. Results are expressed as the mean number (± S.E.) of total cells per lung and for 4 mice per time point and per group. Data were compared using a two-tailed Student's t-test, *p<0.05.
(B) Recruitment of neutrophils to the lungs of W→W and W→K mice during infection with M. tuberculosis. Neutrophils were quantitated by flow cytometry using single cell suspensions stained with anti-CD11b and anti-Gr1 antibodies. Results are expressed as the average number (± S.E.) of CD11bhiGr1hi cells per lung and for 4 mice per time point and per group. Data were compared using a two-tailed Student's t-test, *p<0.05, **p<0.01, ***p<0.005.
(C) Representative dot plots showing the proportion of CD11bhiGr1hi cells in W→W and W→K mice 14 weeks post-infection with M. tuberculosis.
We (Wolf et al., 2007) and others (Gonzalez-Juarrero et al., 2003; Skold and Behar, 2008) have shown that M. tuberculosis induces the recruitment of several subsets of myeloid cells to the lungs. When we compared populations of myeloid cells in the lungs of M. tuberculosis-infected mice, we found that those of W→K mice contained significantly fewer CD11chiCD11bhi myeloid dendritic cells and CD11cloCD11bhi inflammatory/interstitial macrophages during the early stages of infection when compared to the lungs of W→W mice (Suppl. Fig. 4B). The differences between the two groups of mice diminished in the later stages of infection, when these myeloid populations contracted in the W→W mice. There was no significant difference between the two experimental groups in the populations of CD11chiCD11blo alveolar macrophages and CD11c−CD11bhiGr-1− monocytes. During the acute phase of infection, CD11bhiGr-1hi granulocytes were more abundant in lungs of W→K mice than in W→W mice (week 3: 5.0 ± 1.2 × 105 vs. 1.8 ± 0.1 × 105 cells, p=0.031) but by week 4, this population contracted to the same level as in wild type mice (Fig. 2B). At later time points, flow cytometry analysis confirmed the microscopic findings (Fig. 1D and 1E): CD11bhiGr-1hi neutrophils were recruited to the lungs in large numbers. By week 9, the abundance of granulocytes rose sharply in the lungs of W→K mice whereas it remained unchanged in W→W mice (Fig. 2B). At week 14, approximately 2 weeks prior to the average time of death, W→K mice had approximately 30% more cells in their lungs than W→W mice (1.6 ± 0.17 × 107 vs. 1.2 ± 0.17 × 107 cells, p < 0.05), and CD11bhiGr-1hi neutrophils fully accounted for this increase (Fig. 2B and 2C).
Differential gene expression in the lungs of IFNγR chimeric mice during M. tuberculosis infection
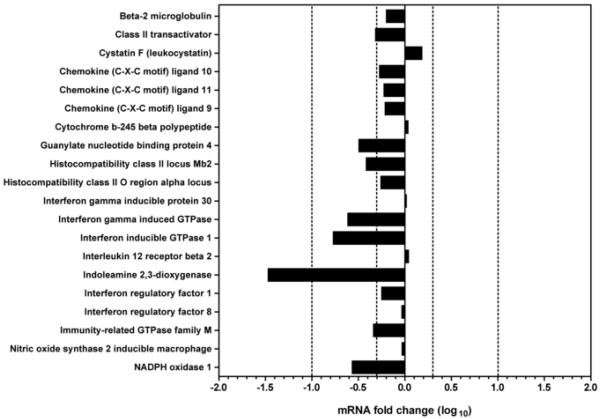
To further understand the mechanisms underlying the immune response to M. tuberculosis in W→K mice, we compared gene expression profiles in the lungs using microarray analysis. We first focused our attention on well-characterized IFNγ-responsive genes (Ehrt et al., 2001; Sana et al., 2005). During the chronic phase of infection, of the 20 IFNγ-responsive genes that we selected and analyzed by microarray, twelve were not differentially expressed between the two experimental groups (i.e., less than 2-fold difference), 7 genes were significantly but moderately (more than 2-fold but less than 10-fold difference) underexpressed in the lungs of W→K mice in comparison to those of W→W mice (Fig. 3). Indoleamine 2,3-dioxygenase (Ido) was the only IFNγ-responsive gene that was underexpressed more than 10-fold: Ido mRNA expression was reduced by 29.4-fold by comparison to W→W mice (p=5 × 10−22) during week 9 post-infection.
Figure 3. Differential expression of IFNγ-responsive genes in the lungs of chimeric mice infected with M. tuberculosis for 9 weeks.
Microarray analysis was conducted on pools of RNA isolated from 4 mice per group and the pools of each group were hybridized against each other. The results are expressed as the fold change in mRNA expression in W→K mice over W→W mice. Dotted lines represent a two-fold change in expression.
To confirm these results using an unbiased set of genes, we performed whole genome expression profiling on the lungs of infected chimeric mice. Of 41,171 genes analyzed, 1,761 were underexpressed and 1,894 genes were overexpressed in W→K mice compared to W→W mice during week 14 post-infection. A large fraction of the underexpressed genes are involved in immune cell recruitment, B cell and NK cell functions and antigen presentation (Table IA), whereas many of the overexpressed genes have roles in tissue remodeling and inflammation (Table IB). At 14 weeks postinfection, Ido remained the most underexpressed gene in the lungs of W→K mice infected with M. tuberculosis; its mRNA was expressed at a level 36.6-fold lower than in the lungs of infected W→W mice (p=2.4 × 10−22).
Table I. Selected genes underexpressed (A) or overexpressed (B) in the lungs of W→K mice compared to W→W mice 14 weeks post-infection with M. tuberculosis.
