Abstract
Purpose
Ovarian cancer (OvCa) recurrence with development of paclitaxel resistance is an obstacle to long term survival. We demonstrated that secretory leukocyte protease inhibitor (SLPI) is a survival factor for OvCa. We hypothesize SLPI may antagonize paclitaxel injury.
Experimental design
Differential SLPI induction in response to paclitaxel, and response to stable forced expression of SLPI was demonstrated in A2780-1A9 cells and their paclitaxel-resistant sublines, PTX10 and PTX22 and confirmed with HEY-A8 cells. SLPI-mediated survival was reduced by the MEK inhibitor, U0126 and a humanized neutralizing monoclonal anti-SLPI antibody, CR012. OVCAR3 xenographs tested the role of CR012 in vivo.
Results
SLPI expression was lower in A2780-1A9 OvCa cells than PTX10 and PTX22 and SLPI was induced by paclitaxel exposure. Stable SLPI expression yielded a proliferation advantage (p=0.01); expression of and response to SLPI in OVCAR3 cells was abrogated by exposure to CR012. SLPI reduced paclitaxel susceptibility of 1A9 and HEY-A8 cells (p≤0.05) and SLPI expression did not increase resistance of PTX10 and −22 cells. Both paclitaxel and SLPI overexpression induced ERK activation. Inhibition of MEK with U0126 increased paclitaxel injury and overcame SLPI-mediated cell protection. It did not reinstate PTX10 sensitivity to paclitaxel, which was associated with AKT activation. Significant inhibition of OVCAR3 xenograft growth was observed with CR012 and paclitaxel, over single agents (p≤0.001).
Conclusions
A two-pronged approach confirmed SLPI overcomes paclitaxel in part through activation of ERK1/2. These results credential SLPI as a molecular target for OvCa and suggest CR012 as a tool for proof of concept.
Keywords: Secretory leukocyte protease inhibitor (SLPI), ovarian cancer, paclitaxel, resistance
INTRODUCTION
Ovarian cancer is the most lethal gynecologic malignancy and the fifth most common cause of cancer-related deaths in women. The estimated number of new ovarian cancer cases in 2008 in the U.S. was 21,650 with an estimated 15,520 deaths (1). The standard treatment for epithelial ovarian cancer consists of cytoreductive surgery followed by a paclitaxel- and platinum-based chemotherapy regimen (2, 3). Paclitaxel-resistance develops leading to treatment failure and death. Mechanisms of drug resistance are complex and may differ between cancers; they may be due to altered pharmacokinetics, tumor micro-environment and/or cancer-cell specific biology and biochemistry (4). Proteins that affect apoptosis, growth-factor and cytokine signaling, and cell-cycle behavior also have been implicated in drug resistance. Signaling proteins with suggested roles in paclitaxel resistance include p53, cyclins, mitogen activated protein kinases (MEK/ERK), and AKT. We sought to examine the role of a novel ovarian cancer growth and survival factor, secretory leukocyte protease inhibitor (SLPI) (5–11) in the paclitaxel resistance of ovarian cancer cells.
SLPI is an 11.7 kDa whey acidic protein (WAP) that is both genomically and transcriptionally upregulated in ovarian cancer at the 20q12-13 WAP locus (12), and many breast cancers (13–16). It is secreted by the mucosal surfaces of the respiratory, gastrointestinal, and reproductive tracts. Its most well known activity is related to its alarm anti-protease (17, 18) and anti-inflammatory properties protecting normal mucosal tissues from the degradative actions of serine proteases, such as elastase, trypsin, and chymotrypsin (19–23). SLPI has an important function in carcinogenesis (7) and has been associated with aggressive and malignant ovarian tumors (15). We have recently described SLPI as a growth and survival factor for ovarian cancer cells in a manner not dependent upon its anti-protease activity (10, 11). We hypothesized that SLPI’s pro-growth and survival behavior would provide protection against paclitaxel-mediated cell injury.
The Ras/Raf/MAPK pathway couples signals from cell surface receptors to transcription factors, regulating gene expression and cellular activity. This cascade triggers or induces many proteins involved in proliferation, differentiation and apoptosis (24). Extracellular signal regulated kinases 1/2 (ERK1/2) are serine/threonine kinases that are activated upon phosphorylation by MEK1/2. Paclitaxel has been shown to transiently activate ERK and AKT in CaOV3 ovarian cancer cells (25). More activated ERK1/2 was demonstrated in paclitaxel-resistant hematopoietic cells (26). These observations suggest that ERK activation enhances survival of paclitaxel-exposed cells. We further hypothesized that the survival function of SLPI would yield protection against paclitaxel through activation of the ERK pathway.
We report SLPI is upregulated in human ovarian cancer cells upon exposure to paclitaxel. Further, overexpression of SLPI decreases susceptibility of A2780 and HEY-A8 human ovarian cancer cells to paclitaxel in a MEK/ERK-dependent fashion. Conversely, exposure of OVCAR3 human ovarian cancer xenografts to neutralizing anti-SLPI antibody increased susceptibility to paclitaxel. These results indicate that high levels of endogenous SLPI and further SLPI induction by paclitaxel are associated with paclitaxel resistance and further credentials SLPI as a molecular target in ovarian cancer.
