Abstract
Background
Adults with aphasia often try mightily to produce specific words, but their word-finding attempts are frequently unsuccessful. However, the word retrieval process may contain rich information that communicates a desired message regardless of word-finding success.
Aims
The original article reprinted here reports an investigation that assessed whether patient-generated self cues inherent in the word retrieval process could be interpreted by listener/observers and improve on communicative effectiveness for adults with aphasia. The newly added commentary identifies and reports tentative conclusions from 18 investigations of self-generated cues in aphasia since the 1982 paper. It further provides a rationale for increasing research on self-generated cueing and notes a surprising lack of attention to the questions investigated in the original article. The original research is also connected with more recent qualitative investigations of interactional, as opposed to transactional, communicative exchange.
Methods & Procedures
While performing single-word production tasks, 10 adults with aphasia produced 107 utterances that contained spontaneous word retrieval behaviours. To determine the “communicative value” of these behaviours, herein designated self cues or self-generated cues, the utterance-final (potential target) word was edited out and the edited utterances were dubbed onto a videotape. Six naïve observers, three of whom received some context about the nature of word retrieval in aphasia and possible topics for the utterances, and three of whom got no information, predicted the target word of each utterance from the word-finding behaviours alone. The communicative value of the self-generated cues was determined for each individual with aphasia by summing percent correct word retrieval and percent correct observer prediction of target words, based on word retrieval behaviours. The newly added commentary describes some challenges of investigating a “communicative value” outcome, and indicates what would and would not change about the methods, if we did the study today.
Outcomes & Results
The observer group that was given some context information appeared to be more successful at predicting target words than the group without any such information. Self-generated cues enhanced communication for the majority of individuals with aphasia, with some cues (e.g., descriptions/gestures of action or function) appearing to carry more communicative value than others (e.g., semantic associates). The commentary again indicates how and why we would change this portion of the investigation if conducting the study at this time.
Conclusions
The results are consistent with Holland’s (1977) premise that people with aphasia do well at communication, regardless of the words they produce. The finding that minimal context information may assist observers in understanding the communicative intent of people with aphasia has important implications for training family members to interpret self-generated cues. The new commentary reinforces these conclusions, highlights potential differences between self cues that improve word-finding success and those that enhance message transmission, and points to some additional research needs.
Interest in the anomic component of aphasia has been widespread and enduring. Numerous investigations have examined factors influencing the production of a desired word (Oldfield and Wingfield, 1965; Goodglass, Klein, Carey and Jones, 1966; Carroll and White, 1973; Gardner, 1973; Mills, Knox, Juola and Salmon, 1979), and behaviors employed by adults with aphasia in word finding attempts (Barton, 1971; Marshall, 1976; Goodglass, Kaplan, Weintraub and Ackerman, 1976).
A range of therapeutic approaches and cueing strategies for patients with aphasia has evolved from this literature (Berman and Peelle, 1967; Whitney, 1975; Mills, 1977; Linebaugh and Lehner, 1977; Pease and Goodglass, 1978). The bulk of therapeutic emphasis has been placed on facilitation of patients’ ability to generate specific words. Successful word retrieval has been the objective of both clinician-imposed cueing regimens and self-cueing strategies. However, this focus disregards the communicative potential of the gestures, descriptions, and other behaviors that a person with aphasia uses in attempting to produce a target utterance. The word-retrieval process may convey as much or more to a listener than the patient’s ultimate word production.
Recent studies of the word-finding behaviors of a diverse group of treated adults with aphasia at the Portland Veterans Administration Medical Center have shown that two-thirds of their self-generated cueing attempts did not result in production of the desired response. In no instance was a patient’s success rate better than 50%, and for one patient, no correct words were emitted in 12 attempts. This information leads to a conclusion similar to Holland’s (1977), that perhaps too much treatment time is directed toward attaining a specific linguistic response at the expense of the communicative value of a patient’s message.
The current investigation attempted to examine information transfer inherent in the total self-cueing process. The following questions were addressed:
Can listeners predict target utterances from observing only self-generated cueing behaviors of adults with aphasia?
Do certain types of self-generated cues have more communicative value than others?
Is the communicative effectiveness of individuals with aphasia increased by considering the information transmitted in the total self-cueing process?
METHOD
Preparation of videotape
One hundred seven samples of self-cueing behaviors from 10 adults with aphasia were randomized for inclusion on a videotape. These behaviors were emitted by the patients in response to a 100-item battery of single-word speaking tasks. Any self-cue, regardless of the accuracy of the patient’s final response, was included on the tape. Each sample was edited such that the patient’s final responses (regardless of accuracy) were eliminated prior to preparation of the master tape. Two speech pathologists independently classified the self-generated cues into one of eight categories, or combinations of the eight categories, with agreement of 93%. Self-cues were also coded as to whether they had culminated in production of the desired word. Classifications and examples of self-generated cues are shown in Table 1.
TABLE 1.
Classifications and examples of self-cueing behaviors
| Categories | Examples |
|---|---|
| Verbal Associations | “eggs” for bacon |
| “cup and” for saucer | |
| Written cues | pantomimes writing action |
| Description of use, context of use, function | “You write with it” |
| “You use it when it’s cold” | |
| Description of form, position in space, outward characteristic | “It’s round” |
| “It’s on the wall” | |
| Gesture of action, or function | pantomimes shaving, climbing a ladder |
| Gesture of shape, location, outward characteristic | points to the wall indicates shape of a book |
| Spelling or letter cues (correct or incorrect) | “B-l-i-c-k” |
| “It starts with A” | |
| Production of sound made by the object | “ding-a-ling-a-ling” for bell |
Subjects
Six normal adults (graduate students and interns) viewed the 107-item videotape. None had received prior formal exposure to patients with aphasia. The six observers were randomly assigned to one of two groups; a control group (N = 3), and a group provided with some training and context (N = 3).
Control group
Members of the control group received limited instructions about the task. They were told that the tape contained 107 samples of adults with aphasia trying to say certain words, and were asked to predict what they thought the patients were trying to say. This condition approximated a situation in which a listener might be approached by an unfamiliar person with aphasia who was attempting to initiate some message.
Context group
Members of the context group were provided a general explanation of word-finding difficulties in aphasia, along with descriptions and examples of the types of self-cues listed in Table 1. Their answer sheets contained four possible responses (the target word and three foils) for each stimulus. Foils for each item were taken from error responses of pilot observers and the errors made by members of the control group. Some foils were semantically or phonetically related to the target body, and others were outright errors. This condition was designed to resemble a situation in which a listener would be familiar with the communication strategies of a person with aphasia, and might have some idea about possible topics of conversation.
Preparation of the data
Only those self-cues that occurred a minimum of four times in the 107-item corpus were included in the analyses. In addition, categories of self-cues were slightly revised to reflect those cueing behaviors (or combinations) which occurred more than four times. The revised classifications are shown in Table 2. The ability of each observer to predict target utterances was determined by scoring each response as correct or incorrect. A correct response indicated that the observer had predicted the intended word from the information contained in the patient’s self-cue; an incorrect response indicated that the observer had not been able to identify the intended word on the basis of the information inherent in the cue.
TABLE 2.