Microarray analysis was conducted on pools of RNA from 5 mice per group and the pools of each group were hybridized against each other. The results are expressed as fold change in mRNA expression.
| Table IA | ||||
|---|---|---|---|---|
| Name | Sequence Description | Accession # | Fold Change | P-value |
| Indo | Indoleamine-pyrrole 2,3 dioxygenase | NM_008324 | −36.59276 | 2.E−22 |
| Il22 | Interleukin 22 | NM_016971 | −5.74921 | 1.E−06 |
| Igj | Immunoglobulin joining chain | NM_152839 | −5.08559 | 3.E−15 |
| Klrb1c | Killer cell lectin-like receptor subfamily B member 1C | NM_008527 | −4.87152 | 3.E−14 |
| Cr2 | Complement receptor 2 | NM_007758 | −4.40919 | 8.E−13 |
| Bank1 | B-cell scaffold protein with ankyrin repeats 1 | NM_001033350 | −4.16530 | 3.E−05 |
| Igtp | Interferon gamma induced GTPase | NM_018738 | −3.44126 | 1.E−11 |
| Cd79b | CD79B antigen | NM_008339 | −3.30180 | 3.E−11 |
| H2-DMb2 | Histocompatibility 2, class II, locus Mb2 | NM_010388 | −3.18093 | 8.E−11 |
| Ighg | Immunoglobulin heavy chain (gamma polypeptide) | BC092269 | −3.15632 | 8.E−11 |
| Klra10 | Killer cell lectin-like receptor subfamily A, member 10 | NM_008459 | −3.10038 | 8.E−09 |
| Klra6 | Killer cell lectin-like receptor, subfamily A, member 6 | NM_008464 | −3.04309 | 2.E−08 |
| Cd19 | CD19 antigen | NM_009844 | −2.88237 | 7.E−10 |
| Trat1 | T cell receptor associated transmembrane adaptor 1 | NM_198297 | −2.77703 | 7.E−09 |
| Gata3 | GATA binding protein 3 | NM_008091 | −2.73872 | 3.E−09 |
| Cd79a | CD79A antigen (immunoglobulin-associated alpha) | NM_007655 | −2.73226 | 3.E−06 |
| Klra7 | Killer cell lectin-like receptor, subfamily A, member 7 | NM_014194 | −2.71811 | 4.E−09 |
| Blr1 | Burkitt lymphoma receptor 1 | NM_007551 | −2.71112 | 3.E−09 |
| Klra8 | Killer cell lectin-like receptor, subfamily A, member 8 | NM_010650 | −2.64265 | 9.E−09 |
| Ifngr1 | Interferon gamma receptor 1 | NM_010511 | −2.63842 | 6.E−09 |
| C2ta | Class II transactivator | NM_007575 | −2.51527 | 2.E−08 |
| Ccr6 | Chemokine (C-C motif) receptor 6 | NM_009835 | −2.47999 | 3.E−08 |
| Lta | Lymphotoxin A | NM_010735 | −2.41145 | 7.E−15 |
| Cxcr3 | Mus musculus chemokine (C-X-C motif) receptor 3 | NM_009910 | −2.40445 | 7.E−08 |
| Card11 | Caspase recruitment domain family, member 11 | NM_175362 | −2.22414 | 5.E−07 |
| Stat2 | Signal transducer and activator of transcription 2 | NM_019963 | −2.21540 | 6.E−07 |
| Tap1 | Transporter 1, ATP-binding cassette, sub-family B (MDR/TAP) | NM_013683 | −2.12481 | 2.E−06 |
| Stat1 | Signal transducer and activator of transcription 1 | NM_009283 | −2.07716 | 3.E−06 |
| Nox1 | NADPH oxidase 1 | NM_172203 | −2.04629 | 4.E−01 |
| Tbx21 | T-box 21 | NM_019507 | −2.02845 | 5.E−06 |
| Psmb9 | Proteosome subunit, beta type 9 | NM_013585 | −2.01649 | 6.E−06 |
| Table IB | ||||
|---|---|---|---|---|
| Name | Sequence Description | Accession # | Fold Change | P-value |
| Stfa1 | Stefin A1 | NM_001001332 | 35.72946 | 3.E−22 |
| Glycam1 | Glycosylation dependent cell adhesion molecule 1 | NM_008134 | 28.69311 | 2.E−20 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | NM_009140 | 27.19208 | 6.E−22 |
| Camp | Cathelicidin antimicrobial peptide | NM_009921 | 16.35017 | 1.E−20 |
| Il17a | Interleukin 17A | NM_010552 | 14.32831 | 2.E−20 |
| Trem1 | Triggering receptor expressed on myeloid cells 1 | NM_021406 | 9.14956 | 9.E−19 |
| Ccl3 | Chemokine (C-C motif) ligand 3 | NM_011337 | 8.84707 | 1.E−18 |
| Csf3 | Colony stimulating factor 3 (granulocyte) | NM_009971 | 6.82582 | 7.E−16 |
| Il1r2 | Interleukin 1 receptor, type II | NM_010555 | 6.37657 | 9.E−17 |
| Selp | Selectin, platelet | NM_011347 | 5.72550 | 0.E+00 |
| Sele | Selectin, endothelial cell | NM_011345 | 5.48462 | 9.E−14 |
| Ccr1 | Chemokine (C-C motif) receptor 1 | NM_009912 | 4.77916 | 1.E−06 |
| Mmp9 | Matrix metallopeptidase 9 | NM_013599 | 3.95812 | 5.E−34 |
| Cxcl4 | Chemokine (C-X-C motif) ligand 4 | NM_019932 | 3.88045 | 7.E−13 |
| Cxcl5 | Chemokine (C-X-C motif) ligand 5 | NM_009141 | 3.76224 | 1.E−12 |
| Il23a | Interleukin 23, alpha subunit p19 | NM_031252 | 3.74914 | 4.E−06 |
| Il1b | Interleukin 1 beta | NM_008361 | 3.39765 | 3.E−27 |
| Chi3l1 | Chitinase 3-like 1 | NM_007695 | 2.72644 | 3.E−09 |
| Il17f | Interleukin 17F | NM_145856 | 2.71424 | 1.E−02 |
| Irak2 | Interleukin-1 receptor-associated kinase 2 | NM_172161 | 2.66109 | 5.E−09 |
| Tnf | Tumor necrosis factor | NM_013693 | 2.60488 | 3.E−17 |
| Il6 | Interleukin 6 | NM_031168 | 2.56139 | 9.E−17 |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 | NM_008176 | 2.37728 | 9.E−08 |
| Col15a1 | Procollagen, type XV | NM_009928 | 2.37712 | 1.E−07 |
| Cd14 | CD14 antigen | NM_009841 | 2.35198 | 1.E−07 |
| Edn1 | Endothelin 1 | NM_010104 | 2.29886 | 2.E−07 |
| Tlr6 | Toll-like receptor 6 | NM_011604 | 2.17960 | 9.E−07 |
| Tlr4 | Toll-like receptor 4 | NM_021297 | 2.05686 | 2.E−10 |
Characterization of the expression of indoleamine 2,3-dioxygenase in the lungs of IFNγR chimeric mice during M. tuberculosis infection
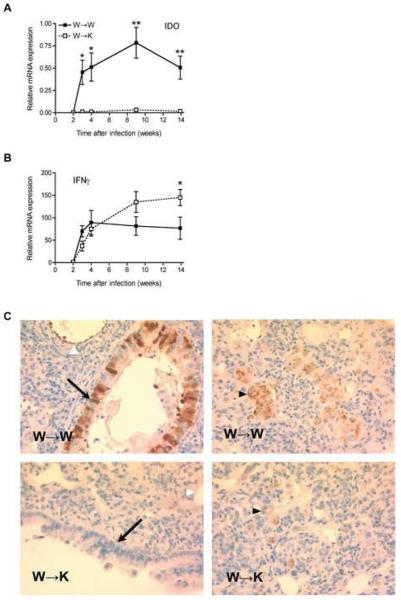
To confirm the results obtained using whole genome expression profiling by microarray, we first quantified the specific expression of Ido mRNA using quantitative real-time PCR during the course of TB infection.