MATERIALS AND METHODS
Cells, culture, transfection
OVCAR3, 4, 8. IGROV1, and SKOV3 human OvCa cells were obtained from ATCC (Manassas, VA). 1A9-A2780 human OvCa parental cell line and its two paclitaxel-resistant sublines, PTX10 and PTX22 were a generous gift from Dr. A. Fojo (NCI) (27) and the HEYA-8 cells from Dr. G. Mills (MD Anderson). The IC50 of paclitaxel in PTX10 and PTX22 cells was reconfirmed at 47 and 48nM, respectively, compared to 2nM for their parental 1A9 line. A2780 cell lines were grown in serum and 25mM HEPES-supplemented RPMI and OVCAR3, 4, 8, IGROV1, and SKOV3 cells in serum-supplemented DMEM, unless otherwise indicated. PTX10 and PTX22 cells were maintained in added 15ng/mL paclitaxel and 5μg/mL verapamil with drug removal 5–7 days prior to the start of an experiment. 3′ HA-tagged SLPI plasmid was generated and transfected as described (11). Cells were propagated in bulk and maintained in medium containing G418 sulfate 1mg/mL except for the passage immediately prior to experimental use. Cells from at least two separate independent transfection series were studied. Stable expression was monitored by immunoblot against the HA-tag. Reduced protease inhibitory activity of the clones has been reported (11). Treatment doses and durations of paclitaxel and U0126 (EMD, Gibbstown, NJ), controlled with DMSO (≤0.01%), and/or rhSLPI (R&D Systems, Minneapolis, MN) are indicated. SLPI concentration was measured using Quantikine Human SLPI Immunoassay kit (R&D Systems) per manufacturer’s instructions after 30X concentration.
Human monoclonal anti-SLPI
The human IgG2-bearing XenoMouse strain was immunized twice weekly by footpad injection with soluble human SLPI (Abgenix, Freemont, CA). Antigen-specific antibodies were selected from up to 2.5 million antibody-producing B cells per immunized mouse. Then, B cells producing antibodies were recovered as described (28) after applying a microplate-based assay to measure and rank antibodies according to binding affinity to hSLPI and measurement of trypsin neutralizing activity.
Western blot and immunoprecipitation
Total cell lysates were prepared with modified RIPA lysis buffer and subjected to immunoprecipitation as described (10). Conditioned medium (CM) was collected, centrifuged to remove cellular debris, aliquoted and frozen at −80°C after the addition of protease inhibitors (aprotinin 10μg/mL, leupeptin 10μg/mL and PMSF 1mM) and where indicated concentrated using centrifugal filtration. Lysates and CM underwent no more than one freeze/thaw cycle. Protein samples were resolved by SDS-PAGE electrophoresis and immunoblotted as described. SLPI rabbit antipeptide antibodies have been reported previously (10). ERK, phospho (p)-ERK, Akt and p-Akt antibodies were from Cell Signaling (Danvers, MA) and p-p38 from Santa Cruz Biotechnology (Santa Cruz, CA). The HA-tag antibody was from Sigma Aldrich (St. Louis, MO). Blots were replicated at least 3 times.
Elastase assay
EnzChek Protease Assay Kit (Molecular Probes, Eugene, OR) was used to measure elastase and trypsin activity. Briefly, 30nM elastase (Calbiochem, La Jolla, CA) was incubated with or without recombinant 170nM SLPI, with or without CR012 (0-240nM), in 100ul of 1x digestion buffer (10mM Tris-HCl, pH 7.8, 0.1mM sodium azide). The substrate BODIPY casein was then added and samples were incubated for 1hour, protected from light according to manufacturers instructions. Fluorescence as a measure of cleavage was read at excitation = 480 ± 25 nm and emission = 530 ± 25 nm.
Flow cytometry
Flow cytometry was done using standard procedure without permeabilization on cells harvested with buffered EDTA and with permeabilization for cell cycle analysis with propidium iodide. Cells were stained 1hr with CR012 240nM or IgG2 isotype and counterstained with peroxidase-conjugated donkey anti-human IgG 1:500 (H+L; Jackson Immunolabs, West Grove, PA). After 30min, cells were washed with FACS buffer and fixed with 1% formaldehyde in PBS. Analysis was done using a FACS Calibur™ flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Proliferation and clonogenic assays
Three proliferation assays were done, XTT (Roche Diagnostics, Indianapolis, IN), trypan blue exclusion, and clonogenic assays. Trypan blue-excluding cell number was determined at successive 24 hour timepoints. For XTT assays, cells were pulsed with paclitaxel for 6 hours and cellular proliferation was assessed 96 hours later. XTT reagent was added for 6 hr prior to quantification of absorbance. Note that the susceptibility to paclitaxel with this pulse approach is different between PTX10 and PTX22 cells. Cells were pretreated with the ERK inhibitor UO126 for 1 hour prior to paclitaxel treatment; drugs were maintained through the assay. OVCAR3, 4, 8, IGROV1, and SKOV3 cells were used for the CR012 clonogenic assays. Recombinant SLPI 170nM was added to serum-containing medium 24 hr after plating, concomitant with CR012 or control IgG. Cells were harvested with trypsin, mixed 1:1 with growth medium and then an aliquot transferred to a culture dish for 7 days. Colonies were stained with crystal violet solution. The data were presented as % of untreated control; data represent mean of 3 separate studies.
Confocal fluorescence microscopy
Cells were grown overnight on 0.1% gelatin-coated slides, serum starved for 24 or 48 hours, fixed in 3.7% formaldehyde and permeabilized with 0.1% Triton X-100. Coverslips were stained with anti-SLPI antibodies and mounted with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Immunofluorescent images were acquired on a Zeiss 510 LSM confocal microscope at 63x/1.4 NA with an oil DIC objective and a scan zoom of 1.
Immunohistochemistry
Formalin fixed, paraffin embedded mouse xenograft specimens (5 um sections) were stained with CR012 or IgG2 isotype control after standard rehydration in graded ethanol and quenching of endogenous peroxides. Stain was developed using DAB reagent, counterstained with hematoxylin, and dehydrated through alcohol dilution series and xylene prior to coverslip.
