Abstract
The process of capacitative or store-operated Ca2+ entry has been extensively investigated, and recently two major molecular players in this process have been described. STIM1 acts as a sensor for the level of Ca2+ stored in the endoplasmic reticulum, and Orai proteins constitute pore-forming subunits of the store-operated channels. Store-operated Ca2+ entry is readily demonstrated with protocols that provide extensive Ca2+ store depletion; however, the role of store-operated entry with modest and more physiological cell stimuli is less certain. Recent studies have addressed this question in cell lines; however, the role of store-operated entry during physiological activation of primary cells has not been extensively investigated, and there is little or no information on the roles of STIM and Orai proteins in primary cells. Hepatocytes respond to physiological levels of glycogenolytic hormones with well characterized intracellular Ca2+ oscillations. Also, the nature of the Ca2+ influx mechanism with hormone activation of hepatocytes is controversial. In the current study, we have utilized both pharmacological tools and RNAi-based techniques to investigate the role of store-operated channels in the maintenance of hormone-induced Ca2+ oscillations in rat hepatocytes. Pharmacological inhibitors of store-operated channels blocked thapsigargin-induced Ca2+ entry but only partially reduced the frequency of Ca2+ oscillations. Similarly, RNAi knock-down of STIM1 or Orai1 substantially reduced thapsigargin-induced calcium entry, and more modestly diminished the frequency of vasopressin-induced oscillations.
Conclusion
Our findings establish that store-operated Ca2+ entry plays a role in the maintenance of agonist-induced oscillations in primary rat hepatocytes, but indicate that other agonist-induced entry mechanisms must be involved to a significant extent.
Keywords: Calcium Channels, Vasopressin Receptor, Stim1, Orai1, Store-operated Channels
Introduction
Calcium signals, which can be induced by a variety of stimuli, control a myriad of functions in the body. In the liver, bile secretion, glucose production, and permeability of tight junctions are all regulated by calcium signals. The liver is mainly composed of hepatocytes, multifunctional cells involved in the regulation of a number of critical homeostatic hormone-controlled pathways. In hepatocytes, Ca2+ signaling occurs in the form of baseline oscillations that stem from periodic openings of Ca2+ channels in the membrane of the endoplasmic reticulum (ER) (1, 2). These oscillations are usually repetitive spikes separated by intervals that can range from a few milliseconds to a few minutes, depending on the type of agonist and its concentration. Moreover, when oscillations become spatially organized, intercellular calcium waves occur, representing an efficient form of communication between cells through which physiological responses can be coordinated (3, 4).
Like most receptor-activated calcium signals, the generation and maintenance of oscillations involves two components- the release of internally stored calcium from the ER and the entry of extracellular calcium to maintain adequate Ca2+ stores in the ER. This second component often involves a process known as capacitative calcium entry or store-operated calcium entry (SOCE), occurring through channels located in the plasma membrane (5, 6). The best characterized of these store-operated channels is the Ca2+-release-activated Ca2+ (CRAC) channel (6-8). The mechanism by which ER Ca2+ levels are sensed to initiate SOCE was unknown until recently, as was the molecular identity of the store-operated channels. Utilization of RNAi screens combined with calcium entry assays identified Drosophila STIM (stromal interaction molecule) (9) or mammalian STIM1 (10) as an essential component of SOCE. Subsequent studies indicated that STIM1, an ER membrane protein with an EF-hand domain in the lumen of the ER, is most likely the calcium sensor that links store depletion to store-operated channels in the plasma membrane (10, 11). This finding was shortly followed by the identification of the protein, Orai1 or CRACM1, as a pore-forming subunit of the CRAC channel in lymphocytes (12-17).