Ido mRNA expression was detected at very low and similar levels in the lungs of W→W and W→K mice after 2 weeks of infection with M. tuberculosis (Fig. 4A). One week later, Ido expression rose sharply in W→W mice and was maintained at a similar level throughout the infection, mirroring the expression of IFNγg mRNA in the lungs of these mice (Fig. 4B). In contrast, Ido expression was 30-fold lower in the lungs of mice whose nonhematopoietic cells are unable to respond to IFNγ, even though the expression of Ifng increased similarly to that in W→W mice after week 3 of infection. These findings imply that IFNγ induces Ido expression by nonhematopoietic cells in the lungs during infection with M. tuberculosis, and are consistent with reports that IFNγ can induce Ido expression in cultured fibroblasts (Pfefferkorn et al., 1986) and epithelial cells (Rapoza et al., 1991). We also confirmed that IFNγ treatment of cultured murine NIH/3T3 fibroblasts induces expression of Ido (Suppl. Fig. 5).
Figure 4. IFNγ-dependent expression of IDO in nonhematopoietic cells is impaired in the lungs of W→K mice infected with M. tuberculosis.
(A-B) Quantitative real-time PCR (qRT-PCR) evaluation of Ido (A) and Ifng (B) mRNA expression in the lungs of chimeric mice after infection with M. tuberculosis. Results are expressed as the average relative level of expression (± S.E.) of specific mRNA after normalization to 18S ribosomal RNA for 4 mice per time point and per group. Data were compared using a two-tailed Student's t-test, *p<0.05, **p<0.01.
(C) Expression of IDO in lung sections of chimeric mice 15 weeks after infection with M. tuberculosis. Positive immunohistochemical staining in airway epithelial (arrows), vascular endothelial (white arrowheads) and myeloid (black arrowheads) cells. Original magnification, x400.
We confirmed the underexpression of IDO at the protein level using immunohistochemistry. In the lungs of W→W mice during the chronic phase of TB, airway epithelial cells and vascular endothelial cells stained intensely with an antibody to murine IDO. In addition, IDO expression was detected in cells localized in granulomas that displayed the morphological characteristics of macrophages and/or dendritic cells (Fig. 4C). In comparison, IDO staining in the lungs of W→K mice 15 weeks post-infection could only be detected in macrophages and/or dendritic cells in granulomas, without any staining of epithelial or endothelial cells (Fig. 4C).
Taken together, these results provide strong evidence that IFNγ-dependent expression of IDO in nonhematopoietic lung cells contributes significantly to the overall expression of IDO in the lungs of mice chronically infected with M. tuberculosis.
In the absence of IFNγR on nonhematopoietic cells, mice develop an excessive IL-17 response to M. tuberculosis
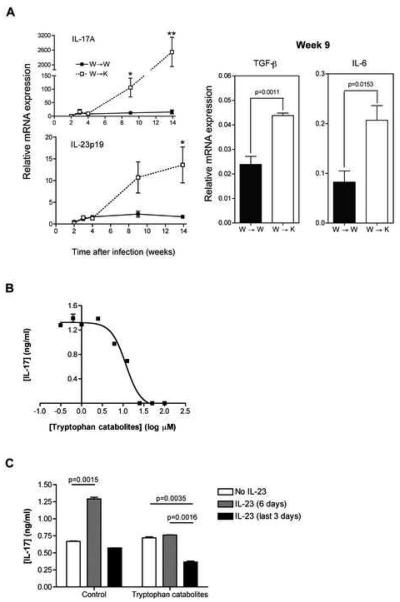
Whole genome expression profiling of the lungs of chimeric mice chronically infected with M. tuberculosis also revealed overexpression of numerous genes involved in inflammation (Table IB). Since we observed an exuberant inflammatory response, including recruitment of neutrophils, during the chronic stage of infection of W→K mice, we characterized the expression of IL-17A in detail, and analyzed the time course of IL17A expression in the lungs of M. tuberculosis-infected mice using quantitative real-time PCR. We also quantitated expression of genes involved in the generation or maintenance of IL-17-secreting cells, i.e. Il6, Tgfb1 and Il23a (Bettelli et al., 2007). The level of expression of Il17a mRNA in the lungs of W→W and W→K mice was low and equivalent until 4 weeks of infection with M. tuberculosis (Fig. 5A). By week 9 post-infection, less than two weeks before the W→K mice started to die, IL17A expression increased more than 10-fold in the lungs of these mice but remained at the same low level in W→W mice. By 14 weeks of infection, expression of IL17A in W→K mice was 163-fold higher than in W→W mice.