Xenograft studies
OVCAR3 tumors were passaged in female CB.17 SCID mice at an AAALAC-certified facility (Piedmont Research Center, Morrisville, NC). Mice (n=40) were implanted subcutaneously with 20–30 mg fragments of OVCAR3 tumor xenografts. Mice were randomized into 4 groups (from 31 mice with tumors) when the tumors reached ~80–120 mm3. IgG2 control and CR012 (1mg/kg) were given intravenously once every 4 days for 4 treatments; paclitaxel (7.5 mg/kg) was given intravenously every other day for a total of 5 treatments. Tumor length and width by caliper, and mouse body weight were measured twice weekly. Tumor volume (TV) was calculated as: TV = (width2 × length)/2. Median tumor volumes were plotted as a function of days. Mice were euthanized when tumors reached ~750 mm3. Tumor growth delay was calculated as increase in time-to-endpoint (TTE) expressed in days calculated as TTE (days) = [log10 (endpoint volume)−b]/m, where b is the intercept and m is the slope of the line obtained by linear regression of a log-transformed tumor growth data set.
Statistical analyses
Experimental differences were tested for statistical significance using ANOVA and Student’s t test. A two-sided p-value of ≤0.05 was considered to be statistically significant.
RESULTS
SLPI increases cell proliferation
SLPI is expressed in most ovarian cancer cells of the NCI 60 cell line screen by U133A expression array (Supplemental Fig. 1). We previously reported SLPI expression, and proliferative and survival activity in OVCAR3, HEYA-8, and SKOV3 ovarian cancer cell lines (10, 11) and now include the A2780-1A9 line and its paclitaxel-resistance sublines, PTX10 and PTX22. Forced expression of HA-tagged SLPI in the 1A9 and PTX10 lines resulted in a small but significant increase in proliferation (Fig. 1A, p≤0.05). Cell cycle analysis confirmed increased cycling cells with a 50% increase in S phase fraction for both 1A9-and PTX10-SLPI compared against controls (p<0.01, 0.03, respectively). CR012, a mouse monoclonal neutralizing anti-SLPI antibody, recognized SLPI in OVCAR3 cell lysates and on cell membranes by flow cytometry (Fig. 1B). SLPI has been demonstrated by our group and others to inhibit serine protease activity. Neutralizing activity of CR012 was confirmed by demonstration that CR012 plus SLPI abrogated the protease inhibition of SLPI against elastase (Fig. 1C). Anti-proliferative activity of CR012 against the OVCAR3 cells was also demonstrated alone against endogenous SLPI and also when recombinant SLPI was included in the culture (Fig. 1D). SLPI expression was examined by IHC in cell lines and qualitative expression graded as ++, + or − as high, medium or no-expression, respectively. Response of OVCAR3, OVCAR4, OVCAR8, IGROV1, SKOV3 cells to CR012 was related to SLPI expression (supplementary table 1). High SLPI expressing OVCAR3 cells were most sensitive to CR012 (IC50 90nM; maximum cell kill 78%) whereas a SLPI non-expressing SKOV3 line was insensitive.
Figure 1. SLPI increases cell proliferation.
A. Forced expression of HA-tagged SLPI stimulates growth. Inset shows HA tag expression. P-values represent the statistical significance for the entire curve. B. CR012 recognizes SLPI. CR012 is credentialed by its recognition SLPI by immunoprecipitation/immunoblot in OVCAR3 lysates (lanes: 1-SLPI antisense-treated cells, 2-scramble-treated, 3-transfection medium control, 4-no exposure), and it illuminates membrane-bound SLPI (B; blue: untreated; green: IgG2 control; red: CR012, 2ug/mL). C, D. CR012 is a neutralizing anti-SLPI antibody. CR012 blocks SLPI inhibition of elastase activity in vitro (C) and reduces proliferation in the presence or absence of added SLPI (D). IgG2 isotype control is used to show specificity. Representative data of at least three independent experiments are shown.
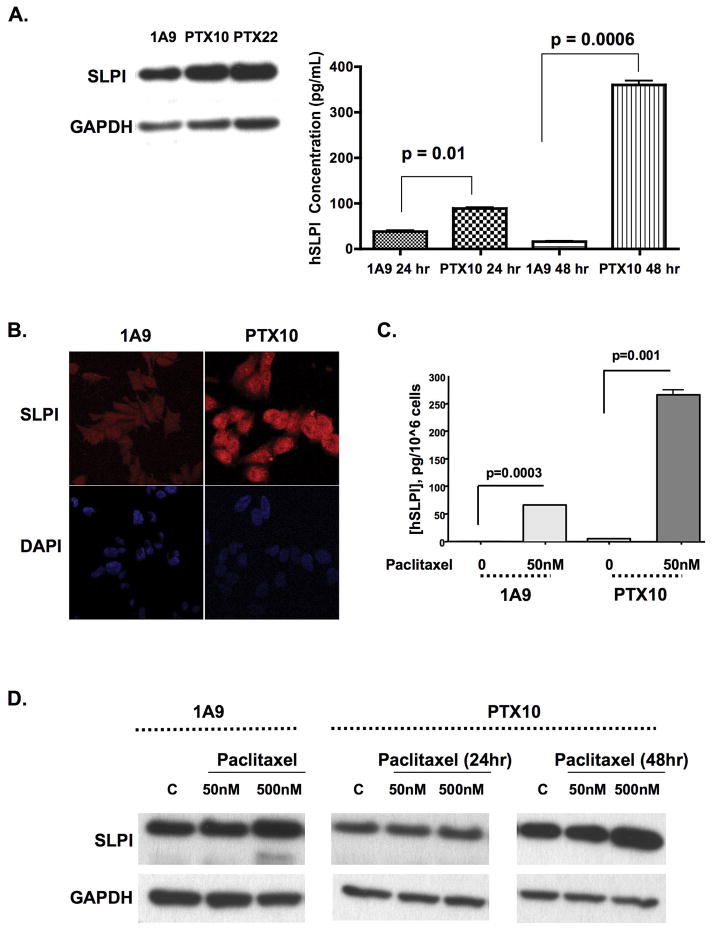
Paclitaxel-resistant cells have increased basal expression of SLPI protein
We hypothesized that SLPI would function as a survival factor against paclitaxel treatment, implying also that SLPI would be upregulated in paclitaxel-resistant cells. The paclitaxel-resistant sublines, PTX10 and PTX22, produce and secrete more SLPI in their CM than their 1A9 parental counterparts (Fig. 2A; p≤0.01). These results were reinforced by confocal microscopy, showing increased SLPI expression in PTX10 compared with 1A9 cells (Fig. 2B). We next examined whether short term paclitaxel exposure induced SLPI. Secreted SLPI was induced by paclitaxel exposure in both 1A9 and PTX10 cells (Fig. 2C; p≤0.001). Immunoblot confirms changes in secreted SLPI with dose and time of exposure to paclitaxel. Prolonged paclitaxel exposure at either dose could not be done with the 1A9 cells due to profound cell loss. (Fig. 2D). These data demonstrate that SLPI is upregulated in response to treatment with or resistant to paclitaxel.