Revised categories of self-cues
|
To determine the communicative efficiency of self-generated cueing behaviors for individual people with aphasia, two computations were carried out. The first involved determining the percentage of self-cues that ultimately led to the production of the intended word, and the second involved calculating the percentage of unsuccessful self-cues for which five of the six observers were able to predict the intended word. For example, in 10 self-cue efforts, a patient might successfully employ a self-cue to retrieve a target word three times (30%). Of his seven unsuccessful efforts, two target utterances might be correctly predicted by five of six observers (20%). The sum of these values (50%) was taken as an overall measure of self-cueing efficiency for the patient.
RESULTS
Prediction of target utterances
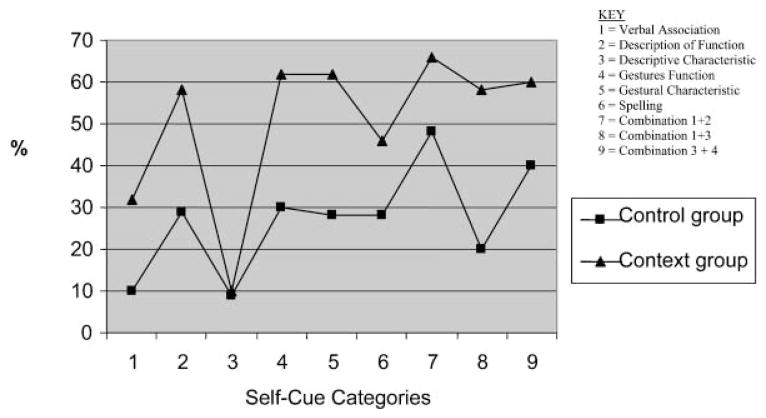
Figure 1 shows the mean percentages of correct predictions for the control and context groups for each of the cueing categories. The mean percentage of correct predictions for the control group ranged from 29–34%, with a mean of 31%. Means of correct prediction for the context group ranged from 47–65% with a mean of 56%.
Figure 1.
Mean percentages of correct predictions for control and context groups for each self-cueing category. Note: Data from the original paper have been extrapolated into this figure.
Self-cue types
Figure 2 shows the percentages of correct predictions for each observer in the control and context groups for each category of self-cues. Although observers provided with context had higher percentages of accurate predictions for most categories, the pattern of successful predictions across the various categories was similar for both individuals (Figure 2) and groups (Figure 1). Visual inspection of Figures 1 and 2 shows that functional description, both gestural categories, and combined cues resulted in higher percentages of correct observer predictions.
Figure 2.

Percentage of correct predictions for individual observers for each category of self-cues. Note: Data from the original paper have been extrapolated into this figure.
Self-cue efficiency
Figure 3 shows the percentages of communicative efficiency for each patient with aphasia. These data show that when the correct predictions of observers are added to the successful word retrieval efforts of the patient, dramatic improvements are seen for three patients (2, 4, and 7), modest improvements for five patients (1, 5, 6, 9, and 10), and no improvement for two patients (3 and 8).
Figure 3.
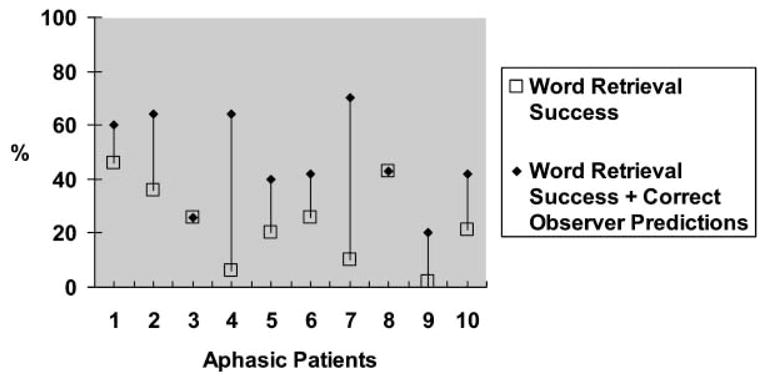
Percentages of communicative efficiency for aphasic patients. Note: Data from the original paper have been extrapolated into this figure.
DISCUSSION
While the absence of statistical analyses necessitates a cautious interpretation of the findings of this study, our data suggest that observers can glean intended meaning from self-cues, even in an artificial situation which allows no hypothesis testing or interaction regarding the desired response of the person with aphasia. Giving a small amount of description and context to observers seems to help them interpret self-cues. This finding has implications for training family members to communicate more effectively, even when a patient’s word-retrieval success may be inconsistent.
Certain types of cues may be more effective than others for message transmission. Support for this conclusion comes from the performance of individual patients and the fact that the overall pattern held for three patients whose communicative efficiency levels increased the most when the information in the total cueing process was considered. For example, subject 4 was a patient with anomic aphasia, approximately five months post-onset, who rarely produced target words. Half his self-cueing attempts were combined cues; the other half were verbal descriptions of action or function. Subject 7 was 8 years post-onset and exhibited moderate nonfluent aphasia and apraxia of speech. He tended to self-cue by combining gestures with phonemic approximations of words within a verbal description. All successful identifications for patient 2 were on combined cues; of his cues that did not successfully communicate the target word, three-fourths were verbal associations. The two patients who showed no increase in communicative level used verbal associations in 75% of their self-cueing efforts. In attempting to predict the target utterances of these subjects, observers tended to be literal when single word associates were given, recording the association itself as the intended response. The ordering of self-cueing effectiveness with regard to information transfer does not correspond to that reported by Pease and Goodglass (1978) in a study of word-retrieval success. They found that cues denoting action, location, and superordinate were least effective in cueing word production for all patients with aphasia. While our investigation showed that verbal associations (including superordinate) cues were ineffective and occasionally misleading for our observers, both verbal and pantomimic indications of action resulted in some of the most accurate observer judgments. Location cues were also effective when gestured, or in combination with verbal associations.
The most relevant result of this experiment indicated that people with aphasia for the most part may “communicate better than they talk” (Holland, 1977). Self-generated cueing behaviors should be regarded as potentially useful ways of transferring information, because a patient’s success at retrieving a specific target word bore no apparent relationship to successful observer identification. Certainly, a therapy focus that encourages the use of clear self-cues regardless of eventual word production should be considered for many adults with aphasia.
Author notes
The original research for this paper was supported by the Research and Development Committee of the Veterans Administration Medical Center, Portland, Oregon. The authors express their appreciation to Sandra Neuburger and Ann Gaddie for their assistance with the research, and to Sara Byers, Kimberly Meigh, and Anita Lewis for their help with this updated manuscript.
In the original paper, the word “aphasic” was used as an adjective; many clinicians and investigators in 1982 still used “aphasic” as a noun. In this update, we have changed relevant phrasing in the original paper, to put the person first.
Authors’ commentary: Introduction
When this article was originally published in 1982 (Tompkins & Marshall, 1982), a “pragmatic revolution” was underway in clinical aphasiology. In a monograph just five years earlier, Holland (1977) had opined that adults with aphasia “probably communicate better than they talk” (p. 173). In 1980, she had published the first edition of the Communicative Abilities in Daily Living test (Holland, 1980; now Communication Activities of Daily Living; Holland, Frattali, & Fromm, 1999), a test that gives credit not only for accuracy of response, but also for functional communication, or “getting the message across”. Along these lines, the study reprinted here was intended to address what Tompkins and Marshall viewed as a major gap in the clinical literature on word retrieval at the time. Word retrieval, especially naming, was probably the most common treatment target for adults with aphasia (Linebaugh & Lehner, 1977), but more often than not, even treated aphasic individuals’ word retrieval attempts were not successful (Marshall & Tompkins, 1981, 1982). To us, the time was ripe to investigate the information conveyed by client-generated, unresolved word retrieval attempts, and the potential contribution to functional communication of the information contained in the word retrieval process itself.