Figure 5. Il17a expression during M. tuberculosis infection in vivo and its regulation by kynurenines in vitro.
(A) qRT-PCR evaluation of Il17a, Il23a, Tgfb1 and Il6 mRNA expression in the lungs of chimeric mice after infection with M. tuberculosis. Results are expressed as the average relative level of expression (± S.E.) of specific mRNA after normalization to 18S ribosomal RNA for 4 mice per time point and per group. IL17a and Il23a mRNA expression was quantitated 3, 4, 9, and 14 weeks post-infection, Tgfb1 and Il6 mRNA expression was assayed on samples harvested on week 9 post-infection. Data were compared using a two-tailed Student's t-test, *p<0.05, **p<0.01 or indicated value.
(B) Dose response of IL-17 production by differentiating Th17 cells in vitro in the presence of increasing concentrations of tryptophan catabolites (L-kynurenine, 3′-hydroxy-DL-kynurenine, 3′-hydroxyanthranilic acid, anthranilic acid and quinolinic acid). A nonlinear regression with variable slope analysis was applied using Prism software (GraphPad) to determine an IC50 value of 11.7±1.1 μM.
(C) IL-17 production by differentiating Th17 cells in vitro in the presence of 15 μM of tryptophan catabolites after 6 days of culture, in the absence of IL-23 (white bars), in the presence of IL-23 for the last 3 days (grey bars) or for 6 days of culture (black bars).
Each condition was assayed in triplicate. The results are expressed as the mean concentration (± S.E.) of IL-17 in the culture supernatants after 6 days of incubation as measured by ELISA and are representative of two independent experiments.
Role of kynurenines in regulating development of Th17 cells
Since we observed marked underexpression of IDO in the lungs of infected W→K mice, and since IDO catabolizes tryptophan to products (collectively termed kynurenines) with immunoregulatory properties (Munn and Mellor, 2007), we determined whether defective generation of tryptophan catabolites might account for the overexpression of IL17A in the lungs. First, we examined the effects of tryptophan catabolites on the development of Th17 cells in vitro.
An equimolar solution of L-kynurenine, 3′-hydroxy-DL-kynurenine, 3′-hydroxyanthranilic acid, anthranilic acid and quinolinic acid caused dose-dependent inhibition of IL-17 production by CD4+ T cells under Th17 differentiating conditions, which was detectable at a concentration of 7.5 μg/ml; complete inhibition was observed at a concentration of 25 μM for the mixture of tryptophan catabolites (Fig. 5B). In these conditions, nonlinear regression analysis revealed an IC50 value of 11.7 ± 1.1 μM (10.4 ± 1.1 μg/ml). When these compounds were tested individually, 3′-hydroxyanthranilic acid was the most potent inhibitor of IL-17 production, with an IC50 of 27.7 ± 3.7 μM, followed by 3′-hydroxy-DL-kynurenine (IC50 = 68.1 ± 1.4 μM) and L-kynurenine (IC50 = 100.8 ± 1.0 μM) (Table II). Anthranilic acid and quinolinic acid had no inhibitory effect within the range of concentrations assayed. The observation that the IC50 for the mixture is lower than for any of the individual compounds suggests that two or more of the individual compounds have distinct targets that affect Th17 differentiation and/or IL-17 secretion. We found that kynurenines were able to inhibit IFNγ production during Th1 differentiation but required substantially higher concentrations (Table II): the most potent inhibitor was 3′-hydroxyanthranilic acid, with an IC50 of 57.6 ± 1.0 μM. The combination of catabolites did not reduce further the production of IFNγ (IC50 = 57.9 ± 1.3 μM), therefore, the combination of tryptophan catabolites was approximately 5-fold more potent for inhibition of Th17 versus Th1 differentiation. In light of our observation that W→K mice also exhibit poorer control of M. tuberculosis growth late in infection (Fig. 1B), we examined kynurenines for the ability to inhibit growth of M. tuberculosis in vitro (Supplemental Fig. 5). While we found that kynurenines inhibited growth of M. tuberculosis at concentrations previously reported to inhibit growth of E. coli, the effective concentrations were approximately 20-fold higher than the concentrations that inhibited Th17 differentiation.
Table II. 3′-hydroxyanthranilic acid is the most potent tryptophan catabolite for the inhibition of IL-17 production by Th17 cells in vitro.
Inhibiting concentrations (IC50) of L-kynurenine, 3′-hydroxy-DL-kynurenine, 3′-hydroxyanthranilic acid, anthranilic acid or quinolinic acid on IFNγ and IL-17 production by differentiating Th1 and Th17 cells in vitro respectively. The results are expressed as the average IC50 values (± S.E.), calculated using a nonlinear regression with variable slope (Prism software, GraphPad).
| IC50 (μM) | ||
|---|---|---|
| Tryptophan metabolites | Th1 | Th17 |
| L-Kynurenine | 154.5 ± 1.2 | 100.8 ± 1.0 |
| 3′-Hydroxy-DL-kynurenine | 153.8 ± 1.1 | 68.1 ± 1.4 |
| 3′-Hydroxyanthranilic acid | 57.6 ± 1.0 | 27.7 ± 3.7 |
| Anthranilic acid | No inhibition | No inhibition |
| Quinolinic acid | 111.5 ± 1.4 | No inhibition |
| All | 57.9 ± 1.3 | 11.7 ± 1.1 |
Since we also observed that IL-23p19 was overexpressed in the lungs of M. tuberculosis-infected W→K mice, and since IL-23 promotes development and maintenance of Th17 cells in peripheral tissues as well as in lymph nodes (McGeachy et al., 2009), we tested the hypothesis that tryptophan catabolites inhibit the action of IL-23 during Th17 differentiation. We confirmed that IL-23 enhanced production of IL-17 under the conditions of our assay, and found that tryptophan catabolites completely abrogated the effect of IL-23, even when they were only included during the latter stages of Th17 differentiation (Figure 5C).