Figure 2. Paclitaxel exposure and resistance stimulate SLPI in ovarian cancer cells.
SLPI is upregulated in paclitaxel-resistant cells. (A) Increased SLPI production and secretion in lysates (immunoblot) and CM (ELISA) in samples collected after 24hr serum starvation. Immunofluorescence (B) confirms upregulation and similar cellular localization. C, D. Exposure to paclitaxel stimulates cellular SLPI (D, immunoblot) and CM (E, ELISA). PTX10 cells were exposed to 50 or 500nM paclitaxel over 24 and 48hr; 1A9 cells were only treated for 24hr. Conditioned medium SLPI concentration is expressed as pg/106 cells to normalize for cell number. Representative data of at least three independent experiments are shown.
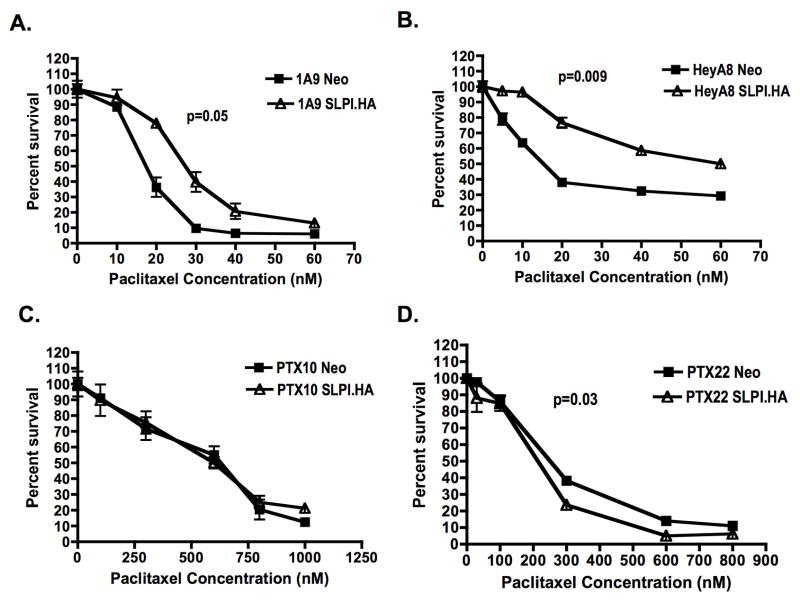
SLPI overexpression confers paclitaxel resistance to wild type but not paclitaxel-resistant cells
The effects of forced SLPI overexpression on paclitaxel susceptibility in 1A9 and PTX10/22 cells was examined. SLPI-transfected 1A9 were statistically significantly less sensitive to a 6hr paclitaxel pulse (Fig. 3A; p=0.05), with 78 v. 36% survival at 20nM paclitaxel. This was tested in HeyA8 cells confirming increased paclitaxel resistance in SLPI-HeyA8 transfectants (Fig. 3B; p=0.009). However, no effect was observed in SLPI-PTX10 cells (Fig. 3C). A paradoxical increased sensitivity was seen in SLPI-PTX22 cells (Fig. 3D; p=0.03). PTX10 and PTX22 cells have a higher basal expression of SLPI and further upregulation of this protein did not confer additional protection from paclitaxel, suggesting a threshold effect.
Figure 3. Overexpression of SLPI confers paclitaxel resistance to wild type but not paclitaxel-resistant cells.
Cells were pulsed with paclitaxel at 0–70nM (A, B) or 1000nM (C, D) for 6hr then allowed to grow for 4d. (A) 1A9; (B) HeyA8; (C) PTX10; (D) PTX22. Representative data of at least three independent experiments are shown. P-values represent the statistical significance for the entire curve.
