Abstract
The efficacy of [bmim][X] ionic liquids (ILs) (X = PF6−, BF4− and NTf2−) as reaction media for methyl oleate metathesis was compared with that of conventional organic solvents (PhCl, PhMe, DCM and DCE) using the well-defined first and second generation Grubbs precatalysts, RuCl2(PCy3)(L)(=CHPh) (L = PCy3 or H2IMes). Best catalytic performance, with excellent selectivity (>98%) at moderate reaction temperatures, was achieved in [bmim][X] ILs compared to conventional solvents. The effects of anion, reaction temperature, solvent polarity, solvent viscosity, and ligand-anion interaction on the reaction are also addressed.
Keywords: metathesis, methyl oleate, [bmim][X], RuCl2(PCy3)(L)(=CHPh)
1. Introduction
Room temperature ionic liquids (RTILs) have recently attracted considerable attention as potential alternatives to conventional organic solvents due to several of their unique physicochemical properties such as high polarity, non-volatility, non-flamability and immiscibility with water and/or organic solvents [1–6]. RTILs also provide excellent solvent media in many homogeneously catalyzed reactions such as olefin hydrogenation, hydroformylation, oligomerization and Pd mediated carbon-carbon coupling reactions and allow for simple recycling and reuse of transition metal catalysts [7–9]. The discovery of the well-defined ruthenium catalysts by Grubbs and coworkers has also advanced olefin metathesis as a powerful tool in organic synthesis [10,11]. However, the use of these catalysts in chemical industry is restricted by the fact that they are non-recyclable and their separation from the product stream still poses a serious challenge. Furthermore, most of the work on RTILs reported in the literature is based on ring closing metathesis (RCM) [12–17] and ring opening metathesis (ROM) [18]. Self- metathesis (SM) and cross-metathesis (CM) reactions in RTILs remain almost unexplored and very few works have been published [19–21].
The present study aims to investigate the self-metathesis of methyl oleate in [bmim][X] type ionic liquids using the well-defined first and second generation Grubbs precatalysts, RuCl2(PCy3)2(=CHPh) (1) and RuCl2(PCy3)(H2IMes)(=CHPh) (2) (Scheme 1). In particular, the influence on the catalytic activity by the anion (X) and other reaction parameters was examined. The study further evaluates the metathesis activity in [bmim][X] ILs against the activity in conventional organic solvents (PhCl, PhMe, DCM and DCE).
Scheme 1.
Grubbs precatalysts and imidazolium ILs.
Fatty acid esters derived from seed oils are an alternative source of chemicals, since their chemical structures are closely related to those of the hydrocarbons in crude oil. Seed oil and their derivatives have recently attracted much attention due to their renewable supply, low cost, versatility and their green chemistry. Fatty acid monoesters, like methyl oleate, are usually derived from transesterification of seed oil with lower alcohols, yielding glycerol as the by-product [22]. Self and cross metathesis of fatty acid esters would result in the formation of monomers, dimers and α-olefins with interesting applications in polymer, pharmaceutical and petrochemical industries [23,24].
2. Results and Discussion
2.1. Self-Metathesis in [bmim][X] Type Ionic Liquids
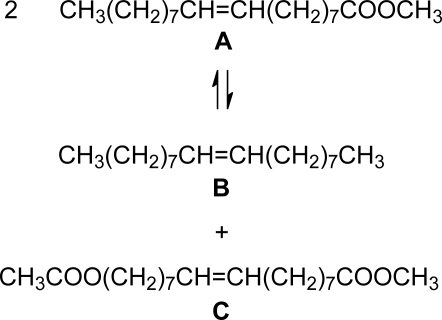
The self-metathesis of methyl oleate in [bmim][X] type RTILs was carried out in presence of Grubbs precatalysts 1 and 2. Different anions (X = hexafluorophosphate (PF6−), tetraflouroborate (BF4−) and bis (trifluoromethylsulphonyl)imide (NTf2−)) in conjunction with a common cation, 1-butyl-3-methylimidazolium (bmim) were tested for their suitability as reaction media. Table 1 presents some of the properties of [bmim][PF6], [bmim][BF4] and [bmim][NTf2]. PF6− and BF4− anions are neutral, weakly coordinating and form highly viscous imidazolium ILs while PF6− and NTf2− form hydrophobic ILs with NTf2− forming a less viscous imidazolium IL [25]. When mixed with methyl oleate all the ILs formed a biphasic system. Two primary metathesis products (PMPs) were obtained in the metathesis of methyl oleate (A), namely, octadec-9-ene (B) and dimethyl octadec-9-ene-1,18-dioate (C), as illustrated in Scheme 2 [26].