DISCUSSION
While IFNγ is essential for immune control of M. tuberculosis, its targets and functions are still incompletely understood. Since macrophages are thought to be a major cellular reservoir for M. tuberculosis, previous studies have focused on the effects of IFNγ on these cells, and Nos2 and Lrg47/Irgm1 are the only two IFNγ-dependent macrophage effector genes known to be essential for the control of tuberculosis (MacMicking et al., 1997; MacMicking et al., 2003).
In the studies reported here, we provided the first direct evidence that macrophages (and possibly other hematopoietic cells) must be able to respond to IFNγ in vivo in order to control growth of M. tuberculosis during the early, acute stage of infection. However, we also found that nonhematopoietic cells must also be responsive to IFNγ for durable control of bacterial growth and survival of the host. We demonstrated that at least one of the mechanisms involving IFNγ-responsive epithelial and endothelial cells is the expression of IDO and the regulation of IL-17 expression and subsequent neutrophilic inflammation in the lungs.
During the acute stage of infection, the adaptive immune response and its capacity to limit bacterial growth was unaffected by the absence of IFNγR on nonhematopoietic cells. In accord with the observation that IFNγ induces cultured epithelial cells to express the chemokines CXCL9, CXCL10, and CXCL11 (Sauty et al., 1999), we observed a transient deficit in the expression of these IFNγ-inducible chemokines, as well as CCL5 (data not shown), but this had no effect on the recruitment of CD4+ and CD8+ T cells to the lungs in W→K mice. The earliest detectable difference between M. tuberculosis-infected W→K and W→W mice was a defect in recruitment and/or differentiation of myeloid dendritic cells and interstitial macrophages in the lungs. This defect, which was detectable by week 3 post-infection, could actually explain the lower bacterial burden that we consistently observed in the lungs of W→K mice at that time. Indeed, a reduced recruitment of potential target cells, such as dendritic cells and macrophages, has been shown to negatively influence the growth of mycobacteria (Davis and Ramakrishnan, 2009). However, this cellular deficiency was a transient phenomenon and by the ninth week of infection, the number of recruited macrophages and myeloid dendritic cells in the lungs was similar between both experimental groups.
Although there were only few measurable differences in the response to M. tuberculosis in W→K compared with W→W mice during the early stages of infection, W→K mice subsequently exhibited poorer control of bacterial growth and selective overexpression of IL-17 and neutrophilic inflammation in the lungs. These observations could have at least two potential general explanations. One is that nonhematopoietic cells in the lungs are underappreciated as cellular reservoirs of M. tuberculosis, and that IFNγ controls growth of the bacteria that reside in these cells. Despite considerable in vitro data showing that M. tuberculosis can be taken up by cultured epithelial and endothelial cells (Bermudez and Goodman, 1996; Debbabi et al., 2005; Mehta et al., 2006), and some evidence that intact M. tuberculosis can be detected in lung epithelial cells in experimental infections (Rivas-Santiago et al., 2008; Teitelbaum et al., 1999), the majority of the existing evidence strongly favors macrophages and dendritic cells in the lungs as the major cellular reservoirs for M. tuberculosis (Wolf et al., 2007). In our present studies, acid-fast staining of the lungs of W→K mice also indicated that most of the bacteria were associated with cells having the morphology of macrophages and/or dendritic cells, and at later stages of infection, bacteria were also associated with neutrophils. Therefore, the in vivo significance of nonhematopoietic cells as reservoirs of M. tuberculosis remains controversial, and it appears unlikely that the course of infection in W→K mice was due to uncontrolled replication of M. tuberculosis in lung epithelial or endothelial cells. In light of the evidence that macrophages and dendritic cells are the major reservoirs of M. tuberculosis, any IFNγ-inducible antimycobacterial activity provided by nonhematopoietic cells would likely be achieved through secreted factors. Although nonhematopoietic cells can respond to M. tuberculosis by secreting TNF, GM-CSF, and MCP-1 (CCL2) (Lin et al., 1998) and they can produce numerous antimicrobial products (Evans et al., 2009), few of these are dependent on IFNγ signaling, e.g. inducible nitric oxide synthase, β-defensins, NADPH oxidases, and indoleamine 2,3-deoxygenase. Among those, we found that indoleamine 2,3-deoxygenase (IDO) was the most underexpressed IFNγ-responsive gene in W→K mice during chronic TB, and that airway epithelial cells and vascular endothelial cells were the major source of this enzyme at that time. IDO catalyzes the first step of the degradation pathway of tryptophan and is highly induced by IFNγ in a wide variety of cells (Carlin et al., 1989). Its antimicrobial activity was originally described as a consequence of tryptophan depletion (Pfefferkorn, 1984). Such mechanism is unlikely to account for our observations, since M. tuberculosis is capable of synthesizing tryptophan (Parish and Stoker, 2002). More recent data indicate that the tryptophan catabolites generated by IDO, or kynurenines, possess broad spectrum bacteriostatic activity (Narui et al., 2009), although we found that inhibition of M. tuberculosis required kynurenine concentrations that are at least 20-fold higher than the concentrations that inhibit Th17 differentiation in vitro. Likewise, since macrophages and dendritic cells in both W→W and W→K mice are competent to respond to IFNγ and express IDO in vivo (Fig. 4C), it is unlikely that kynurenines mediate antimycobacterial activity strictly in an intracellular environment. Other IFNγ-inducible molecules, expressed in non-hematopoietic cells and with a potential role in bacteriostasis include inducible nitric oxide synthase (NOS2) and members of the β-defensin family. The expression level of these genes in the lungs did not suggest any involvement in the mortality experienced by infected W→K mice. Lastly, the expression of NADPH oxidase Nox1, also produced by non-hematopoietic cells in response to IFNγ and implicated in the control of bacterial infection (Leto and Geiszt, 2006; Robbins et al., 1994), was only moderately reduced in W→K mice. The importance of Nox1 in the control of M. tuberculosis is currently unknown but mice deficient in the closely related phagocyte NADPH oxidase Nox2 do not succumb to TB (Nathan and Shiloh, 2000).
