Abstract
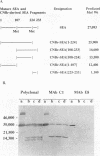
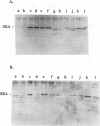
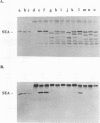
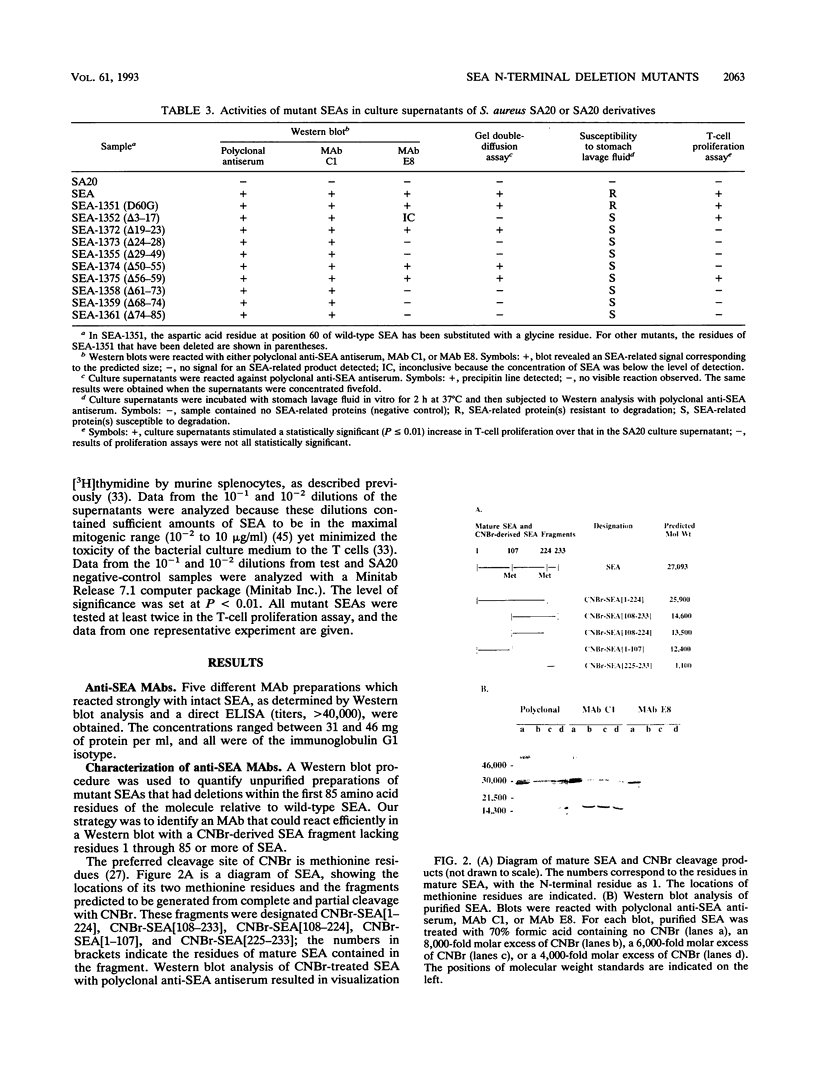
Previous findings indicate that the N-terminal region of staphylococcal enterotoxin type A (SEA) is required for its ability to induce T-cell proliferation. To better localize internal peptides of SEA that are important for induction of murine T-cell proliferation, SEA mutants that had internal deletions in their N-terminal third were constructed. A series of unique restriction enzyme sites were first engineered into sea; only one of these changes resulted in an amino acid substitution (the aspartic acid residue at position 60 of mature SEA was changed to a glycine [D60G]). Because the D60G substitution had no discernible effect on serological or biological activity, the sea allele encoding this mutant SEA was used to construct a panel of mutant SEAs lacking residues 3 to 17, 19 to 23, 24 to 28, 29 to 49, 50 to 55, 56 to 59, 61 to 73, 68 to 74, or 74 to 85. Recombinant plasmids with the desired mutations were constructed in Escherichia coli and transferred to Staphylococcus aureus. Staphylococcal culture supernatants containing the mutant SEAs were examined. Western immunoblot analysis with polyclonal anti-SEA antiserum revealed that each of the recombinant S. aureus strains produced a mutant SEA of the predicted size. All the mutant SEAs exhibited increased sensitivity to monkey stomach lavage fluid in vitro, which is consistent with these mutants having conformations unlike that of wild-type SEA or the SEA D60G mutant. In general, deletion of internal peptides had a deleterious effect on the ability to induce T-cell proliferation; only SEA mutants lacking either residues 3 to 17 or 56 to 59 consistently produced a statistically significant increase in the incorporation of [3H]thymidine. In the course of this work, two monoclonal antibodies that had different requirements for binding to SEA in Western blots were identified. The epitope for one monoclonal antibody was contained within residues 108 to 230 of mature SEA. Binding of the other monoclonal antibody to SEA appeared to be dependent on the conformation of SEA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Borst D. W., Regassa L. B. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol. 1992;55:1–35. [PubMed] [Google Scholar]
- Betley M. J., Löfdahl S., Kreiswirth B. N., Bergdoll M. S., Novick R. P. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5179–5183. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Handley J. P., Schlievert P. M. Biological and immunological properties of the carboxyl terminus of staphylococcal enterotoxin C1. Infect Immun. 1989 Jan;57(1):23–28. doi: 10.1128/iai.57.1.23-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. doi: 10.1128/iai.57.7.2249-2252.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Bowness P., Moss P. A., Tranter H., Bell J. I., McMichael A. J. Clostridium perfringens enterotoxin is a superantigen reactive with human T cell receptors V beta 6.9 and V beta 22. J Exp Med. 1992 Sep 1;176(3):893–896. doi: 10.1084/jem.176.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K. W., Watson D. W. Pyrogenic specificity of streptococcal exotoxins, staphylococcal enterotoxin, and gram-negative endotoxin. Infect Immun. 1974 Aug;10(2):347–351. doi: 10.1128/iai.10.2.347-351.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow R., O'Hehir R. E., Schreifels R., Kummerehl T. J., Riley G., Lamb J. R. Localization of the immunologic activity in the superantigen Staphylococcal enterotoxin B using truncated recombinant fusion proteins. J Immunol. 1992 Jan 1;148(1):1–6. [PubMed] [Google Scholar]