Table 1.
Polarity (ETN), solubility and viscosity of [bmim][X] ILs [25].
| X | Solubility in H2O | Viscosity (cP)(25 °C) | |
|---|---|---|---|
| PF6 | 0.667 | Insoluble | 207 |
| BF4 | 0.673 | Soluble | 233 |
| NTf2 | 0.642 | Insoluble | 52 |
Scheme 2.
Primary metathesis products from self-metathesis of methyl oleate.
Figure 1 shows a typical gas chromatogram resulting from the metathesis of methyl oleate with nonadecane as internal standard (IS).
Figure 1.
Typical gas chromatogram resulting from the metathesis of methyl oleate.
2.1.1. Influence of RTIL Anion [X]
Anions play a significant role in determining the properties of ILs. For example, [bmim][PF6] is immiscible with water, whereas, [bmim][BF4] is soluble in water. Furthermore, the anions determine viscosity, density, hydrophobicity and solvation of ILs [27]. The effect of varying the anion counterpart in [bmim][X] type RTILs on the metathesis of methyl oleate using Grubbs catalysts has been studied. Figure 2 shows the influence of anion on the catalytic activity of 1. The metathesis activity showed a decreasing trend with catalyst 1 according to the solvent/anion order BF4− > NTf2− ~ PF6−. For catalyst 2 the trend in metathesis activity decreased in the order NTf2− ~ BF4− > PF6− as illustrated in Figure 3.
Figure 2.
Influence of [bmim][X] type ILs on the activity of 1 at 20 °C.
Figure 3.
Influence of [bmim][X] type ILs on the activity of 2 at 20 °C.
Clearly only a small difference in catalytic activity occurred in different ILs for both 1 and 2. The effect on solvent efficacy and the catalytic activity upon changing the ligand L on the catalyst was evident from a change in the activity trend as PCy3 replaced H2IMes. However, no simple correlation could be deduced between solvent polarity and catalytic activity like it was the case for conventional organic solvents [26]. On the other hand it could be that the coordination ability of the anion to the catalyst was important in determining the activity pattern as observed [28].
2.1.2. Influence of Reaction Temperature
Table 2 summarizes the activity of 1 and 2 in 3a–3c at different reaction temperatures in a closed system. Increasing the reaction temperature from 20–100 °C resulted in a rate enhancement with optimum reaction temperature reached at almost 80 °C in 3a. Excellent selectivity (>98%) was achieved at moderate reaction temperatures (≤60 °C) but decreased at higher temperatures with the formation of secondary metathesis products (SMPs) (F to J) shown in Scheme 3 [23]. The formation of SMPs at high reaction temperatures was evidence of double bond isomerization (A to D and E) occurring.
Table 2.
Activity of 1 and 2 in 3a–3c after 4 hour reaction time.
| RTIL | Catalyst | Temp. (°C) | Conv (%) | Selec[a](%) | TON |
|---|---|---|---|---|---|
| 3a | 1 | 20 | 50.1 | 100 | 50.1 |
| 2 | 52.9 | 100 | 52.8 | ||
| 1 | 40 | 57.1 | 100 | 57.1 | |
| 2 | 64.9 | 90 | 64.9 | ||
| 1 | 60 | 59.4 | 99 | 59.4 | |
| 2 | 74.9 | 81 | 74.9 | ||
| 1 | 80 | 61.6 | 92 | 61.6 | |
| 2 | 79.0 | 60 | 79.0 | ||
| 1 | 100 | 62.0 | 92 | 62.0 | |
| 2 | 79.1 | 47 | 79.1 | ||
| 3b | 1 | 20 | 54.4 | 100 | 54.4 |
| 2 | 58.8 | 100 | 58.8 | ||
| 1 | 60 | 61.1 | 95 | 61.1 | |
| 2 | 78.2 | 87 | 78.2 | ||
| 3c | 1 | 20 | 51.2 | 100 | 51.2 |
| 2 | 60.5 | 99 | 60.5 | ||
| 1 | 60 | 60.5 | 99 | 60.5 | |
| 2 | 72.7 | 72 | 72.7 | ||
| 3a* | 1 | 60 | 23.0 | 88 | 2300 |
| 2 | 62.0 | 85 | 6200 | ||
MO/Ru ratio = 100,
selectivity towards PMPs.
MO/Ru molar ratio = 10,000.
Scheme 3.
SMPs resulting from the metathesis of methyl oleate.