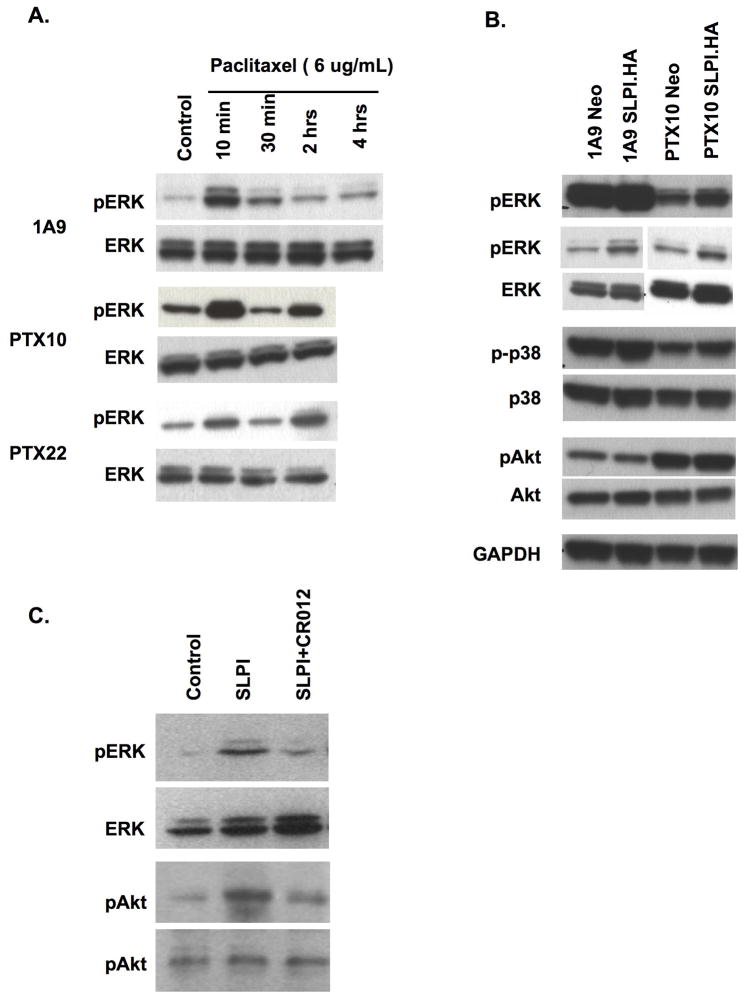
SLPI and paclitaxel treatment upregulate ERK activation
Next, the A2780 cell series was pulsed with higher doses of paclitaxel for up to 4 hr and activation of ERK assessed. A transient activation of ERK was observed in the 1A9 cells as early as ten minutes into exposure with a biphasic effect seen in resistant cells, concomitant with the known behavior of ERK with paclitaxel resistance (Fig. 4A). No clear activation of p38 was seen and no effect was observed on JNK (not shown). In contrast, increased activation of AKT was seen only in the paclitaxel-resistant cells (Fig. 4B). OVCAR3 cells had upregulation of both pERK and pAKT upon SLPI treatment which was reversed by co-exposure to CR012 (Fig. 4C). CR012 treatment also reduced activation of pAKT, induced in the OVCAR3 cells exposed to SLPI. SLPI overexpression was not associated with AKT activation in the A2780 cell set.
Figure 4. Both paclitaxel treatment and transfection of SLPI.HA activate ERK.
A. Paclitaxel pulse induces phospho-ERK. Paclitaxel (6ug/mL) was pulsed over 10min to 4hr prior to cell harvesting. A biphasic activation is observed in the resistant cells. B. Paclitaxel and SLPI induce ERK activation, but only paclitaxel resistance is associated with activation of AKT in the A2780 cell set. Two panels of pERK are presented, independent experiments, one showing a darker view and one a shorter exposure. Representative data of at least three independent experiments are shown. GAPDH represents the loading control. C. CR012 reduces activation of ERK and AKT.
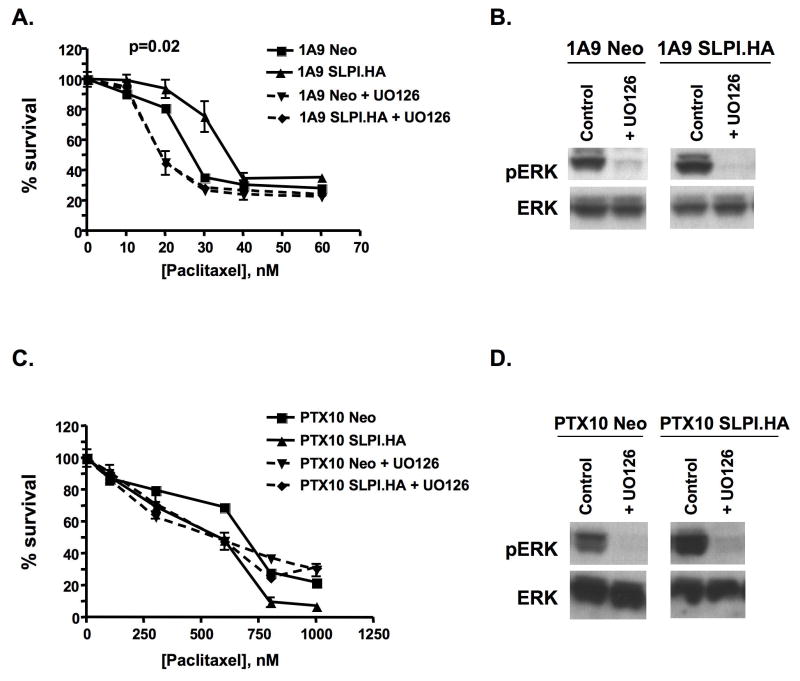
Treatment with the MAPK inhibitor, UO126 reverses paclitaxel resistance caused by SLPI overexpression
In the previous experiments, we showed SLPI overexpression conferred paclitaxel resistance and ERK activation in 1A9 cells. This suggested that MEK inhibition would overcome SLPI effects. We examined this with the MAPK inhibitor, UO126 (Fig. 5). Cells were pretreated with 10uM UO126 prior to paclitaxel treatment and continuously maintained during the assay duration. UO126 completely reversed SLPI-induced paclitaxel resistance in 1A9 SLPI.HA cells collapsing the 1A9-Neo and 1A9-SLPI growth curves below the basal 1A9 phenotype (p=0.02; Fig. 5A). Immunoblot demonstrated loss of pERK in these UO126-treated cells (Fig. 5B). No significant changes were noted in PTX10 Neo and SLPI.HA cells exposed to UO126, suggesting that resistance in these cells is not dependent on the ERK pathway, although ERK activation is reduced (Fig. 5C,D).