It is well-known that store-operated calcium channels are required for the replenishment of calcium following extensive depletion of ER Ca2+, but their role with submaximal, more physiological stimuli that generally result in cytoplasmic calcium oscillations is controversial (18-20). Furthermore, in the rat hepatocyte, the relative contribution of store-operated entry to the Ca2+ signals seen with receptor activation has been questioned (21, 22). It has been shown in many cell types, including hepatocytes, that extracellular calcium is indeed required for the maintenance of oscillations since they diminish after a few minutes in nominally calcium-free medium (23-25). In one study pharmacological evidence was presented implicating SOCE in the maintenance of agonist-induced oscillations in rat hepatocytes (26). In HEK-293 cells, the frequency of oscillations was significantly reduced in the presence of the two SOCE inhibitors, Gd3+ and 2APB (25). The recent discoveries that the STIM1 and Orai1 proteins are essential for CRAC channel activation provide a more succinct molecular signature for at least one type of store-operated channel, the archetypical CRAC channel. In the current study, we have utilized pharmacological inhibitors as well as molecular manipulations of STIM and Orai proteins to investigate the role of store-operated Ca2+ channels in oscillations in primary rat hepatocytes. To our knowledge this is the first investigation of the roles of these key signaling proteins in the process of Ca2+ signaling in the liver1, and the first investigation of their role in Ca2+ oscillations in any primary mammalian cell.
Materials and Methods
Hepatocyte Isolation, Cell Culture and Transfection
The liver was isolated from adult male Sprague-Dawley rats and digested with the collagenase perfusion method developed by Berry and Friend (27). After collagenase treatment, the liver was excised, minced in balanced salt solution, and centrifuged at 50 g for 3 min. Immediately following isolation, hepatocytes were transfected using the Amaxa Nucleofector protocol with plasmids coding small hairpin RNAs (shRNA, 3 μg each) against STIM1, STIM2, Orai1, Orai2, or Orai3 or transfected with the empty vector (all HuSH-29 shRNA constructs, Origene Technologies, Inc; Rockville, MD) and co-transfected with 0.2 μg cDNA for GFP (see Table I for targeted gene sequences). In some experiments, full-length STIM2 cDNA plasmid in the pCMV6-XL5 vector (Origene) was transfected into hepatocyte cultures. All transfected hepatocytes were then resuspended in Williams E Medium containing penicillin (100 units/ml), streptomycin (100 μg/ml), 2 mM glutamine, 10 mM HEPES, ph 7.4, and 10% fetal bovine serum and plated on collagen-coated 30-mm round glass coverslips at a density of 1200 cells/10 μL, and maintained for 48 hours in a 95% air-5% CO2, 37° C incubator. Non-transfected hepatocytes were used in pharmacological studies. These hepatocytes were also plated on collagen-coated coverslips, cultured in the same medium as described for transfected hepatocytes and maintained in culture for 24 hours after isolation.
Table I.
RNAi sequences Used to Achieve Knockdown in Hepatocytes
| Gene of Interest | RNAi Sequence |
|---|---|
| Orai1 | GACCGACAGTTCCAGGAGCTCAACGAGCT |
| Orai2 | AGACGCTAGCCACGAGCCGGAGCGGAGAA |
| GTGCAGGCCCTGTCCTGGAGGAAGCTGTA | |
| Orai3 | TGGCATTTAGTGCCTGCACCACTGTGTTA |
| Stim1 | ACAGTGAAACACAGCACCTTCCATGGTGA |
| Stim2 | ATTGAGAAGATCTGTGGCTTTCAGATAGC |
| AGACACAGCTTCAGAATGTGACTCCTTAA |
RNA Isolation and Real Time PCR
For each transfection condition, total RNA was isolated 48 hours post-transfection with a mini-prep kit using the manufacturer’s instructions (Quiagen). For the reverse transcriptase step, 1 μg of DNase-treated total RNA for each sample was used according to the manufacturer’s protocol (SuperArray). STIM1, STIM2, Orai1, Orai2, and Orai3 mRNA levels were semi-quantified in control-transfected hepatocytes. In addition, STIM1 and Orai mRNA levels were quantified for each knockdown condition. Quantitative real-time PCR was carried out with the ABI Prism 7000 Instrument (Applied Biosystems) using a PCR master mix (SuperArray) and SYBR Green probe. All primers were obtained from SuperArray (proprietary sequences). The reaction volume for each sample, 25 μl, was run in triplicate in 96-well PCR plates. Standard curves were generated for each gene by preparing ten-fold serial dilutions using pooled cDNAs containing the message of interest. The following program was used to amplify the reactions: i) Melting, 95 °C, 10 min; ii) melting, 95 °C, 15 sec; iii) annealing, 60 °C, 60 sec (SYBR Green fluorescence detected); iv) repeat to step ii for 40 cycles. Real time data was collected by the ABI software program and raw data was exported to Excel for analysis. All transfection conditions were run in parallel. The mRNA levels were normalized against the amount of a housekeeping gene transcript, GAPDH. The normalized ratio levels for each knockdown condition were divided by the normalized ratio level for the control-transfected condition to obtain a ratio of the expression level relative to the wild-type condition.