We note here that some of these word retrieval attempts clearly were conscious, strategic patient-generated self cues, while others likely were not strategic at all. Although the term “self cue” connotes a strategy to many, we dub such word retrieval behaviours “self cues” or “self-generated cues” in this article and commentary for ease of exposition. As the reader will see, all relevant behaviours were produced spontaneously and without prompting by patients with aphasia, in response to single-word production tasks.
Research on word retrieval/naming continues to flourish in the aphasiology literature. A variety of newer efforts have explored word retrieval behaviours in aphasia (e.g., Beeson, Holland, & Murray, 1997; Ferguson, 1993; Marshall & Freed, 1995; Watamori, Fukusako, Monoi, & Sasanuma, 1991), but one of the most lasting investigative interests involves the effects of clinician-imposed cues on word retrieval success. Among such cues are semantic (e.g., Saito & Takeda, 2001; Stimley & Noll, 1991); phonologic/phonemic (e.g., Fink, Brecher, Schwartz, & Robey, 2002); lexical (Faroqi-Shah & Thompson, 2003); rhyme (Spencer, Doyle, McNeil, Wambaugh, Park, & Carroll, 2000); prosodic (Seron, Van der Kaa, Vanderlinden, Remits, & Feyereisen, 1982); gestural (Drummond & Rentschler, 1981); and various cue combinations (e.g., Wambaugh, Cameron, Kalinyak-Fliszar, Nessler, & Wright, 2004; Weidner & Jinks, 1983).
In contrast to the considerable continued interest in clinician-generated cueing and naming in aphasia, only a modest amount of work has investigated client self-generated cueing. A table in the Appendix documents 18 post-1982 aphasia investigations that address self-generated cueing in some form, although some (several of the Feature Analysis studies) rather indirectly. We identified these studies using both electronic searches (PsychInfo, Ovid, Medline, Clinical Aphasiology website; Keywords: aphasia, adult, cue, self-cue, naming) and extensive manual searches (going back at least 10 years) of the subject indexes and/or tables of contents of six specific journals (American Journal of Speech-Language Pathology; Aphasiology; Brain & Language; Clinical Aphasiology; Journal of Medical Speech-Language Pathology; and Journal of Speech, Language, and Hearing Research). The overwhelming majority of these articles on self-generated cueing were published at least a decade after this original 1982 manuscript. Marshall, Freed, and colleagues’ systematic studies dominate the landscape, but papers by other research groups also pepper the literature, particularly in more recent years. While the performance targets, subject characteristics, and other methods vary widely across this body of research, results converge on the tentative conclusion that self-generated cues appear to foster durable generalisation to untrained stimuli. Generalisation to connected speech and daily life interaction appears to remain elusive, however.
Why might clinical aphasiology benefit from more attention to self-generated cueing? In addition to the promising results documented in the Appendix, it is clear from studies of normal cognition and learning that self-generated cues, whether verbal or gestural, trump other-generated cues, including clinician supplied, for immediate and delayed recall performance (e.g., Frick-Horbury, 2002). Self-generated cues also aid recall even in incidental/unintended learning contexts (e.g., Mantyla, 1986); and the benefits of self-generated cues are long-lasting, being evident after delays of weeks’ duration (e.g., Backman & Mantyla, 1988; Mantyla, 1986; Sauzeon, N’Kaoua, & Claverie, 2001).
As noted above, adults with aphasia often engage in word retrieval behaviours that do not ultimately result in production of a desired word. The durability of learning/memory that derives from the self-generated cueing process suggests that such cues may help address the eternally thorny problems of generalisation and maintenance of treatment gains in aphasia. Much of the evidence in the Appendix appears to be consistent with that notion, although the real-world generalisation of self-generated cues remains an empirical issue in need of well-designed treatment research. It may be that to be most effective, self-generated cues would need to address the presumed functional lesion in the patient’s word-retrieval system (e.g., rhyme cues for phonological output lexicon deficit; Spencer et al., 2000). But this need not be the case (e.g., Boyle, 2004; Francis, Clark, & Humphreys, 2002; Hillis, 1998). For example, Francis and colleagues (2002) gave only general instructions to their patient with a moderate deficit in accessing the phonological output lexicon, to continue to “talk around” a word until she could retrieve it (though it appears that Francis et al. also often repeated the patient’s self-cues to the patient, to keep her engaged in this process). Indeed, most of the investigations in the Appendix, as well the studies of normal cognition cited above, suggest that the idiosyncratic semantic content of a self-cue is the key to its success. However, more investigation is needed to determine whether, for adults with aphasia, self- and other-generated cues that are equated for quality and quantity of semantic content would differ in their immediate and longer-term effectiveness, including clinical significance.
If the work on self-generated cueing in aphasia has been limited since 1982, further research on the communicative value of such cues, or of word retrieval behaviors in general, is nearly nonexistent. We identified only two relevant articles using the search process described above. A year after this original article, part of an investigation by Golper and Rau (1983) assessed how effectively the self-cues of an individual with aphasia could communicate information unknown by his interactant (see the Appendix). And in 1993 Ferguson examined 21 conversations between 7 adults described as having mild-moderate fluent aphasia and 14 non-brain-damaged familiar and less-familiar communication partners, to describe how often and in what circumstances communication partners supply words to adults with aphasia. Familiarity did not affect a listener’s formation of hypotheses about target concepts, but at specific trouble spots, less-familiar partners supplied more words than did familiar interactants.
We continue to view the paucity of such work as a major gap in clinical aphasiology research. In light of the broader pragmatics/functional communication boom (e.g., Aten, 1986; Davis, 2005; Davis & Wilcox, 1985; Elman & Bernstein-Ellis, 1995; Frattali, 1998; Holland et al., 1999; Hough & Pierce, 1994; Lomas, Pickard, Bester, Elbard, Finlayson, & Zoghaib, 1989) and the limitations of the original Tompkins and Marshall (1982) study, we find this gap somewhat puzzling, though there is no doubt that it is difficult to design appropriate empirical studies of “communicative value” outcomes.
Progress is occurring on some related fronts, however; particularly in work rooted in qualitative analysis of the communicative value of aspects of interaction (as in Ferguson, 1993) rather than the transfer of information (as in this original article and the studies in the Appendix). For example, using an ethnographic approach to analyse dyadic interactions, Simmons-Mackie and Damico (1995) identified certain interjections and automatic social responses that had value for communication regulation and social affiliation. Another example comes from Oelschlaeger’s (1999) conversation analysis of the ways in which collaborative interactions lead to successful hypotheses of content and intent by the communication partners of people with aphasia, which allow a conversation to continue despite specific linguistic difficulties. Qualitative research of this kind is costly and time-consuming, but has clear promise to illuminate the nature and potential effectiveness of the rich “hints” (Lubinski, Duchan, & Weitzner-Lin, 1980) to be found in the communication attempts of adults with aphasia.