- CLARK W. G., BORISON H. L. PYROGENIC EFFECT OF PURIFIED STAPHYLOCOCCAL ENTEROTOXIN. J Pharmacol Exp Ther. 1963 Nov;142:237–241. [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Fast D. J., Schlievert P. M., Nelson R. D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989 Jan;57(1):291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Griggs N. D., Pontzer C. H., Jarpe M. A., Johnson H. M. Mapping of multiple binding domains of the superantigen staphylococcal enterotoxin A for HLA. J Immunol. 1992 Apr 15;148(8):2516–2521. [PubMed] [Google Scholar]
- Grossman D., Cook R. G., Sparrow J. T., Mollick J. A., Rich R. R. Dissociation of the stimulatory activities of staphylococcal enterotoxins for T cells and monocytes. J Exp Med. 1990 Dec 1;172(6):1831–1841. doi: 10.1084/jem.172.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D., Van M., Mollick J. A., Highlander S. K., Rich R. R. Mutation of the disulfide loop in staphylococcal enterotoxin A. Consequences for T cell recognition. J Immunol. 1991 Nov 15;147(10):3274–3281. [PubMed] [Google Scholar]
- Hedlund G., Dohlsten M., Herrmann T., Buell G., Lando P. A., Segrén S., Schrimsher J., MacDonald H. R., Sjögren H. O., Kalland T. A recombinant C-terminal fragment of staphylococcal enterotoxin A binds to human MHC class II products but does not activate T cells. J Immunol. 1991 Dec 15;147(12):4082–4085. [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hufnagle W. O., Tremaine M. T., Betley M. J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect Immun. 1991 Jun;59(6):2126–2134. doi: 10.1128/iai.59.6.2126-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. J., Hudson K. R., Fraser J. D., Gascoigne N. R. Enterotoxin residues determining T-cell receptor V beta binding specificity. Nature. 1992 Oct 29;359(6398):841–843. doi: 10.1038/359841a0. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Magazine H. I. Potent mitogenic activity of staphylococcal enterotoxin A requires induction of interleukin 2. Int Arch Allergy Appl Immunol. 1988;87(1):87–90. doi: 10.1159/000234654. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Herman A., Clements J., Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Feb 1;175(2):387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Blackman M., Kushnir E., Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990 Feb 1;171(2):455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L. Microbial "superantigens". Infect Immun. 1990 Aug;58(8):2409–2413. doi: 10.1128/iai.58.8.2409-2413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Pier G. B. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis. 1986 Jul;154(1):55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R., Gould S., Bergdoll M. Detecting the enterotoxigenicity of Staphylococcus aureus strains. Appl Microbiol. 1974 Dec;28(6):946–950. doi: 10.1128/am.28.6.946-950.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978 Dec 25;253(24):8787–8791. [PubMed] [Google Scholar]
- Stuart P. M., Woodward J. G. Yersinia enterocolitica produces superantigenic activity. J Immunol. 1992 Jan 1;148(1):225–233. [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V. Spleen cell analysis and optimal immunization for high-frequency production of specific hybridomas. Methods Enzymol. 1983;92:26–36. doi: 10.1016/0076-6879(83)92006-2. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- Tomai M., Kotb M., Majumdar G., Beachey E. H. Superantigenicity of streptococcal M protein. J Exp Med. 1990 Jul 1;172(1):359–362. doi: 10.1084/jem.172.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Enzyme-linked fluorescence assay: Ultrasensitive solid-phase assay for detection of human rotavirus. J Clin Microbiol. 1979 Sep;10(3):317–321. doi: 10.1128/jcm.10.3.317-321.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]