Intracellular Calcium Measurements
In preparation for [Ca2+]i measurements, coverslips were rinsed twice with Hepes buffered salt solution (HBSS) containing 121 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM glucose, 20 mM HEPES, and 1.8 mM Ca2+ in the presence of 250 μM sulfinpyrazone. Hepatocytes were then loaded with 5 μM Fura-5F for 45 minutes at 37° C and 5% CO2. The coverslips with Fura-5F-loaded cells were placed onto the stage of a Nikon TS-100 inverted microscope equipped with a 20× objective and a light-sensitive CCD camera to monitor changes in fluorescence. Changes in intracellular calcium ([Ca2+]i) were monitored by alternately exciting the dye at 340 and 380 nm, and collecting the emission at 510 nm using InCytIm2 imaging platform (Intracellular Imaging Inc., Cincinnati, OH). For all experiments, ratio values were corrected for autofluorescence, measured after treatment of cells with 5 μM ionomycin and 20 mM MnCl2. Only cells in the field of view that were GFP-positive were chosen as regions of interest. The background-corrected ratio of the 510 nM emission fluorescence was calculated from 25-35 cells in the field. For Ca2+ free experiments, measurements were made in nominally calcium-free HBSS. In experiments utilizing Gd3+, the buffer was modified to contain no sulfate, phosphate or bicarbonate. To analyze agonist-induced calcium oscillations, we focused on the oscillation frequency of a field of individual cells recorded over a period of 25 minutes following agonist stimulation. For analysis, this 25-minute time period was then divided into 5-minute intervals. Within each 5-minute interval, the number of oscillations was determined. For all cells that responded in a given field, the number of Ca2+ spikes per 5-minute interval was then averaged to generate an average frequency plot for each knockdown condition or treatment (25, 28).
RESULTS
Calcium Dependence of Oscillations in Hepatocytes
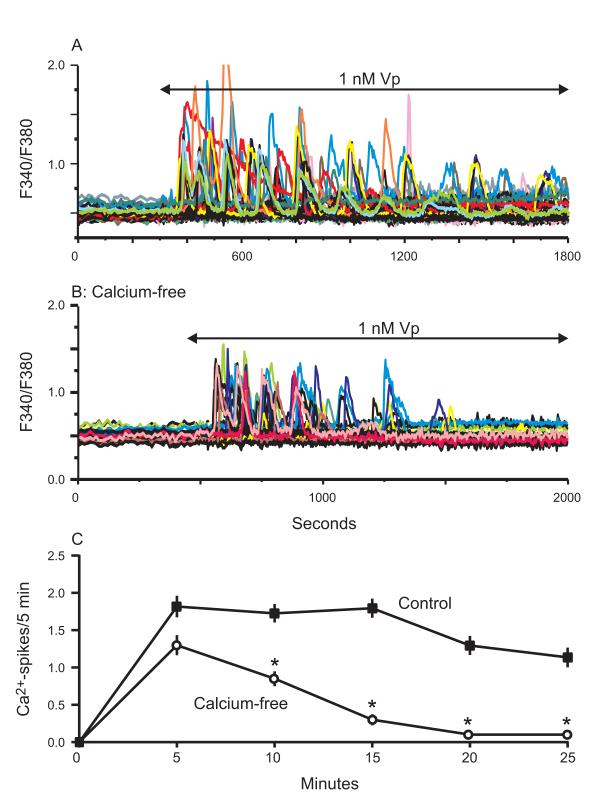
Rat hepatocytes express the V1a subtype of vasopressin receptor, and when stimulated with 1 nM vasopressin, individual cells displayed a spectrum of [Ca2+]i responses that included baseline oscillations, sustained responses, or failure to respond (Figure 1A). On average, 65-70% of cells responded to vasopressin, and approximately 20% of these responses involved sustained elevations in baseline Ca2+ (data not shown). The oscillations monitored in individual cells displayed a range of frequencies sustained over 25 minutes (Figure 1C, Control). In nominally calcium-free conditions (Figure 1B), the frequency of oscillations was not sustained beyond 10 minutes following agonist stimulation (Figure 1C, Calcium-free), supporting previously published observations that calcium entry across the plasma membrane is required to maintain oscillations (29).