Figure 5. The MAPK inhibitor, UO126, reverses the SLPI-mediated paclitaxel resistance only in wild type cells.
A, C. Paclitaxel survival curves and downregulation of pERK with U0126 (10μM) in 1A9 or PTX10 cells. Neo and SLPI.HA cells were pretreated with UO126 for 1hr then UO126 was maintained throughout the assay. After the first hour, cells had a 6hr pulse of paclitaxel; the XTT assay was performed 4d later. B, D. Immunoblot demonstration of loss of pERK activation. Western immunoblot demonstrates ERK and pERK expression in cells treated with UO126 for 24hr in serum-free medium. Representative data of at least three independent experiments are shown. P-values represent the statistical significance for the entire curve.
Downregulation of SLPI with CR012 augments paclitaxel effects
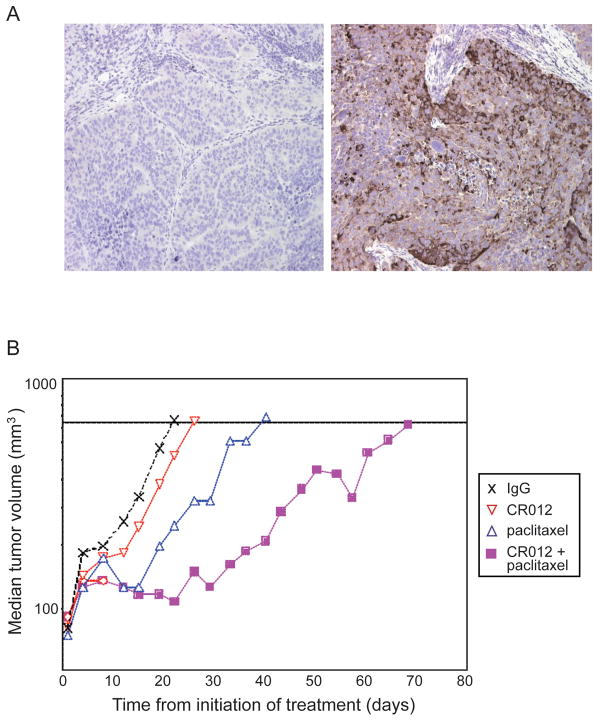
OVCAR3 tumor fragments were xenografted as described. Treatment was initiated when tumors were on average 100mm3, and mice euthanized when tumors reached ~750mm3. Human SLPI produced by the xenografts was recognized by CR012 as shown by immunohistochemical staining in control IgG2-treated OVCAR3 xenografts (Fig. 6A). Tumor growth was monitored over time and median tumor volumes plotted (Fig. 6B). Time to end (TTE) for all treatments was compared against the IgG2 control and paclitaxel alone against CR012+paclitaxel. TTE for IgG2, paclitaxel, CR012, and paclitaxel+CR012 were 23, 39, 28 and 68 days, respectively; a nearly 3-fold improvement in TTE was observed for CR012+paclitaxel compared with IgG2 treatment. Single agent paclitaxel afforded a 16 day improvement (p<0.05) and the combination 45 days (p<0.0001) over IgG2 alone. CR012+paclitaxel resulted in a significant increase in TTE over paclitaxel alone of 29 days (p<0.001). Limited weight loss (7–11%) was recorded in paclitaxel-treated groups and no treatment related deaths were observed (data not shown). Thus, CR012 with paclitaxel delays recurrent tumor growth with the treatment schedule applied.
Figure 6. CR012 augments antiproliferative effects of paclitaxel in xenografts.
A. CR012 recognizes SLPI expression in OVCAR3 xenografts. Immunohistochemical staining with CR012 (right) or IgG2 isotype control (left) indicates specific staining. B. CR012 augments anticancer activity of paclitaxel. Median tumor volumes are plotted as a function of time. The combination of CR012 and paclitaxel yielded a T/C of 298% (p<0.001). Treatment started on day 1: IgG2 or CR012, 1 mg/kg by tail vein every 4 days × 4 doses; paclitaxel 7.5 mg/kg by tail vein every other day × 5 doses.
DISCUSSION
SLPI is a serine protease inhibitor overexpressed in epithelial ovarian cancers and shown to be associated with aggressive and malignant phenotypes (15). In certain environments, SLPI functions as both a growth factor and a survival factor (10). We hypothesized that the pro-survival function of SLPI play a role in paclitaxel-resistance in ovarian cancer cells. We showed that paclitaxel-resistant cells have a higher baseline expression of SLPI and that exposure to paclitaxel in wild type cells upregulated SLPI. Forced expression of SLPI resulted in a reduced sensitivity to paclitaxel that was overcome by blockade of MEK activity. However, this was not observed in the paclitaxel resistant cells, PTX10. These cells have a higher basal SLPI expression and further upregulation of SLPI did not result in further paclitaxel resistance. CR012, a humanized neutralizing anti-SLPI antibody, recognizes human SLPI produced in OVCAR3 xenografts and blocked the protease inhibitory activity of SLPI and reduced growth in vitro. Addition of CR012 to paclitaxel prolonged time to target tumor size nearly 3-fold in xenografts. These data demonstrate the survival and proliferative activity of SLPI and further credentials SLPI as a molecular target for ovarian cancer therapy.