The second general mechanism that may account for the premature death of W→K mice involves dysregulation of the immune response during the second month of infection with M. tuberculosis. While recent evidence indicates that IL-10 contributes to regulation of immunity to TB during the chronic stage of infection (Higgins et al., 2009), we did not observe any defect in IL-10 expression in infected W→K mice. However, this report emphasizes the importance of immune regulation in the late stage of infection with M. tuberculosis. Coincidentally, IDO has been increasingly implicated in the regulation of the immune response in cancer, transplantation, autoimmunity, allergies and chronic infections (Brandacher et al., 2008; Katz et al., 2008; Le and Broide, 2006; Zelante et al., 2009). Much attention has been focused on the production of IDO by regulatory dendritic cells (Mellor and Munn, 2004) but several lineages of nonhematopoietic cells derived from human lungs have been reported to regulate T cell proliferation via IDO-dependent mechanisms in vitro (Heseler et al., 2008). In our studies, Ido mRNA was detectable at high levels in the lungs throughout infection with M. tuberculosis but the expression of the protein, as revealed by immunohistochemistry, was limited to myeloid cells during the acute phase (data not shown). Maximum IDO expression appeared after nine weeks of infection, in an IFNγR-dependent fashion and in cells of nonhematopoietic origin, i.e. airway epithelial cells and vascular endothelial cells. This pattern suggests the existence of tissue specific, post-transcriptional regulation mechanisms and it may explain why IDO is only required during the later stages of M. tuberculosis infection. The observation that epithelial and endothelial cells in the lungs of W→K did not express detectable amounts of IDO at a time when IDO was readily detected in epithelial and endothelial cells of W→W mice provides strong evidence that these nonhematopoietic cells in the lungs were of recipient, and not donor origin. This is consistent with the published observation that bone marrow cells do not differentiate into lung epithelial cells at a detectable frequency (Kotton et al., 2005).
Two other significant observations in chronically infected chimeric mice were the dramatic increase in IL-17 expression, induction of CXCL2, and the massive influx of neutrophils in the lungs, both starting at week 9 and peaking at week 14 of infection. The role of IL-17 on neutrophil recruitment has been extensively studied (Linden et al., 2005), but the relationship between M. tuberculosis infection and IL-17 has only been recently examined (Khader and Cooper, 2008). Most evidence reported to date has indicated a beneficial role for IL-17 in immunity to M. tuberculosis. In particular, IL-17 production is required for a protective memory response following subunit vaccination (Khader et al., 2007). In addition, gene delivery of IL-23 has been shown to positively influence the outcome of M. tuberculosis infection (Happel et al., 2005). One study showed that infection of IFNγ−/− mice with BCG leads to increased frequency of IL-17 producing cells but the investigators did not report the outcome of this imbalance in terms of pathology or bacterial control (Cruz et al., 2006). In our experiments, given the timing and the amplitude of the neutrophilic inflammation associated with the expression of IL-17 in infected W→K mice, it is highly likely that dysregulation of IL-17 expression contributes to the death of W→K mice, through extensive tissue damage and subsequent impairment of respiratory function. Unchecked Th17 responses have been associated with several deleterious inflammatory conditions. In particular, a murine model of the severe inflammation observed in chronic granulomatous disease (CGD) patients infected with Aspergillus fumigatus revealed an excessive Th17 response provoked by the impaired conversion of tryptophan into kynurenines (Romani et al., 2008). In accordance with this observation, we found that individual kynurenines have a direct and additive inhibitory effect on the development of Th17 cells in vitro, at concentrations that have been found in vivo during viral pneumonia (Christen et al., 1990). This effect on Th17 differentiation was selective, and at least in part mediated by inhibition of the effects of IL-23; while kynurenines also exerted some inhibitory effect on Th1 differentiation, much higher concentrations were required.
We therefore propose a model in which IFNγR1−/− nonhematopoietic cells, unable to respond to IFNγ during the chronic phase of M. tuberculosis infection, fail to express the enzyme IDO and to initiate the conversion of tryptophan to kynurenines. The ongoing bacterial replication characterizing chronic TB in the murine model (Gill et al., 2009) provides sustained pro-inflammatory signals which, in the absence of kynurenines, triggers excessive production of IL-17 and lethal lung neutrophilia.
Since the human response to infection with M. tuberculosis can vary greatly, from asymptomatic latent infection to progressive lung destruction with formation of pulmonary cavities and extensive disability among survivors (de Valliere and Barker, 2004), evidence that defective expression of IDO in pulmonary parenchymal cells, with overexpression of IL-17 and excessive inflammation contributes to fatal infection in mice, provides an opportunity to determine whether these mechanisms contribute to the morbidity and mortality in humans with tuberculosis.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 congenic CD45.1+ wild type (W) and CD45.2+ IFNgR1−/− (K) mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). They were bred as homozygotes and maintained under specific pathogen-free conditions at the New York University Medical Center (NYUMC, New York, NY). For infections with Mycobacterium tuberculosis, mice were housed under barrier conditions in the ABSL-3 facility at NYUMC. All mice used were females, between 8 and 12 weeks of age at the beginning of the experiment. For tissue harvest, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. All experiments were performed with the prior approval of NYUMC Institutional Animal Care and Use Committee.