Figure 1. Calcium oscillations run down in the absence of extracellular Ca2+.
A) Vasopressin induced calcium oscillations in hepatocytes loaded with fura-5F. An ensemble of 30-35 individual cells displayed a range of frequencies in response to 1 nM vasopressin, with a few cells exhibiting sustained signals. B) Vasopressin-induced oscillations are significantly diminished in Ca2+-free media, where N=30 cells. C) For each condition described above, the oscillation frequency is summarized as the number of Ca2+ spikes per 5 minute interval following agonist-stimulation. The data is taken from 3 independent experiments and is reported as the mean ± SEM, where N=53 and 54 cells for the 1.8 mM and nominally- free Ca2+ conditions, respectively. Student’s t-test revealed a significant change in the number of Ca2+ spikes/5 min for each time point, denoted by “*” (p < 0.05).
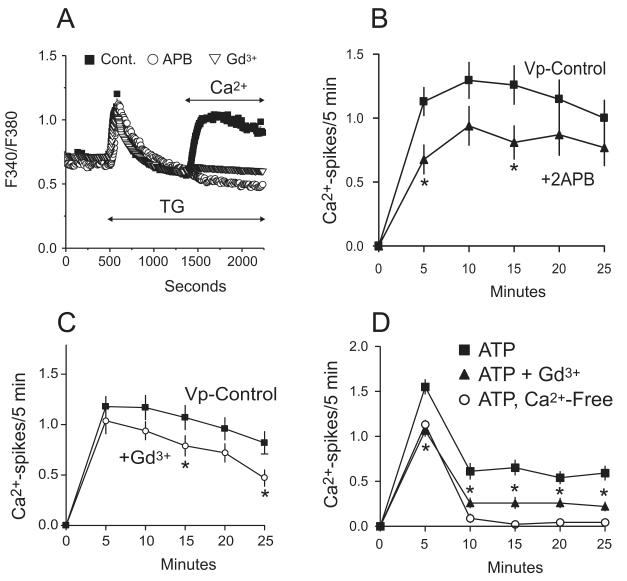
Gadolinium (Gd3+) and 2-aminoethyldiphenyl borate (2APB), known pharmacological inhibitors of SOCE, were used to block the action of thapsigargin in order to provide clues as to which entry pathway(s) may be involved in agonist-induced oscillations in hepatocytes. To confirm the pharmacological efficacy of these inhibitors, we utilized a protocol in which thapsigargin was added to hepatocytes in the absence of Ca2+, to induce Ca2+ release, followed by addition of Ca2+ to assess store-operated Ca2+ entry. Pre-incubation with either 30 μM 2APB or 1 μM Gd3+ did not affect calcium release, but the entry was almost completely abolished in the presence of these inhibitors (Figure 2A). Treatment with either SOCE inhibitor also significantly reduced the frequency of both vasopressin and ATP-induced oscillations, although they were not completely blocked as was observed in the absence of extracellular Ca2+ (Figure 2b-d). We also monitored the percentage of responding cells as a measure of upstream, receptor-dependent steps in the signaling pathways (28). The mean percentage of cells that responded to vasopressin was reduced by both 2APB and Gd3+, but this decrease was statistically significant only for Gd3+. For 2APB treatment, the percentage of cells responding to vasopressin was 47.33 ± 5.37, compared to 63.8% ± 5.6 for untreated cells. Although we used 2APB to investigate its action on calcium influx, this drug was originally reported as an inhibitor of IP3 receptors (30). Because reports in the literature investigating its specific role varied (31-33), we examined the action of 2APB on the release of Ca2+ by a high (1 μM) vasopressin