At 60 °C, the best catalytic performance occurred in 3b with 2 generally displaying superior activity than 1 in all the runs. However, 2 suffered a significant loss in selectivity at temperatures >60 °C compared to 1, with the result that comparatively higher yields of SMPs were obtained. A radical change in the activity trend from NTf2− > BF4− > PF6− at 20 °C to BF4− > PF6− > NTf2− at 60 °C in the case of 2 was quite remarkable, especially for NTf2− which showed more sensitivity towards a change in reaction temperature. It appears in this instance, given the inverse relationship that exists between temperature and viscosity of ILs [25], that the lowering in the viscosity of [bmim][NTf2] through an increase in reaction temperature might have compromised its solvent efficacy and performance. Such an effect was, however, not so much pronounced for BF4− and PF6− whose viscosities at elevated temperatures remained relatively high to that of NTf2− given their extreme high viscosities at room temperature. If this was to be true for NTf2−, then BF4− and PF6− would be seen as more suited for high temperature reactions and NTf2− for reactions at low temperatures.
For MO/Ru molar ratio of 10,000, TONs of 2,300 and 6,200 were obtained at 60 °C in 3a for 1 and 2, respectively. The difference in TONs could be attributed to the short lifetime and poor thermal stability of 1 compared to 2 [29,30]. In spite of significantly enhanced activity of 2 relative to 1, catalyst 2 displayed poor selectivity, especially at high reaction temperatures. High selectivities and TONs are some of the important indicators if the process is to find industrial application.
2.2. [bmim][X] ILs vs. Conventional Organic Solvents
Self-metathesis of MO in organic solvents was carried out in presence of 1 and 2 and the results are summarized in Table 3. The solvents used for this study were DCM, DCE, PhMe and PhCl. The best catalytic performance among the conventional solvents occurred in DCM and the lowest in PhMe. The activity of 1 and 2 were found to increase in the order PhMe < PhCl < DCE ~ DCM in accordance to an increase in solvent polarity. Selectivity towards PMPs was 100% in all the organic solvents. These results are in agreement with the work done by Buchowicz and Mol [31] and Marvey et al. [26].
Table 3.
Activity of 1 and 2 in conventional organic solvents.
| Entry | Solvent | Catalyst | Conv (%) | Selec (%) |
|---|---|---|---|---|
| 1 | PhCl | 1 | 47.1 | 100 |
| 2 | 50.6 | 100 | ||
| 2 | PhMe | 1 | 45.0 | 100 |
| 2 | 48.0 | 100 | ||
| 3 | DCM | 1 | 49.0 | 100 |
| 2 | 51.3 | 100 | ||
| 4 | DCE | 1 | 48.9 | 100 |
| 2 | 50.7 | 100 | ||
MO/Ru ratio 100, 20 °C, 4 h.
Compared to [bmim][X] ILs, the conventional organic solvents were less efficient in that relatively lower methyl oleate conversions were obtained for both 1 and 2. Figures 4 and 5 compare the activities of 1 and 2, respectively, in [bmim][BF4], DCM and DCE.
Figure 4.
Activity of 1 in [bmim][BF4], DCM and DCE at 20 °C.
Figure 5.
Activity of 2 in [bmim][BF4], DCM and DCE at 20 °C.
From the results obtained, it is clear that a significant rate enhancement occurred in [bmim][BF4] as opposed to the best of the conventional solvents, namely, DCM and DCE. A further substantial rate enhancement occurred in [bmim][BF4] at a higher reaction temperature (60 °C). Therefore, [bmim][X] ILs provide excellent reaction media for methyl oleate metathesis and can be used as convenient substitutes for conventional organic solvents due to their “green” characteristics and possibilities for easy product separation and catalyst recycling [9].
3. Experimental Section
3.1. Materials and Apparatus
Chlorobenzene (PhCl), dichloromethane (DCM), 1,2-dichloroethane (DCE), toluene (PhMe), 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]), 1-butyl-3-methylimidazolium tetra-fluoroborate ([bmim][BF4]), 1-butyl-3-methylimidazolium bis (trifluoromethylsulphonyl)imide ([bmim][NTf2]) were all reagent grade from Sigma-Aldrich. Methyl oleate (≥ 99%) was obtained from Sigma-Aldrich and was treated with activated alumina and stored under N2 at a subzero temperature. Ethyl vinyl ether was purchased from Fluka. Nonadecane purchased from Fluka was used as the internal standard (IS). Grubbs precatalysts 1 and 2 were stored under N2 and used as purchased from Sigma-Aldrich. Chromatograms were obtained using Varian Star 3400 CX GC equipped with a DB-624 capillary column (J&W Scientific, 30 m × 0.53 mm) and a flame ionization detector (FID). The oven temperature was held at 200 °C and then increased to 270 °C at a rate of 20 °C min−1. The injector temperature was set at 270 °C and the detector temperature at 300 °C with N2 as carrier gas.