We have demonstrated a prosurvival and confirmed here a pro-growth activity of SLPI. We have previously shown that this is independent of SLPI’s protease inhibitory activity (10, 11). SLPI has been characterized as an alarm anti-protease, a protease inhibitor-induced under stress conditions that may include exposure to pharmacotoxins, such as paclitaxel (17). This concept was confirmed when exposure to the microtubule-stabilizing agent paclitaxel induced SLPI expression and secretion, and was supported by the finding that paclitaxel-resistant cells had increased basal SLPI concentration. That SLPI would antagonize paclitaxel to promote survival in the wild type cells was a logical extension of these findings. Demonstration that the neutralizing anti-huSLPI antibody was more than additive with paclitaxel also suggests that both may signal through common pathways, as we showed.
Paclitaxel is a mainstay of treatment for ovarian cancer and other cancers, such as lung cancer (29, 30), both of which highly express SLPI (7, 16, 31). Paclitaxel treatment causes the activation of numerous signal events, including activation of MEK resulting in phosphorylation of ERK (32). Prolonged activation of ERK is proposed to be at least partially responsible for paclitaxel resistance (26). This role for phosphorylated ERK is controversial. Paradoxically, we observed paclitaxel resistant PTX10 cells had lower pERK expression compared to the parental 1A9 cells. ERK activation may serve as a prosurvival signal in our model, as evidenced by the initial upregulation of pERK seen in the first few hours of paclitaxel exposure and secondary induction later in the time course in the resistant cells. SLPI also contributed to the activation of ERK which may have strengthened the survival signal and thus led to paclitaxel resistance. This would explain why initial upregulation of pERK induced by SLPI causes paclitaxel resistance in the 1A9 cells, but when cells have a persistent paclitaxel resistant phenotype (i.e. PTX10 cells), overexpression of SLPI and upregulation of pERK has no added effect on chemoresistance. However, other studies have shown that the progressive loss of ERK activation can lead to the emergence of chemoresistance (33). This dichotomous finding may be interpreted as a model or selective cancer-specific event.
The development of MEK/ERK small molecule inhibitors opens new directions for combination therapy approaches (34–36). Studies have shown that the use of ERK inhibitors enhances the cytotoxic effects of chemotherapeutic drugs (36, 37). We confirm SLPI expression results in paclitaxel resistance in 1A9 and HeyA8 cells and also causes activation of ERK, which is reversed by the MAPK inhibitor, UO126. When 1A9 SLPI.HA cells are treated with UO126 prior to treatment with paclitaxel, the paclitaxel resistance conferred by overexpression of SLPI is reversed. This does not hold true for the PTX10 cells, possibly because these cells have lost dependence on pERK and thus are not as susceptible to the effects of UO126. No effect on paclitaxel susceptibility was observed when inhibitors of JNK or p38 MAPKs were used (p38 inhibitor SB220025, or JNK inhibitor SP600125; data not shown). A direction for later clinical consideration is combination of CR012 with a MEK inhibitor, affecting two potentially important and interacting targets for ovarian cancer.
We can also suggest that protection through induction of SLPI may occur in response to alarms other than paclitaxel exposure, those that are more difficult to model in vitro. These events may be simple tumor presence, tumor injury by hypoxia or agents, or in response to local microenvironmental cues such as activated stroma. Further, SLPI production by tumor may result in other survival effects, those that may be protective from paclitaxel or other chemotherapy injury. We reported protection/induction of the partner protein of SLPI, progranulin, with increased SLPI presence (11). Progranulin has survival activity for ovarian cancer; its ability to protect from paclitaxel is unknown. It has been suggested that the alarm anti-proteases such as SLPI, may also be part of the adaptive immune response. SLPI may be synthesized and secreted by cells local to the site of alarm, such as inflammation or in response to tissue injury (38, 39). Thus, it is possible that in an applied clinical approach, reduction of SLPI by CR012 may shift the behavior of the local inflammatory response and increase effectiveness of anti-cancer agents. While these postulates will not explain a direct effect of SLPI against an M-phase specific regulator, such as paclitaxel, they do suggest further directions for preclinical and clinical investigation to support credentialing of SLPI for future clinical targeting.
Supplementary Material
Acknowledgments
NR, JC, ND, and EK are supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project is a research collaboration with the CuraGen investigators.