concentration. As with thapsigargin, we found that the high vasopressin-induced calcium entry was essentially completely blocked by 30 μM 2APB (not shown). The release of Ca2+, indicative of activation of IP3 receptors, was slightly but significantly attenuated (see Supplementary Figure 3). The percentage of cells that responded to this high vasopressin dose in the presence of 2APB was also reduced to 73.3% ± 10.96 in comparison to 96.6% ± 1.67 for the control condition. Thus, an effect of 2APB on intracellular IP3 receptors, or other upstream signaling steps, may contribute in a minor way to the inhibitory action of this drug on the frequency of Ca2+ oscillations. For treatment with 1 μM Gd3+, 45% ± 7.83 of the total cell population responded to vasopressin, while 68% ± 4.42 of control cells responded. The number of cells responding to 5 μM ATP was 82.5%. Treatment with 1 μM Gd3+ reduced this number to 66%, although this change was not statistically significant. We attribute this reduction in cell responsiveness to inhibition of the receptor or possibly phospholipase C activity by the antagonist. The basis for this inhibition by Gd3+ was not investigated further. However, the data for Gd3+ and 2APB for both vasopressin and ATP suggest that at least a component of the Ca2+ entry supporting hepatocyte oscillations involves store-operated channels.
Figure 2. Effect of SOCE inhibitors on thapsigargin (TG) induced entry and agonist-induced Ca2+ oscillations.
A) Shown is a trace of 30-35 hepatocytes, representative of 3 experiments, under control conditions (filled squares) or pre-treated with either 30 μM 2APB (open circles) or 1 μM Gd3+ (open triangles) before adding thapsigargin in the absence of Ca2+ followed by addition of 1.8 mM Ca2+. Treatment with 2APB or Gd3+ completely blocks the Ca2+ entry. B-C) The frequency of vasopressin-induced oscillations is diminished by 2APB or Gd3+. The data is taken from 3 independent experiments and is reported as the mean ± SEM, where N=54 and 50 cells for 2APB or Gd3+, respectively. One-Way ANOVA revealed which time points had a significant change in the number of Ca2+ spikes, denoted by “*” (p < 0.05).
D) ATP-induced oscillations in Ca2+ free media or 1 μM Gd3+ follow trends similar to that observed when using vasopressin. The data, taken from 3 independent experiments, is reported as the mean ± SEM where N=98, 82, and 60 for 5 μM ATP, 1 μM Gd3+, or nominally-free Ca2+, respectively. One-Way Anova revealed a significant change in the number of Ca2+ spikes for each time point, denoted by “*” (p < 0.0001).