3.2. Metathesis Experiments
All the reactions were performed under a N2 atmosphere in a glass reactor fitted with a thermometer and a rubber septum. For the reaction in organic solvents, 12.4 mg of 1 (0.015 mmol) or 2 (0.014 mmol) was dissolved in 2.0 mL of organic solvent followed by 0.1 g of internal standard and 0.5 mL substrate. For the reaction in RTILs, 0.5 mL of the substrate was added to 1 mL of ionic liquid and stirred for 10 min to attain the reaction temperature. An internal standard (0.05 g) was added followed by the addition of 12.4 mg of 1 or 2. Both catalysts were soluble in ILs with the substrate forming a biphasic mixture with ILs. Samples were withdrawn by a syringe at regular time intervals for up to 5 hours. The reaction was terminated by immediately quenching with a few drops of ethyl vinyl ether [31]. The quenched sample was diluted with solvent (S) and analyzed by GC. The following formulas were used in the calculations:
where n0 = number of moles of substrate at the beginning of the reaction. nt = number of moles of substrate after time t.
TON = MO/Ru ratio x substrate conversion; TON is the number of moles of substrate that a mole of catalyst can convert before becoming inactivated.
4. Conclusions
The efficacy of [bmim][X] ILs as reaction media in methyl oleate metathesis was evaluated and the effects of anion, reaction temperature, solvent polarity, viscosity and ligand-anion interaction on the reaction have been addressed. The nature of the anion in [bmim][X] ILs proved to have a slight influence on catalytic performance, suggesting that only a small degree in rate enhancement could be expected from the variation of the anion. The results obtained further indicate that reaction temperature has a significant effect on solvent efficacy with anions displaying varied sensitivities upon a change in ligand L and reaction temperature. While excellent selectivity is achieved at a moderate reaction temperature, a loss in selectivity occurs at higher reaction temperatures with the formation of SMPs and is more pronounced for L = H2IMes. Indeed [bmim][X] ILs outperformed conventional solvents as reaction media for the ruthenium-catalysed self-metathesis of fatty acid methyl esters and have demonstrated their potential as excellent substitutes to these solvents with an added advantage that they meet greener character requirements and allow for easy catalyst separation and recycling.
Acknowledgments
The National Research Foundation (NRF) of South Africa for financial support.
References and Notes
- 1.Welton T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999;99:2071–2083. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- 2.Dupont J, de Souza RF, Suarez PAZ. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002;102:3667–3692. doi: 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes JS. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A: Chem. 2004;214:11–17. [Google Scholar]
- 4.Pârvulescu VI, Hardacre C. Catalysis in ionic liquids. Chem. Rev. 2007;107:2615–2665. doi: 10.1021/cr050948h. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury S, Mohan RS, Scott JL. Reactivity of ionic liquids. Tetrahedron. 2007;63:2363–2389. [Google Scholar]
- 6.D’Anna F, Frenna V, La Marca S, Noto R, Pace V, Spinelli D. On the characterization of some [bmim][X]/co-solvent binary mixtures: A multidisciplinary approach by using kinetic, spectrophotometric and conductometric investigations. Tetrahedron. 2008;64:672–680. [Google Scholar]
- 7.Favre F, Olivier-Bourrbigou O, Commereuc D, Saussine L. Hydroformylation of 1-hexene with rhodium in non-aqueous ionic liquids: How to design the solvent and the ligand to the reaction. Chem Commun. 2001:1360–1361. [Google Scholar]
- 8.Song CE, Yoon MY, Choi DS. Significant improvement of catalytic efficiencies in ionic liquids. Bull. Korean Chem. Soc. 2005;26:1321–1330. [Google Scholar]
- 9.Hardacre C, Holbrey JD, Katdare SP, Seddon KR. Alternating copolymerization of styrene and carbon monoxide in ionic liquids. Green Chem. 2002;4:143–146. [Google Scholar]