Authors’ commentary: Method
One of the primary difficulties in performing this kind of study is that the behaviours of interest comprise an opportunity sample. As such, there is no way to control for differences in self-generated cue frequency or content within or between speakers, and a very big corpus of word-retrieval behaviours is necessary to elicit a sample of self-generated cues that is large and varied enough for a rigorous assessment of research aims. The original Tompkins and Marshall (1982) investigation included a relatively large sample of self-generated cue utterances (N = 107), but did not require each individual adult with aphasia to contribute a minimum number of self-cues to the sample.
Another complication with research of this type is that so much of the observed value of self-generated cues would depend on the individual listeners’ verbal and nonverbal communication attitudes and aptitudes, as well as the nature and quality of the interaction between speaker and listener. We will return to this issue in the Discussion commentary.
If we were conducting this study and writing this paper today, we would change a number of aspects of the method. First, making a case in the Introduction for the hypothesis that a semantic base is the potent aspect of self-generated cues, we would establish rules and reliability for defining each type of self cue, so we could analyse the relationship of semantic content to the communicative effectiveness of such cues. This process would also allow us to begin to tease out the relative contributions of various aspects of combined self cues. Second, we would not use the term “efficiency” to describe the dependent measures of interest. There are various definitions of “efficiency”, but most are concerned with some measure per unit of time, which was not the focus of this work. Rather, we would tag the outcomes of interest with a term like “effectiveness”, which we will use in ensuing commentary.
The rest of the modifications would reflect more general research principles (see, e.g., Tompkins & Lustig, 2001). For example, we would describe fully the individuals with aphasia who produced the self-generated cues, providing information such as their major relevant behavioural characteristics and the basis for their diagnoses. This would allow us to assess the effect of individual differences on study results in a more rigorous manner than we did, and to improve the generalisability of the findings. We would use more observers to predict target utterances, perhaps six to eight per group. We would administer reliable and valid instruments to characterise our observers’ communication attitudes and aptitudes, if any such measures exist, and we would report the observers’ other relevant characteristics as well. It would also be important to work from specific operational definitions of the categories of self-cueing behaviours, rather than just the examples provided in the paper, and to address observer reliability for target utterance predictions. Finally, to address the adequacy of the behaviour sample, we would include self-generated cues only from individuals with aphasia who produced a specified minimum number of such cues, perhaps 10–15; and we would increase the total sample of self-cues such that the cues to be analyzed would occur more than four times each (perhaps at least six to eight times, though this is still a small number of occurrences).
We would not, however, change the way we provided “context” to our context group of observers. We acknowledge that the results of a study with this design may have limited generality to everyday, real-world interactions, though that is an empirical question. At the same time, we are satisfied that we approximated some potentially potent contextual variables (re: listener and topic knowledge) without sacrificing the experimental control afforded by our methods (using single-word tasks where the target word is known or knowable; limiting the pre-target information provided by the individuals with aphasia). Our choice reflects our bias in the paradigmatic tension between experimental control and investigation of natural context. That is, we deem strong experimental control necessary for determining if some phenomenon is real (valid), rather than an artifact of something left uncontrolled. If the results of a new study done today, modified in the ways noted above, were similar to those of the original study, we might then design systematic extensions that would include and manipulate elements of a natural context.
The question of the ecological validity of the methods in the original Tompkins and Marshall (1982) investigation is undoubtedly an important one. However, to state the obvious, it is far from straightforward to incorporate and analyse contextual effects on communication. The term “context” is typically undefined, and much of the available research on aphasia and performance in context focuses on macro-variables like topic familiarity (Li, Williams, & Della Volpe, 1995; Williams, Li, Della Volpe, & Ritterman, 1994) or communication-partner familiarity (e.g., Ferguson, 1998; Li et al., 1995; Williams et al., 1994), that may be proxies for variables that are more relevant (e.g., topic expertise) and/or less objectifiable (e.g., extent and nature of shared life experiences, beliefs, etc., Oelschlaeger, 1999). Other work addresses context by varying the communication task (e.g., word retrieval in single-word vs connected speech tasks, Mayer & Murray, 2003; Pashek & Tompkins, 2002), the communication setting (e.g., discourse in a controlled environment vs a cafeteria, Lustig & Tompkins, 2002), or the sample of behaviour available to communication partner (e.g., longer vs shorter clips for social validity ratings, Lustig & Tompkins, 2002). Interactional studies of the kind cited in the Introduction Commentary (e.g., Ferguson, 1993; Oelschlaeger, 1999; Simmons-Mackie & Damico, 1995) bring rich descriptions to the fore, for possible incorporation into controlled (quasi-)experiments. Nonverbal cues of both speaker and listener, including gestures, intonation, and facial expression, are natural candidates for investigation as well. Avenues for follow-up, thus, are extensive and varied.
Authors’ commentary: Results
This section illustrates starkly how much times have changed! If we were conducting this study today we certainly would not claim quantitative differences between the two observer groups or draw conclusions about differences in effectiveness of various self-generated cues based on simple percentage figures (and only means at that!) or visual inspection of the data rather than appropriate statistical analysis. It is interesting nonetheless that some other work with aphasia reports the communicative value of action/function based self-cues (Golper & Rau, 1983), even if they are not necessarily effective for the retrieval of specific words (Pease & Goodglass, 1978). Such cues are also highly salient in object descriptions of adults with and without traumatic brain injury (Constantinidou & Kreimer, 2004).
Today we would also perform statistical analyses of effect size (e.g., Cohen, 1977) to buttress our claims about the extent of gains in communicative effectiveness of self-generated cues (dramatic, modest) for individual adults with aphasia. We would attend to other individual differences as well; evaluating, for example, the relationships of individual characteristics of both patients and observers to the extent of gain in communicative effectiveness, as previewed in the Method commentary. Analyses of individual factors that might contribute to successes or failures, in general, deserve more attention in aphasia research (Tompkins & Lustig, 2001).
Authors’ commentary: Discussion
At the 1982 Clinical Aphasiology Conference where this paper was presented, one of the questions after the presentation noted an apparent discrepancy between these results and earlier findings that in spontaneous productions, “delay” was the most effective word-retrieval strategy for adults with aphasia (Marshall, 1976). This gave us a chance to underscore that there is not necessarily a concurrence between the types of self-generated cues that are most beneficial for communication and those that improve word finding in aphasia. We concluded this paper by suggesting that it might be beneficial to address communicatively clear self-generated cues in treatment. Such self-cues might be considered as productive delays for communication, as opposed to productive delays for word retrieval. Silent delay, while productive for word finding for many adults with aphasia, obviously holds no communicative value for a listener, and delays filled by other than productive cues may be distracting to a listener (Whitney & Goldstein, 1989), perhaps affecting that listener’s patience and comfort with the exchange. Single-subject experimental designs, with prior in-depth analysis of the communicatively productive self-cues used by individuals with aphasia, would help to determine whether treatment could shape their use and long-term effectiveness.