Real-Time PCR Measurements
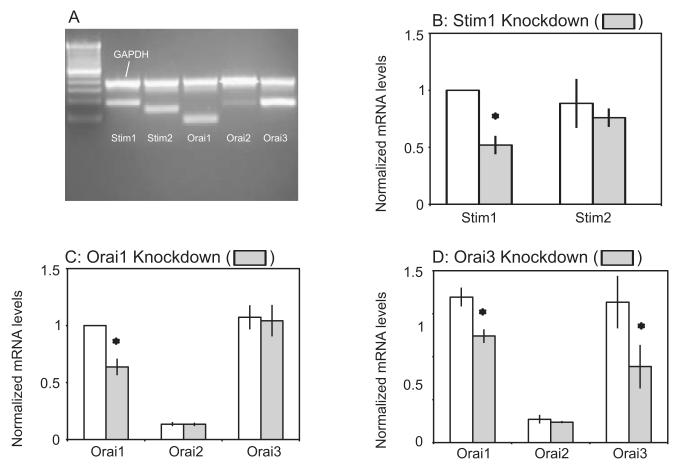
Before considering the molecular basis for the store-operated entry supporting Ca2+ oscillations in hepatocytes, we sought to determine which of the possible players are expressed in this cell type. Using conventional PCR, we detected mRNA for each of the STIM and Orai homologues (Figure 3A). However, Orai2 appeared to be present at much lower levels than Orai1 or 3. Real-time PCR was used to verify reduction of the transcript of interest by RNAi. For control-transfected hepatocytes, STIM1 and STIM2 messages were detected in roughly equal amounts, and the STIM1 mRNA transcript was reduced by approximately 50% in STIM1 shRNA-transfected cells (Figure 3B). As shown in Figure 3C, there was roughly a 45% reduction in mRNA message in Orai1-knockdown cells, and this was specific for Orai1. Orai3 message was reduced by 46% in Orai3 shRNA-transfected cells (Figure 3D). Surprisingly, the RNAi used against Orai3 also significantly reduced the Orai1 message by 27%. Because this result was unexpected, we tried 5 different RNAi plasmids (shRNA’s ). Each of these additional 5 plasmids significantly reduced Orai3 message, but in each case, Orai1 message was reduced as well. Thus, there may be some degree of regulation of Orai1 message transcription or stability by Orai3 message or protein.
Figure 3. Presence of messenger RNA for STIM and Orai homologues in primary hepatocytes and real-time PCR verification of message knockdown by shRNA.
A) RT-PCR reveals mRNA message for STIM1, STIM2, Orai1, Orai2, and Orai3. Using GAPDH as a housekeeping gene, STIM1 and STIM2 were expressed in relatively equal amounts as well as Orai1 and Orai3. In comparison, the message for Orai2 was low but detectable in hepatocytes. B) STIM1 shRNA-transfected cells significantly reduced the STIM1, but not the STIM2 message as revealed using One-Way ANOVA, denoted by “*” (p < 0.05). C) The mRNA message in Orai1-knockdown cells was significantly reduced, but did not affect Orai2 or Orai3 message levels as revealed using One-Way ANOVA, denoted by “*” (p < 0.05). D) Orai3 shRNA significantly reduced both the Orai3 and Orai1 message as revealed using One-Way ANOVA, denoted by “*” (p < 0.05). Real-time PCR measurements were made from RNA extracted from 2 independent transfection experiments, each in triplicate.
Because some experiments were carried out with cells after 24 hours in culture, others after 48 hours, we carried out real-time PCR from RNA extracted at 24 and 48 hours post-isolation (See Supplementary Figure 1). Interestingly, message for STIM1 and STIM2 appeared to decrease after 24 hours in culture; however, there was no statistically significant difference in message levels between 24 and 48 hours for any of the three genes of interest.