Generation of bone marrow chimeras
Donor wild type and IFNγR1−/− mice were euthanized using CO2 asphyxiation and cervical dislocation, and femurs and tibias were removed aseptically. Bone marrow was flushed with cold DMEM (Invitrogen), supplemented with 10% heat-inactivated FCS (Invitrogen) and 2 mM L-glutamine (Invitrogen). Cells were washed twice with PBS without calcium and magnesium (Invitrogen), supplemented with 1% FCS. The suspension was depleted of mature T cells by treatment with microbeads coated with anti-Thy1.2 antibody (Miltenyi Biotec) followed by magnetic activated cell sorting according to the manufacturer's recommendations. Viable cells were counted in a hemacytometer using a Trypan blue exclusion assay. Recipient wild type and IFNgR1−/− mice were irradiated with 10 Gy in split doses at 2-hour intervals. They were reconstituted no later than 6 hours after the last irradiation with 2 × 106 wild type (W→W and W→K mice) or IFNγR1−/−(K→W and K→K mice) cells by intravenous injection. Mice were given sulfamethoxazole (150 mg/ml) and trimethoprim (30 mg/ml) in drinking water for the first 3 weeks of reconstitution. Chimeras were used no earlier than 6 weeks after transplantation. Prior to infection with M. tuberculosis, we confirmed that mice reconstituted with congenic bone marrow stem cells had achieved a satisfactory level of chimerism by assessing the number of CD45.1+ (wild type) and CD45.2+ (IFNγR1−/−) leukocytes in the lungs, using flow cytometry (Suppl. fig. 1). In this organ, the proportion of donor-derived leukocytes averaged 86.6±1% over multiple experiments, after the transfer of either wild type or IFNγR1−/− marrow, and did not vary over time after M. tuberculosis infection. The same result was found in the spleen (data not shown).
Bacterial infection
The H37Rv strain of Mycobacterium tuberculosis was grown as previously described (Banaiee et al., 2006). Chimeras were infected via the aerosol route, using an inhalation exposure system (Glas-Col) (Wolf et al., 2007), calibrated to deliver 150 colony forming units (CFU) per animal. The infectious dose was confirmed on day 1 by plating whole lung homogenates from 5 sentinel mice on Middlebrook 7H11 agar. CFU were counted after incubation at 37° C for 2-3 weeks. To determine the bacterial load throughout the infection, the right lung, MLN and spleen were harvested from chimeric mice, homogenized and serial dilutions were plated on Middlebrook 7H11 agar.
Histology and immunohistochemistry
The left lung was excised, fixed in 4% paraformaldehyde (PFA) for one week at room temperature then embedded in paraffin. Sections of 5 μm were cut and stained with hematoxylin and eosin. Alternatively, sections were treated with an acid-fast Kinyoun's stain to reveal the presence of M. tuberculosis and counterstained with Brilliant Green.
For indoleamine 2,3-deoxygenase (IDO) immunostaining, PFA-fixed, paraffin-embedded lung sections were deparaffinized using CitriSolv solution (Fisher Scientific) and rehydrated by successive baths of decreasing ethanol concentration. Endogenous peroxidase activity was blocked by treatment with 3% H2O2. Endogenous avidin/biotin activity was blocked using a commercially available kit (Vector Laboratories). Non-specific binding sites were saturated by a solution of bovine serum albumin 2%. IDO was stained using a polyclonal rabbit anti-murine IDO antibody (Alexis Biochemicals). Subsequently, the sections were incubated with biotinylated polyclonal goat anti-rabbit IgG, streptavidin-conjugated horseradish peroxidase and DAB chromogenic substrate (Vector Laboratories). The tissues were counterstained with hematoxylin, dehydrated and mounted using PerMount solution (Fisher Scientific).
Cell isolation and flow cytometry
The right lung as well as the MLN were removed and processed for flow cytometry as previously described (Wolf et al., 2007). Antibodies conjugated to various fluorophores and directed against the following surface markers were used: CD4 (RM4-5, BioLegend), CD8α (53-6.7, BD Biosciences), CD62L (MEL-14, BioLegend), CD45RA (14.8, BioLegend), Dx5 (BD Biosciences), B220 (RA3-6B2, BD Biosciences), CD19 (6D5, BioLegend), CD11c (HL3, BD Biosciences), CD11b (M1/70, BD Biosciences), Gr-1 (RB6-8C5, BD Biosciences), CD45.2 (104, BD Biosciences) A minimum of 200,000 events per sample, gated on live cells using forward and side scatter parameters, was acquired using an LSRII and the FACSDiva software (BD Biosciences). Data were analyzed using the FlowJo software (TreeStar).
Microarray analysis
Total RNA was extracted from the left lung using TriZol reagent (Invitrogen) and was processed as previously described for removal of contaminating genomic DNA and reverse transcription (Banaiee et al., 2006). RNA integrity of individual samples was confirmed by ribosomal RNA profiles at the Genomics Facility of the Cancer Institute of NYU Langone Medical Center. RNA samples of each group of mice were pooled and the resulting pools were hybridized against each other for differential gene expression analysis using Agilent Whole Mouse Genome Oligo Microarray (Miltenyi Biotec).
Real-time quantitative RT-PCR
Total RNA was extracted from lung tissues as described above. The cDNA equivalent of 50 ng of total RNA was analyzed for specific gene expression in triplicate for each sample by quantitative real-time PCR using Platinum SYBR Green qPCR SuperMix (Invitrogen) on an MJ Research Opticon 2. Sequences of the primer pairs can be found in Supplementary Table I. Thermal cycling conditions were 95°C for 10 min then 40 cycles at 94°C for 45 s, 58°C for 30 s and 72°C for 30 s. For quantitation, the relative values were determined by comparing the threshold cycle C(t) of each sample to a standard curve consisting of serial dilutions of a positive control cDNA sample. Results were normalized using 18S rRNA expression as an internal standard for each sample.
Cell culture and inhibition studies
For the in vitro differentiation of Th17 cells, CD4+ T cells were isolated from spleen and lymph nodes of C57BL/6 mice followed by magnetic cell sorting using anti-CD4+ antibody-coated microbeads (Miltenyi Biotec). Cells were re-suspended in RPMI supplemented with 10% FBS, hamster anti-murine CD3 antibody (0.25 μg/ml), hamster anti-murine CD28 antibody (1 μg/ml), anti-murine IL-4 neutralizing antibody (1 μg/ml), anti-murine IFNγ neutralizing antibody (1 μg/ml), recombinant murine IL-6 (20 ng/ml, PeproTech), recombinant human TGFβ (0.5 μg/ml, PeproTech) and recombinant murine IL-23 (20 ng/ml, eBioscience). All antibodies were purchased from BioLegend. The cells were seeded at a density of 105 cells/well in 96-well plates pre-coated with 0.12 μg/ml of goat anti-hamster IgG antibody (Vector Laboratories) and cultured for 6 days at 37°C in 5% CO2. In some experiments, IL-23 was omitted or only added on the third day of culture. The concentration of IL-17 in culture supernatants was measured by ELISA (R&D Systems).