- 10.Nguyen ST, Grubbs RH, Ziller JW. Syntheses and activities of new single-component, ruthenium-based olefin metathesis catalysts. J. Am. Chem. Soc. 1993;115:9858–9859. [Google Scholar]
- 11.Scholl M, Ding S, Lee CW, Grubbs RH. Synthesis and activity of new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 12.Buijsman RC, van Vuuren E, Sterrenburg JG. Ruthenium-catalyzed olefin metathesis in ionic liquids. Org. Lett. 2001;3:3785–3787. doi: 10.1021/ol016769d. [DOI] [PubMed] [Google Scholar]
- 13.Rix D, Clavier H, Coutard Y, Gulajski L, Grela K, Mauduit M. Activated pyridinium-tagged ruthenium complexes as efficient catalysts for ring-closing metathesis. J. Organomet. Chem. 2006;691:5397–5405. [Google Scholar]
- 14.Audic N, Clavier H, Mauduit M, Guillemin J-C. An ionic liquid-supported ruthenium carbine complex: A robust and recyclable catalyst for ring-closing olefin metathesis in ionic liquids. J.Am. Chem. Soc. 2003;125:9248–9249. doi: 10.1021/ja021484x. [DOI] [PubMed] [Google Scholar]
- 15.Clavier H, Audic N, Guillemin J-G, Mauduit M. Olefin metathesis in room temperature ionic liquids using immidazolium-tagged ruthenium complexes. J. Organomet. Chem. 2005;690:3585–3599. [Google Scholar]
- 16.Consorti CS, Aydos LPG, Ebeling G, Dupont J. On the immobilization of ruthenium metathesis catalysts in imidazolium ionic liquids. Organometallics. 2009;28:4527–4533. [Google Scholar]
- 17.Śledź P, Maduit M, Grela K. Olefin metathesis in ionic liquids. Chem. Soc. Rev. 2008;37:2433–2442. doi: 10.1039/b711482f. [DOI] [PubMed] [Google Scholar]
- 18.Csihony S, Fischmeister C, Bruneau C, Horvath IT, Dixneuf PH. First ring-opening metathesis polymerization in an ionic liquid. Efficient recycling of a catalyst generated from a cationic ruthenium allenylidene complex. New. J. Chem. 2002;26:1667–1670. [Google Scholar]
- 19.Williams DBG, Ajam M, Ranwell A. Highly selective metathesis of 1-octene in ionic liquids. Organometallics. 2006;25:3088–3090. [Google Scholar]
- 20.Ding X, Xianhai L, Hui B, Chen Z, Xiao M, Guo B, Tang W. Olefin self-cross metathesis catalyzed by the second-generation grubbs carbene complex in room temperature ionic liquids. Tetrahedron Lett. 2006;47:2921–2924. [Google Scholar]
- 21.Clavier H, Nolan SP, Maduit M. Ionic liquid anchored “boomerang” catalysts bearing saturated and unsaturated NHCs: Recyclability in biphasic media for cross metathesis. Organometallics. 2008;27:2287–2292. [Google Scholar]
- 22.Flagella Z, Caterina D, Monteleone R, Giuzio L, Pompa M, Tarantino E, Rotunno T. Potentials for sunflower cultivation for fuel production in Southern Italy. HELIA. 2006;29:81–88. [Google Scholar]
- 23.Mol JC. Applications of olefin metathesis in oleo chemistry: An example of green chemistry. Green Chem. 2004;4:5–13. [Google Scholar]
- 24.Marvey BB. Sunflower-based feedstocks in nonfood applications: Perspectives from olefin metathesis. Int. J. Mol. Sci. 2008;9:1393–1406. doi: 10.3390/ijms9081393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikami K, editor. Green Reaction Media in Organic Synthesis. Wiley-Blackwell; Oxford, UK: 2005. pp. 22–23. [Google Scholar]
- 26.Marvey BB, Segakweng CK, Vosloo HCM. Ruthenium carbene mediated metathesis of oleate-type fatty compounds. Int. J. Mol. Sci. 2008;9:615–625. doi: 10.3390/ijms9040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennecke JF, Maginn EJ. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001;47:2384–2388. [Google Scholar]
- 28.Ajam M.Metathesis and hydroformylation reactions in ionic liquids. MSc dissertation, 200561–65.
- 29.Forman GS, McConnell AE, Hanton MJ, Slawin AMZ, Tooze RP, van Rensberg WJ, Meyer WH, Dwyer C, Kirk MM, Serfontein DW. A stable Ruthenium catalyst for productive olefin metathesis. Organometallics. 2004;23:4824–4827. [Google Scholar]
- 30.Forman GS, Bellabarba RM, Tooze RP, Slawin AMZ, Karch R, Winde R. Metathesis of renewable unsaturated fatty acid esters catalyzed by a phoban-indenylidene ruthenium catalyst. J. Organomet. Chem. 2006;691:5513–5516. [Google Scholar]
- 31.Buchowicz W, Mol J. Catalytic activity and selectivity of Ru(=CHPh)Cl2(PCy3)2 in the metathesis of linear olefins. J. Mol. Catal. A: Chem. 1999;148:97–103. [Google Scholar]