Effect of STIM1 or Orai knockdown on SOCE
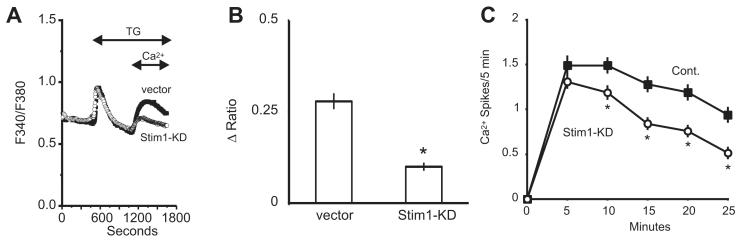
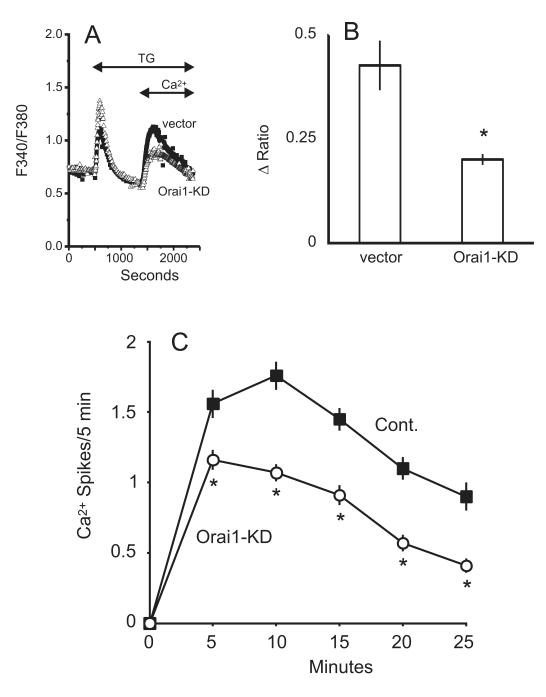
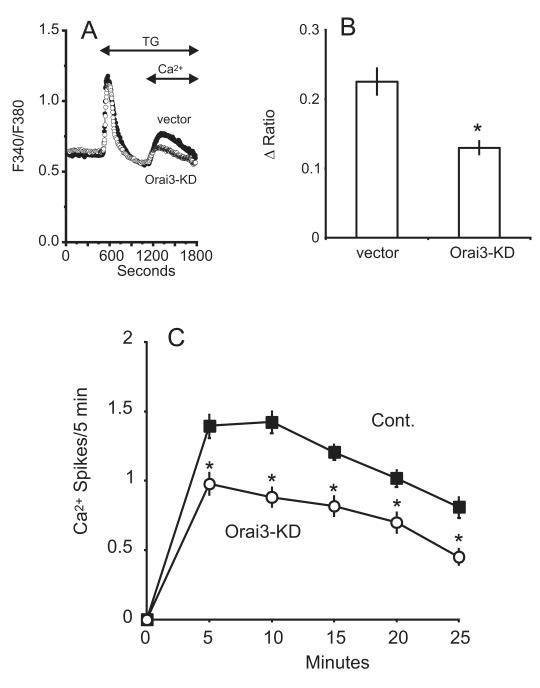
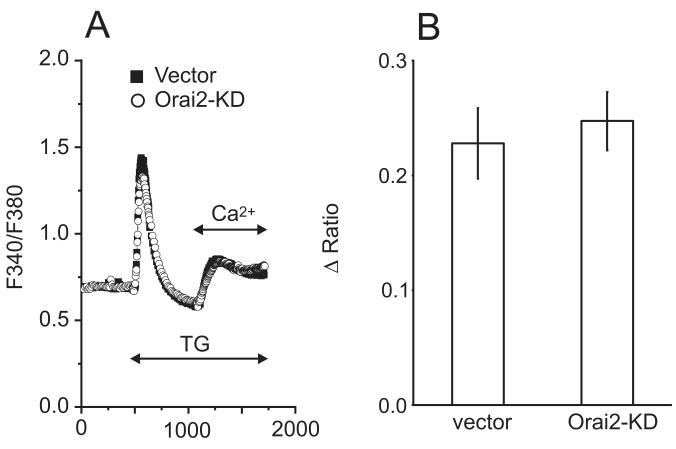
STIM1 is considered the primary ER Ca2+ sensor, and of the three Orai channel subunits, message for Orai1 and 3 suggested these are the major candidates for store-operated channels in hepatocytes. Knockdown of endogenous STIM1, Orai1, or Orai3 was achieved by using shRNAs targeting each gene. Compared to hepatocytes transfected with an empty control vector, a significant reduction in thapsigargin-induced calcium entry was observed following knockdown of STIM1, Orai1 or Orai3. For STIM1 knockdown, the reduction in calcium entry was approximately 64% (Figure 4a-b). A significant reduction in the frequency of vasopressin-induced oscillations was observed 10 minutes after agonist-addition for STIM1-knockdown cells (Figure 4c). Thapsigargin-induced calcium entry was reduced by approximately 53% in Orai1-knockdown hepatocytes (Figure 5a-b) and a significant reduction in frequency was observed after the initial 5 minute period following agonist application (Figure 5c). Thapsigargin-induced calcium entry was reduced by approximately 43% in Orai3-knockdown hepatocytes (Figure 6a-b) and a significant reduction in oscillation frequency was observed after the initial 5 minute period following agonist application (Figure 6c). Transfection with a plasmid encoding shRNA against Orai2 had no effect on the thapsigargin-induced entry in hepatocytes (Figure 7). Compared to control-transfected cells, there was no difference in the number of responding cells for the any of the knockdown conditions discussed above (data not shown).