Among the elements of long-term effectiveness that future research should address is the generalisation of communicative gains via self-generated cues to daily life or real-world interactions. This was one of the main themes of the discussion that followed the presentation of the original paper. One questioner’s thought, stemming from the finding that verbal association alone was weak in communicative value regardless of context group, was that natural context might provide enough meaning to enable listeners to narrow the choices provided by association cues, which were often taken literally by the observers in this study. This is a good point—and another empirical question, and as noted in the Method Commentary, interactant variables, may be crucial to addressing it: for example, how apt is the listener to offer “guesses” prematurely, that might throw off the individual with aphasia? It is our guess that when an individual with aphasia ends the word-retrieval process with real, interpretable lexical items, such as associations or semantic paraphasias, listeners have a strong propensity to take those words literally, with the potential exception of listeners most attuned to that individual’s communication patterns. But this is another empirical question—just what makes research so much fun!
It remains encouraging that the minimal information provided to the context group of observers was apparently beneficial for interpreting the self-generated cues of these 10 individuals with aphasia. In this time of insurance reimbursement limitations and decreased lengths of stay in both acute and rehabilitative settings, meaningful yet speedy results are the ideal. We also commented in the original article on the implications of our results for family training; we echo that sentiment these many years later. Family training has been evaluated and advocated for some time (e.g., Simmons, Kearns, & Potechin, 1987), with renewed attention in recent years (e.g., Cranfill, Simmons-Mackie, & Kearns, 2005; Kagan, Winckel, Black, Duchan, Simmons-Mackie, & Square, 2004; Cunningham & Ward, 2003; Robey & Schultz, 1998). To assess our assertion, well-conceived and rigorously designed studies are needed of the benefits of communication partner/family training to capitalise on the communicative value of self cues in aphasia.
APPENDIX
Articles that address self-generated cueing in adult aphasia since Tompkins and Marshall (1982)
| Authors | Sample descriptiona;b | Tasks and stimuli | Cue conditions | Results of primary interest |
|---|---|---|---|---|
| Boyle, 2004 | Chronic anomic aphasia (N = 1) Chronic Wernicke’s aphasia (N = 1) |
Confrontation naming Black-and-white line drawings of nouns (and other unspecified pictured nouns for Subject 1) |
Self-generated semantic descriptors, elicited using Semantic Feature Analysis diagram | Generalisation to untrained stimuli, maintained at 1-month follow-up Generalisation to connected speech: correct information units per minute (Subject 1); average number of correct information units (Subject 2) |
| Boyle & Coelho, 1995 | Chronic Broca’s aphasia (N = 1) | Confrontation naming Black-and-white line drawings of nouns |
Self-generated semantic descriptors, elicited using Semantic Feature Analysis diagram | Generalisation to untrained stimuli, maintained at 2-month follow-up No generalisation to connected speech Social validity: gains (rated by daughter) were clinically significant |
| Coelho, McHugh, & Boyle, 2000 | Chronic, moderate fluent aphasia (N = 1) | Confrontation naming Black-and-white line drawings of nouns |
Self-generated semantic descriptors, elicited using Semantic Feature Analysis diagram | Generalisation to untrained stimuli, maintained at 1-month and 2-month follow-up Questionable generalisation to connected speech |
| DeDe, Parris, & Waters, 2003 | Chronic, moderate-severe nonfluent aphasia; moderate apraxia of speech (N = 1) Primary impairment in phonological output lexicon; mild semantic impairment |
Confrontation naming Colour photographs of nouns (1–2-syllable) judged client-relevant by clinician |
Self-generated written name and tactile placement cues, plus clinician-supplied phonological cues (e.g., first sound and syllable) | No generalisation to untrained stimuli; questionable generalisation to other naming tasks No self-cues noted at 6-week follow-up; fair-to-poor maintenance of gains |
| Francis, Clark, & Humphreys, 2002 | Moderate anomia, ~ 2 MPO (N = 1) Primary impairment of access to phonological output lexicon; mild semantic and some visual recognition deficit |
Confrontation naming Black-and-white line drawings of low-frequency animate and manmade items |
Self-generated circumlocution, “talking around” the item until its name was accessed, without clinician-supplied cues | Generalisation to untrained stimuli; appeared to be maintained at 2.5-week follow-up Untreated deficits in oral reading did not improve; effects not solely spontaneous recovery |
| Freed, Celery, & Marshall, 2004 | Chronic moderate Broca’s aphasia with agrammatism (N = 1) Same as above, plus severe apraxia of speech (N = 1) Chronic moderate Wernicke’s aphasia (N = 1) |
Confrontation naming Colour photos and black- and-white line drawings of familiar objects |
Personalised self-cue, drawing on “something personally relevant” (p. 746) about the item Clinician supplied phonologic cue (number of syllables; initial phoneme) |
Maintenance of treatment gains up to 3-month follow-up, for items trained with personalised cues only No generalisation to untrained control items |
| Freed & Marshall, 1995a | Chronic, mild-moderate aphasia (N = 14, 7 per cue condition) | Symbol labelling Black-and-white non-iconic symbols; paired with concrete nouns for training |
Personalised self-cue, drawing on semantics and perceptual characteristics Clinician-supplied cue: based on other subject group’s personalised cues |
Both cue types effective up to final (72-hour) follow-up Self-cue group generalised gains to untrained control items |
| Freed & Marshall, 1995b | Chronic, mild-moderate aphasia (N = 10) | Confrontation naming Colour photos of unknown subordinate members of the ‘dog’ and ‘bird’ categories |
Personalised self-cue, drawing on semantics and perceptual characteristics | Maintenance of trained performance at 1-week and 1-month follow-up Generalisation to untrained stimuli; also maintained at 1-week and 1-month follow-up |
| Freed, Marshall, & Nippold, 1995 | Chronic, mild-moderate aphasia (N = 30, 15 per cue condition) | Symbol labelling Black-and-white non-iconic symbols; paired with concrete nouns for training |
Personalised self-cue, drawing on semantics and perceptual characteristics Clinician-supplied cue: based on other subject group’s personalised cues |
Both cue types effective Maintenance of trained performance at 72-hour follow-up At 30 day follow-up performance had declined, but still above baseline |
| Golper & Rau, 1983 | Moderately-severe aphasia, 2 MPO (N = 1) Mild nonfluent aphasia; moderate apraxia of speech, 3 MPO (N = 1) |