For comparison studies with Th1 cells, T cells were differentiated in the same in vitro conditions, with only anti-murine IL-4 neutralizing antibody, recombinant murine IL-2 (10 ng/ml, eBioscience) and recombinant murine IL-12p70 (20 ng/ml, BD Biosciences) and CD3/CD28 stimulation. The concentration of IFNγ in culture supernatants was measured by ELISA (BD Biosciences).
L-kynurenine, 3′-hydroxy-DL-kynurenine, 3′-hydroxyanthranilic acid, anthranilic acid and quinolinic acid (Sigma) were dissolved in RPMI medium under agitation at 1mM concentration and sterile filtered. Tryptophan metabolites were added individually or in combination, in two-fold dilutions, to the differentiating medium of Th1 or Th17 cells for the 6 days of culture.
Statistical analysis
Results are expressed as mean and standard error. Student's two-tailed t-test was used to compare experimental groups, unless otherwise stated, with P <0.05 considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Juan J. LaFaille for invaluable assistance in making these studies possible, and for helpful comments on the manuscript. We also thank Eleanor Allen, Everett Hayes, and Sabriya Stukes for expert technical assistance. Supported by NIH grants R03 AI053074 and R01 AI059667.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR, Jr., Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J Immunol. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infection and immunity. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nature immunology. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Brandacher G, Margreiter R, Fuchs D. Clinical relevance of indoleamine 2,3-dioxygenase for alloimmunity and transplantation. Curr Opin Organ Transplant. 2008;13:10–15. doi: 10.1097/MOT.0b013e3282f3df26. [DOI] [PubMed] [Google Scholar]
- Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989;45:535–541. doi: 10.1007/BF01990503. [DOI] [PubMed] [Google Scholar]
- Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Valliere S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:767–771. [PubMed] [Google Scholar]
- Debbabi H, Ghosh S, Kamath AB, Alt J, Demello DE, Dunsmore S, Behar SM. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274–279. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G, Nathan C. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. The Journal of experimental medicine. 2001;194:1123–1140. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Scott BL, Clement CG, Larson DT, Kontoyiannis D, Lewis RE, Lasala PR, Pawlik J, Peterson JW, Chopra AK, et al. Stimulated Innate Resistance of Lung Epithelium Protects Mice Broadly Against Bacteria and Fungi. American journal of respiratory cell and molecular biology. 2009 doi: 10.1165/rcmb.2008-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. The Journal of experimental medicine. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Seminars in immunology. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of experimental medicine. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Happel KI, Lockhart EA, Mason CM, Porretta E, Keoshkerian E, Odden AR, Nelson S, Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infection and immunity. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heseler K, Spekker K, Schmidt SK, MacKenzie CR, Daubener W. Antimicrobial and immunoregulatory effects mediated by human lung cells: role of IFN-gamma-induced tryptophan degradation. FEMS immunology and medical microbiology. 2008;52:273–281. doi: 10.1111/j.1574-695X.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Lee EJ, Orme IM, Gonzalez-Juarrero M. Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2009;89:149–157. doi: 10.1016/j.tube.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, et al. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. The Journal of clinical investigation. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature immunology. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. American journal of respiratory cell and molecular biology. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AV, Broide DH. Indoleamine-2,3-dioxygenase modulation of allergic immune responses. Curr Allergy Asthma Rep. 2006;6:27–31. doi: 10.1007/s11882-006-0006-7. [DOI] [PubMed] [Google Scholar]
- Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang M, Barnes PF. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infection and immunity. 1998;66:1121–1126. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science (New York, N.Y. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nature immunology. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PK, Karls RK, White EH, Ades EW, Quinn FD. Entry and intracellular replication of Mycobacterium tuberculosis in cultured human microvascular endothelial cells. Microb Pathog. 2006;41:119–124. doi: 10.1016/j.micpath.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. The Journal of clinical investigation. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J Immunol. 2003;171:4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- Narui K, Noguchi N, Saito A, Kakimi K, Motomura N, Kubo K, Takamoto S, Sasatsu M. Anti-infectious activity of tryptophan metabolites in the L-tryptophan-Lkynurenine pathway. Biol Pharm Bull. 2009;32:41–44. doi: 10.1248/bpb.32.41. [DOI] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish T, Stoker NG. The common aromatic amino acid biosynthesis pathway is essential in Mycobacterium tuberculosis. Microbiology. 2002;148:3069–3077. doi: 10.1099/00221287-148-10-3069. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986;6:267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- Rapoza PA, Tahija SG, Carlin JP, Miller SL, Padilla ML, Byrne GI. Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Invest Ophthalmol Vis Sci. 1991;32:2919–2923. [PubMed] [Google Scholar]
- Rivas-Santiago B, Contreras JC, Sada E, Hernandez-Pando R. The potential role of lung epithelial cells and beta-defensins in experimental latent tuberculosis. Scandinavian journal of immunology. 2008;67:448–452. doi: 10.1111/j.1365-3083.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- Robbins RA, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Adcock IM, Riveros-Moreno V, Moncada S, Polak J, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochemical and biophysical research communications. 1994;198:835–843. doi: 10.1006/bbrc.1994.1119. [DOI] [PubMed] [Google Scholar]
- Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- Sana TR, Janatpour MJ, Sathe M, McEvoy LM, McClanahan TK. Microarray analysis of primary endothelial cells challenged with different inflammatory and immune cytokines. Cytokine. 2005;29:256–269. doi: 10.1016/j.cyto.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Skold M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol. 2008;181:6349–6360. doi: 10.4049/jimmunol.181.9.6349. [DOI] [PubMed] [Google Scholar]
- Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, McMurray DN, Bloom BR. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–3906. [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. The Journal of experimental medicine. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. The Journal of experimental medicine. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 2009;11:133–141. doi: 10.1016/j.micinf.2008.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.