Footnotes
Statement of Translational Relevance
Epithelial ovarian cancer (OvCa) presents in late stage and nearly all late stage patients will suffer disease recurrence death. Molecular therapeutics requires credentialed and validated targets. Secretory leukocyte protease inhibitor, SLPI, is an alarm anti-protease genomically upregulated in a large proportion of OvCa. We have shown previously SLPI functions as a pro-survival factor, stimulate growth, inhibits apoptosis, and may augment dissemination of disease. We hypothesized that the survival function of SLPI was responsible in part for growth and loss of susceptibility to paclitaxel, a mainstay of OvCa treatment. Our findings indicate SLPI is upregulated in response to paclitaxel; SLPI forced expression reduces effectiveness of paclitaxel in an ERK-mediated fashion. A humanized neutralizing monoclonal anti-SLPI antibody, CR012, confirmed these findings and augmented the anti-tumor effect of paclitaxel in OvCa xenografts. These findings credential SLPI as an OvCa molecular target, suggesting CR012 as a mechanism for clinical proof of concept.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol. 1992;10:1748–53. doi: 10.1200/JCO.1992.10.11.1748. [DOI] [PubMed] [Google Scholar]
- 3.du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 5.Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001;61:3869–76. [PubMed] [Google Scholar]
- 6.Ota Y, Shimoya K, Zhang Q, et al. The expression of secretory leukocyte protease inhibitor (SLPI) in the Fallopian tube: SLPI protects the acrosome reaction of sperm from inhibitory effects of elastase. Hum Reprod. 2002;17:2517–22. doi: 10.1093/humrep/17.10.2517. [DOI] [PubMed] [Google Scholar]
- 7.Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci U S A. 2003;100:5778–82. doi: 10.1073/pnas.1037154100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann N Y Acad Sci. 2004;1028:380–9. doi: 10.1196/annals.1322.044. [DOI] [PubMed] [Google Scholar]
- 9.Sugino T, Yamaguchi T, Ogura G, et al. The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood-borne metastasis via an invasion-independent pathway. The Journal of pathology. 2007;212:152–60. doi: 10.1002/path.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpkins FA, Devoogdt NM, Rasool N, et al. The alarm anti-protease, secretory leukocyte protease inhibitor, is a proliferation and survival factor for ovarian cancer cells. Carcinogenesis. 2008;29:466–72. doi: 10.1093/carcin/bgm212. [DOI] [PubMed] [Google Scholar]
- 11.Devoogdt N, Rasool N, Hoskins E, Simpkins F, Tchabo N, Kohn EC. Overexpression of protease inhibitor-dead secretory leukocyte protease inhibitor causes more aggressive ovarian cancer in vitro and in vivo. Cancer science. 2009;100:434–40. doi: 10.1111/j.1349-7006.2009.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner MM, Grenman S, Koul A, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–9. [PubMed] [Google Scholar]
- 13.Kluger HM, Chelouche Lev D, Kluger Y, et al. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. 2005;65:5578–87. doi: 10.1158/0008-5472.CAN-05-0108. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Takamoto N, Hongo A, et al. Secretory leukoprotease inhibitor inhibits cell growth through apoptotic pathway on ovarian cancer. Oncology reports. 2008;19:1085–91. [PubMed] [Google Scholar]
- 15.Israeli O, Goldring-Aviram A, Rienstein S, et al. In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genet Cytogenet. 2005;160:35–42. doi: 10.1016/j.cancergencyto.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Use of serum secretory leukocyte protease inhibitor levels in patients to improve specificity of ovarian cancer diagnosis. Gynecol Oncol. 2005;96:516–9. doi: 10.1016/j.ygyno.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 18.King AE, Morgan K, Sallenave JM, Kelly RW. Differential regulation of secretory leukocyte protease inhibitor and elafin by progesterone. Biochem Biophys Res Commun. 2003;310:594–9. doi: 10.1016/j.bbrc.2003.08.151. [DOI] [PubMed] [Google Scholar]
- 19.Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun. 2005;73:1271–4. doi: 10.1128/IAI.73.3.1271-1274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier F, Fryksmark U, Ohlsson K, Bieth JG. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982;700:178–83. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith CE, Johnson DA. Human bronchial leucocyte proteinase inhibitor. Rapid isolation and kinetic analysis with human leucocyte proteinases. Biochem J. 1985;225:463–72. doi: 10.1042/bj2250463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fink E, Nettelbeck R, Fritz H. Inhibition of mast cell chymase by eglin c and antileukoprotease (HUSI-I). Indications for potential biological functions of these inhibitors. Biol Chem Hoppe Seyler. 1986;367:567–71. doi: 10.1515/bchm3.1986.367.2.567. [DOI] [PubMed] [Google Scholar]
- 23.Junger WG, Hallstrom S, Redl H, Schlag G. Inhibition of human, ovine, and baboon neutrophil elastase with Eglin c and secretory leukocyte proteinase inhibitor. Biol Chem Hoppe Seyler. 1992;373:119–22. doi: 10.1515/bchm3.1992.373.1.119. [DOI] [PubMed] [Google Scholar]
- 24.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu L, Di W, Jiang Q, et al. Targeted inhibition of transient activation of the EGFR-mediated cell survival pathway enhances paclitaxel-induced ovarian cancer cell death. Int J Oncol. 2005;27:1441–8. [PubMed] [Google Scholar]
- 26.McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–25. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 28.Babcook JS, Leslie KB, Olsen OA, Salmon RA, Schrader JW. A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc Natl Acad Sci USA. 1996;93:7843–8. doi: 10.1073/pnas.93.15.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reck M, von Pawel J, Zatloukal P, et al. Phase III Trial of Cisplatin Plus Gemcitabine With Either Placebo or Bevacizumab As First-Line Therapy for Nonsquamous Non Small-Cell Lung Cancer: AVAiL. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 31.Tanner MM, Grenman S, Koul A, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–9. [PubMed] [Google Scholar]
- 32.Okano J, Rustgi AK. Paclitaxel induces prolonged activation of the Ras/MEK/ERK pathway independently of activating the programmed cell death machinery. J Biol Chem. 2001;276:19555–64. doi: 10.1074/jbc.M011164200. [DOI] [PubMed] [Google Scholar]
- 33.Villedieu M, Deslandes E, Duval M, Heron JF, Gauduchon P, Poulain L. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol Oncol. 2006;101:507–19. doi: 10.1016/j.ygyno.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Chung EJ, Brown AP, Asano H, et al. In vitro and In vivo Radiosensitization with AZD6244 (ARRY-142886), an Inhibitor of Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase 1/2 Kinase. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2954. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haass NK, Sproesser K, Nguyen TK, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–9. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 37.Zelivianski S, Spellman M, Kellerman M, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–85. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–78. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 39.Nukiwa T, Suzuki T, Fukuhara T, Kikuchi T. Secretory leukocyte peptidase inhibitor and lung cancer. Cancer Sci. 2008;99:849–55. doi: 10.1111/j.1349-7006.2008.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.