Subject 1: Confrontation naming of pictures of common objects, out of clinician’s view Subject 2: Oral reading of sentences |
Subject 1: Self-generated initial phoneme, grapheme, gesture, association, description, and/or delay cues Subject 2: Self-generated rhythm/pacing cues |
Association and description cues less frequent but most often led to accurate clinician inference (Subject 1) Treatment focused on subject-generated cues may improve target performance, spontaneous use of self-cues, and communication to listener |
| Howard & Harding, 1998 | Chronic aphasia with severe naming impairment (N = 1) Hypothesised naming deficit in mapping from semantics to lexical phonology |
Confrontation naming Line drawings of targets varying in frequency and length |
First letter self-cue, subject-selected from alphabet chart Silent delay (extra 5 seconds) Clinician-supplied cues: Initial letter, spoken name of initial letter, initial phoneme |
Alphabet self-cue better than silent delay, spoken name of initial letter Written letter and initial phoneme as good as alphabet self-cue |
| Lowell, Beeson, & Holland, 1995 | Chronic, moderate conduction aphasia (N = 2) Chronic, moderate anomic aphasia (N = 1) |
Confrontation naming Black-and-white line drawings of picturable nouns |
Semantic descriptors, both self- and examiner-generated but then subject-selected for target nouns. Descriptors elicited using Semantic Feature Analysis diagram | Rapid training effects for 2 subjects; no effect for 1 diagnosed with conduction aphasia Maintenance of treatment gains at 1-week follow-up Generalisation to untrained items: complete for anomic aphasia subject, partial for other subject |
| Marshall, Freed, & Karow, 2001 | chronic, mild-moderate aphasia (N = 30, 15 per cue condition) | Confrontation naming Colour photos of unknown dog breeds |
Personalised self-cue, drawing on semantics of target word and perceptual characteristics Clinician-supplied phonologic cue (number of syllables; initial phoneme) |
Both cue types effective for learning Self-cue group had better maintenance at 1-week, 1-month, and 6-month follow-up Both groups essentially back to baseline at 6-month follow-up |
| Marshall, Freed, & Phillips, 1994 | Chronic, mild-moderate aphasia (N = 8) | Symbol labelling Black-and-white non-iconic symbols; paired with words for training |
Personalised self-cue, drawing on semantics of target word and perceptual characteristics Clinician-supplied phonologic cue (number of syllables; initial phoneme) |
Both cue types effective for learning Self-cue condition yielded better generalisation to untrained stimuli, and maintenance at 24-hour follow-up |
| Marshall, Karow, Freed, & Babcock, 2002 | Chronic, mild-moderate aphasia (N = 15) | Confrontation naming Colour photos of unknown dog breeds |
Personalised self-cues, drawing on semantics of target word and perceptual characteristics Subdivided into 5 cue forms: feature, visual/literal, experiential, rhyme, and combination cues |
Cues containing semantic information more effective than those containing phonological information, at 1-week, 1-month, and 6-month follow-up |
| Marshall, Neuburger, & Phillips, 1991 | Chronic, mild-moderate aphasia (N = 22) | Symbol labelling Black-and-white non-iconic symbols; paired with words for training |
Personalised self-cue in form of association, mnemonic, phrase, or other internal cue Also clinician-supplied simple, complex, and repetition cues |
Self-cue condition gains retained to 1-week follow-up No maintenance for clinician-supplied cues |
| Marshall, Neuburger, & Phillips, 1992 | Chronic, mild-moderate aphasia (N = 23) | Symbol labelling Black-and-white non-iconic symbols; paired with words |
Personalised self-cue; unclear what instructions or guidance provided Also clinician-supplied determinate sentence, indeterminate sentence, and repetition cues |
Self-cue condition poorest as a training task. Self-cue condition best at 1-week follow-up, but not due to maintenance of trained performance: fell to the level of the first training probe, in which it was already better than other cues. |
| Massaro & Tompkins, 1994 | Chronic communication deficits resembling Broca’s aphasia (N = 2) | Topic description Topics of interest representing nouns and verbs in 3 categories |
Self-generated semantic descriptors, elicited using Semantic Feature Analysis diagram | Generalisation to untrained stimuli No generalisation to unfamiliar listeners; performance better with familiar listener Social validity: gains (rated by unfamiliar listeners) in organisation and completeness (Subject 1); cohesion (Subject 2) |
Chronic=≥4 months post-onset; in most cases much longer aphasia duration. MPO=months post-onset.
Excludes any non-brain-damaged control participants.
Diagnostic labels taken directly from the cited articles.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Connie A. Tompkins, University of Pittsburgh, PA, USA
Victoria L. Scharp, University of Pittsburgh, PA, USA
Robert C. Marshall, University of Kentucky, KY, USA
REFERENCES FOR THE 1982 PAPER
- Barton M. Recall of generic properties of words in aphasic patients. Cortex. 1971;7:73–82. doi: 10.1016/s0010-9452(71)80023-0. [DOI] [PubMed] [Google Scholar]
- Berman M, Peelle L. Self-generated cues: A method for aiding aphasic and apractic patients. Journal of Speech and Hearing Disorders. 1967;32:372–376. doi: 10.1044/jshd.3204.372. [DOI] [PubMed] [Google Scholar]
- Carroll J, White M. Word frequency and age of acquisition as determiners of picture-naming latency. Quarterly Journal of Experimental Psychology. 1973;25:85–95. [Google Scholar]
- Gardner The contribution of operativity to naming capacity in aphasic patients. Neuropsychologia. 1973;11:213–220. doi: 10.1016/0028-3932(73)90010-9. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Weintraub S, Ackerman N. The “tip-of-the tongue” phenomenon in aphasia. Cortex. 1976;12:145–153. doi: 10.1016/s0010-9452(76)80018-4. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Klein B, Carey P, Jones K. Specific semantic word categories in aphasia. Cortex. 1966;2:74–89. [Google Scholar]
- Holland A. Some practical considerations in aphasia rehabilitation. In: Sullivan M, Kommers MS, editors. Rationale for Adult Aphasia Therapy. Nebraska: Univ. of Neb. Med. Center; 1977. [Google Scholar]
- Linebaugh C, Lehner L. Cueing hierarchies and word retrieval: A therapy program. In: Brookshire R, editor. Clinical Aphasiology Conference Proceedings, 1977. Minneapolis, MN: BRK Publishers; 1977. [Google Scholar]
- Mills R. The effects of environmental sound on the naming performance of aphasic subjects. In: Brookshire R, editor. Clinical Aphasiology Conference Proceedings, 1977. Minneapolis, MN: BRK Publishers; 1977. [Google Scholar]
- Mills R, Knox A, Juola J, Salmon S. Cognitive loci of impairments in picture naming by aphasic subjects. Journal of Speech and Hearing Research. 1979;22:73–87. doi: 10.1044/jshr.2201.73. [DOI] [PubMed] [Google Scholar]
- Oldfield R, Wingfield A. Response latencies in naming objects. Quarterly Journal of Experimental Psychology. 1965;17:273–281. doi: 10.1080/17470216508416445. [DOI] [PubMed] [Google Scholar]
- Pease D, Goodglass H. The effects of cuing on picture naming in aphasia. Cortex. 1978;14:178–189. doi: 10.1016/s0010-9452(78)80043-4. [DOI] [PubMed] [Google Scholar]
- Whitney J. Developing aphasics’ use of compensatory strategies. Paper presented at the annual convention of the American Speech and Hearing Association; Washington, DC, USA. 1975. [Google Scholar]
COMMENTARY REFERENCES
- Aten J. Functional communication treatment. Language intervention strategies in adult aphasia. Baltimore: Williams & Wilkins; 1986. [Google Scholar]
- Backman L, Mantyla T. Effectiveness of self-generated cues in younger and older adults: The role of retention interval. International Journal of Aging and Human Development. 1988;26(4):241–248. doi: 10.2190/TQWD-W1AQ-1NV2-P73G. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Holland AL, Murray LL. Naming famous people: An examination of tip-of-the-tongue phenomena in aphasia and Alzheimer’s disease. Aphasiology. 1997;11(4–5):323–336. [Google Scholar]
- Boyle M. Semantic feature analysis treatment for anomia in two fluent aphasia syndromes. American Journal of Speech-Language Pathology. 2004;13:236–249. doi: 10.1044/1058-0360(2004/025). [DOI] [PubMed] [Google Scholar]
- Boyle M, Coelho CA. Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology. 1995;4:94–108. [Google Scholar]
- Coelho CA, McHugh RE, Boyle M. Semantic feature analysis as a treatment for aphasic dysnomia: A replication. Aphasiology. 2000;14(2):133–142. [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- Constantinidou F, Kreimer L. Feature description and categorization of common objects after traumatic brain injury: The effects of a multi-trial paradigm. Brain and Language. 2004;89:216–225. doi: 10.1016/S0093-934X(03)00399-7. [DOI] [PubMed] [Google Scholar]
- Cranfill TB, Simmons-Mackie N, Kearns KP. Preface to “Treatment of aphasia through family member training”. Aphasiology. 2005;19(6):577–582. [Google Scholar]
- Cunningham R, Ward CD. Evaluation of a training programme to facilitate conversation between people with aphasia and their partners. Aphasiology. 2003;17(8):687–707. [Google Scholar]
- Davis GA. PACE revisited. Aphasiology. 2005;19(1):21–38. [Google Scholar]
- Davis GA, Wilcox M. Adult aphasia rehabilitation. San Diego, CA: College-Hill Press; 1985. [Google Scholar]
- DeDe G, Parris D, Waters G. Teaching self-cues: A treatment approach for verbal naming. Aphasiology. 2003;17(5):465–480. [Google Scholar]
- Drummond SS, Rentschler GJ. The efficacy of gestural cueing in dysphasic word-retrieval responses. Journal of Communication Disorders. 1981;14:287–298. doi: 10.1016/0021-9924(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Elman RJ, Bernstein-Ellis E. What is functional? American Journal of Speech-Language Pathology. 1995;4(4):115–117. [Google Scholar]
- Faroqi-Shah Y, Thompson CK. Effect of lexical cues on the production of active and passive sentences in Broca’s and Wernicke’s aphasia. Brain and Language. 2003;85:409–426. doi: 10.1016/s0093-934x(02)00586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Conversational repair of word-finding difficulty. In: Lemme ML, editor. Clinical aphasiology. Vol. 21. Austin, TX: Pro-Ed; 1993. pp. 299–307. [Google Scholar]
- Ferguson A. Conversational turn-taking and repair in fluent aphasia. Aphasiology. 1998;12(11):1007–1031. [Google Scholar]
- Fink RB, Brecher A, Schwartz MF, Robey RR. Rehabilitation of spoken word production in aphasia. Aphasiology. 2002;16(10–11):1061–1086. [Google Scholar]
- Francis DR, Clark N, Humphreys GW. Circumlocution-induced naming (CIN): A treatment for effecting generalisation in anomia? Aphasiology. 2002;16(3):243–259. [Google Scholar]
- Frattali CM, editor. Measuring outcomes in speech-language pathology. New York: Thieme; 1998. [Google Scholar]
- Freed DB, Celery K, Marshall RC. Effectiveness of personalized cueing and phonological cueing on long-term naming performance by aphasic subjects: A clinical investigation. Aphasiology. 2004;18(8):743–757. [Google Scholar]
- Freed DB, Marshall RC. The effect of cue origin on the facilitation of aphasic subjects’ verbal labeling. In: Lemme ML, editor. Clinical aphasiology. Vol. 23. Austin, TX: Pro-Ed; 1995a. [Google Scholar]
- Freed DB, Marshall RC. The effect of personalized cueing on long-term naming of realistic visual stimuli. American Journal of Speech-Language Pathology. 1995b;4(4):105–108. [Google Scholar]
- Freed DB, Marshall RC, Nippold MA. Comparison of personalized cueing and provided cueing on the facilitation of verbal labeling by aphasic patients. Journal of Speech and Hearing Research. 1995;38:1081–1090. doi: 10.1044/jshr.3805.1081. [DOI] [PubMed] [Google Scholar]
- Frick-Horbury D. The use of hand gestures as self-generated cues for recall of verbally associated targets. American Journal of Psychology. 2002;115(1):1–20. [PubMed] [Google Scholar]
- Golper LC, Rau MT. Systematic analysis of cuing strategies in aphasia: Taking your “cue” from the patient. In: Brookshire RH, editor. Clinical aphasiology. Vol. 13. Minneapolis, MN: BRK Publishers; 1983. pp. 52–64. [Google Scholar]
- Kagan A, Winckel J, Black S, Duchan JF, Simmons-Mackie N, Square P. A set of observational measures for rating support and participation in conversation between adults with aphasia and their conversation partners. Topics in Stroke Rehabilitation. 2004;11(1):67–83. doi: 10.1310/CL3V-A94A-DE5C-CVBE. [DOI] [PubMed] [Google Scholar]
- Hillis A. Treatment of naming disorders: New issues regarding old therapies. Journal of the International Neuropsychological Society. 1998;4:648–660. doi: 10.1017/s135561779846613x. [DOI] [PubMed] [Google Scholar]
- Holland AL. Some practical considerations in aphasia rehabilitation. In: Sullivan M, Kommers MS, editors. Rationale for adult aphasia therapy. Omaha, NE: University of Nebraska Medical Center; 1977. pp. 167–180. [Google Scholar]
- Holland AL. Communicative Abilities in Daily Living. 2. Austin, TX: Pro-Ed; 1980. [Google Scholar]
- Holland AL, Frattali CM, Fromm D. Communication Activities of Daily Living. 2. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Hough MS, Pierce RS. Pragmatics and treatment. In: Chapey R, editor. Language intervention strategies in adult aphasia. Baltimore: Williams & Wilkins; 1994. pp. 246–268. [Google Scholar]
- Howard D, Harding D. Self-cueing of word retrieval by a woman with aphasia: Why a letter board works. Aphasiology. 1998;12(4–5):399–420. [Google Scholar]
- Li EC, Williams SE, Della Volpe A. The effects of topic and listener familiarity on discourse variables in procedural and narrative discourse tasks. Journal of Communication Disorders. 1995;28(1):39–55. doi: 10.1016/0021-9924(95)91023-z. [DOI] [PubMed] [Google Scholar]
- Linebaugh CW, Lehner L. Cueing hierarchies and word retrieval: A therapy program. In: Brookshire RH, editor. Clinical aphasiology. Vol. 7. Minneapolis, MN: BRK Publishers; 1977. pp. 19–31. [Google Scholar]
- Lomas J, Pickard L, Bester S, Elbard H, Finlayson A, Zoghaib C. The communicative effectiveness index: Development and psychometric evaluation of a functional communication measure for adult aphasia. Journal of Speech and Hearing Disorders. 1989;54:113–124. doi: 10.1044/jshd.5401.113. [DOI] [PubMed] [Google Scholar]
- Lowell S, Beeson PM, Holland AL. The efficacy of a semantic cueing procedure on naming performance of adults with aphasia. American Journal of Speech-Language Pathology. 1995;4(4):109–114. [Google Scholar]
- Lubinski R, Duchan J, Weitzner-Lin . Analysis of breakdowns and repairs in aphasic adult communication. In: Brookshire RH, editor. Clinical aphasiology. Vol. 10. Minneapolis, MN: BRK Publishers; 1980. pp. 111–116. [Google Scholar]
- Lustig AP, Tompkins CA. A written communication strategy for a speaker with aphasia and apraxia of speech: Treatment outcomes and social validity. Aphasiology. 2002;16(456):507–521. [Google Scholar]
- Mantyla T. Optimizing cue effectiveness: Recall of 500 and 600 incidentally learned words. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12(1):66–71. [Google Scholar]
- Marshall RC. Word retrieval behavior of aphasic adults. Journal of Speech and Hearing Disorders. 1976;44:444–451. doi: 10.1044/jshd.4104.444. [DOI] [PubMed] [Google Scholar]
- Marshall RC, Freed DB. Lexical retrieval behaviors of subjects with aphasia and subjects without brain damage on a rebus riddle task. American Journal of Speech-Language Pathology. 1995;4:164–168. [Google Scholar]
- Marshall RC, Freed DB, Karow CM. Learning of subordinate category names by aphasic subjects: A comparison of deep and surface-level training methods. Aphasiology. 2001;15(6):585–598. [Google Scholar]
- Marshall RC, Freed DB, Phillips DS. Labeling of novel stimuli by aphasic subjects: Effects of phonologic and self-cueing procedures. In: Lemme ML, editor. Clinical aphasiology. Vol. 22. Austin, TX: Pro-Ed; 1994. pp. 335–343. [Google Scholar]
- Marshall RC, Karow CM, Freed DB, Babcock P. Effects of personalized cue form on the learning of subordinate category names by aphasic and non-brain damaged subjects. Aphasiology. 2002;16(7):763–771. [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Experimental analysis of aphasia treatment tasks: A preliminary report. In: Prescott TE, editor. Clinical aphasiology. Vol. 20. Austin, TX: Pro-Ed; 1991. [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Effects of facilitation and cueing on labelling of ‘novel’ stimuli by aphasic subjects. Aphasiology. 1992;6(6):567–583. [Google Scholar]
- Marshall RC, Tompkins CA. Identifying behavior associated with verbal self-corrections of aphasic clients. Journal of Speech and Hearing Disorders. 1981;46(2):168–173. doi: 10.1044/jshd.4602.168. [DOI] [PubMed] [Google Scholar]
- Marshall RC, Tompkins CA. Verbal self-correction behaviors of fluent and nonfluent aphasic subjects. Brain and Language. 1982;15:292–306. doi: 10.1016/0093-934x(82)90061-x. [DOI] [PubMed] [Google Scholar]
- Massaro M, Tompkins CA. Feature analysis for treatment of communication disorders in traumatically brain-injured patients: An efficacy study. In: Lemme ML, editor. Clinical aphasiology. Vol. 22. Austin, TX: Pro-Ed; 1994. pp. 245–256. [Google Scholar]
- Mayer JF, Murray LL. Functional measures of naming in aphasia: Word retrieval in confrontation naming versus connected speech. Aphasiology. 2003;17(5):481–497. [Google Scholar]
- Oelschlaeger ML. Participation of a conversation partner in the word searches of a person with aphasia. American Journal of Speech-Language Pathology. 1999;8(1):62–71. [Google Scholar]
- Pashek GV, Tompkins CA. Context and word class influences on lexical retrieval in aphasia. Aphasiology. 2002;16(3):261–286. [Google Scholar]
- Pease DM, Goodglass H. The effects of cuing on picture naming in aphasia. Cortex. 1978;14:178–189. doi: 10.1016/s0010-9452(78)80043-4. [DOI] [PubMed] [Google Scholar]
- Robey RR, Schultz MC. A model for conducting clinical-outcome research: An adaptation of the standard protocol for use in aphasiology. Aphasiology. 1998;12(9):787–810. [Google Scholar]
- Saito A, Takeda K. Semantic cueing effects on word retrieval in aphasic patients with lexical retrieval deficit. Brain and Language. 2001;77:1–9. doi: 10.1006/brln.2000.2388. [DOI] [PubMed] [Google Scholar]
- Sauzeon H, N’Kaoua B, Claverie B. The effect of self generated category cues on organized processing in the recall performance of young, middle-old, and old adults. Current Psychology Letters: Behaviour, Brain and Cognition. 2001;(5):65–78. [Google Scholar]
- Seron X, Van der Kaa MA, Vanderlinden M, Remits A, Feyereisen P. Decoding paralinguistic signals: Effects of semantic and prosodic cues on aphasics’ comprehension. Journal of Communication Disorders. 1982;15:223–231. doi: 10.1016/0021-9924(82)90035-1. [DOI] [PubMed] [Google Scholar]
- Simmons NN, Kearns KP, Potechin G. Treatment of aphasia through family member training. In: Brookshire RH, editor. Clinical aphasiology. Vol. 17. Minneapolis, MN: BRK Publishers; 1987. [Google Scholar]
- Simmons-Mackie NN, Damico JS. Communication competence in aphasia: Evidence from compensatory strategies. In: Lemme ML, editor. Clinical aphasiology. Vol. 23. Austin, TX: Pro-Ed; 1995. [Google Scholar]
- Spencer KA, Doyle PJ, McNeil MM, Wambaugh JL, Park G, Carroll B. Examining the facilitative effects of rhyme in a patient with output lexicon damage. Aphasiology. 2000;14(5–6):567–584. [Google Scholar]
- Stimley MA, Noll JD. The effects of semantic and phonemic prestimulation cues on picture naming in aphasia. Brain and Language. 1991;41:496–509. doi: 10.1016/0093-934x(91)90170-6. [DOI] [PubMed] [Google Scholar]
- Tompkins CA, Lustig AP. Research principles for the clinician. In: Chapey R, editor. Language intervention strategies in aphasia and related neurogenic communication disorders. 4. Baltimore: Lippincott Williams & Wilkins; 2001. pp. 129–147. [Google Scholar]
- Tompkins CA, Marshall RC. Communicative value of self-cues in aphasia. In: Brookshire RH, editor. Clinical aphasiology. Vol. 12. Minneapolis, MN: BRK Publishers; 1982. pp. 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J, Cameron R, Kalinyak-Fliszar M, Nessler C, Wright S. Retrieval of action names in aphasia: Effects of two cueing treatments. Aphasiology. 2004;18(11):979–1004. [Google Scholar]
- Watamori TS, Fukusako Y, Monoi H, Sasanuma S. Confrontation naming performance in dementia and aphasia. In: Prescott TE, editor. Clinical aphasiology. Vol. 20. Austin, TX: Pro-Ed; 1991. [Google Scholar]
- Weidner WE, Jinks AFG. The effects of single versus combined cue presentations on picture naming by aphasic adults. Journal of Communication Disorders. 1983;16:111–121. doi: 10.1016/0021-9924(83)90042-4. [DOI] [PubMed] [Google Scholar]
- Whitney JL, Goldstein H. Using self-monitoring to reduce disfluencies in speakers with mild aphasia. Journal of Speech and Hearing Disorders. 1989;54(4):576–586. doi: 10.1044/jshd.5404.576. [DOI] [PubMed] [Google Scholar]
- Williams SE, Li EC, Della Volpe A, Ritterman SI. The influence of topic and listener familiarity on aphasic discourse. Journal of Communication Disorders. 1994;27(3):207–222. doi: 10.1016/0021-9924(94)90001-9. [DOI] [PubMed] [Google Scholar]