Figure 4. Effect of STIM1-shRNA knockdown on thapsigargin (TG) induced calcium entry and vasopressin-induced Ca2+ oscillations.
A) Thapsigargin-induced calcium entry was compared in hepatocytes transfected with the empty vector (filled symbols) or shRNA against STIM1 (open symbols). The traces are averages of 25 -30 cells from a single experiment, representative of 5 experiments. B) For the vector and STIM1 shRNA-transfected cells, summarized data are shown for the change in the peak Ca2+ entry amplitude. Student’s t-test revealed a significant decrease in the thapsigargin-induced entry in STIM1 knockdown cells, denoted by “*” (p < 0.05). C) The average frequency of vasopressin-induced calcium oscillations is shown for STIM1 shRNA-transfected vs. control-transfected cells, where N=107 and 100 cells, respectively. Student’s t-test revealed a significant change in the number of Ca2+ spikes for each time point after the initial 5-minute period, denoted by “*” (p < 0.05).
Figure 5. Effect of Orai1-shRNA knockdown on thapsigargin (TG) induced calcium entry and vasopressin-induced Ca2+ oscillations.
A) Thapsigargin-induced calcium entry was compared in hepatocytes transfected with the prs vector (filled symbols) or shRNA against Orai1 (open symbols). The traces are averages of 25 -30 cells from a single experiment, representative of 5 experiments. B) For control and Orai1 shRNA- transfected cells, summarized data are shown for the change in the peak Ca2+ entry amplitude. Student’s t-test revealed a significant decrease in the thapsigargin-induced entry in Orai1 knockdown cells, denoted by “*” (p < 0.05). C) The average oscillation frequency is shown for Orai1 shRNA-transfected vs. control-transfected cells, where N=123 and 107 cells, respectively. Student’s t-test revealed a significant change in the number of Ca2+ spikes for each time point following agonist-addition, denoted by “*” (p < 0.05).
Figure 6. Effect of Orai3-shRNA knockdown on thapsigargin (TG) induced calcium entry and vasopressin-induced Ca2+ oscillations.
A) Thapsigargin-induced calcium entry was compared in hepatocytes transfected with the prs vector (filled symbols) or shRNA against Orai3 (open symbols). The traces are averages of 25 -30 cells from a single experiment, representative of 5 experiments. B) For control and Orai3 shRNA- transfected cells, summarized data are shown for the change in the peak Ca2+ entry amplitude. Student’s t-test revealed a significant decrease in the thapsigargin-induced entry in Orai3 knockdown cells, denoted by “*” (p < 0.05). C) The average oscillation frequency is shown for the Orai3 shRNA-transfected condition vs. control-transfected, where N=93 and 111 cells, respectively. Student’s t-test revealed a significant change in the number of Ca2+ spikes for each time point following agonist-addition, denoted by “*” (p < 0.05).
Figure 7. Effect of Orai2-shRNA knockdown on thapsigargin (TG) induced calcium entry.
A) Thapsigargin-induced calcium entry was compared in hepatocytes transfected with the prs vector (filled symbols) or shRNA against Orai2 (open symbols). The traces are averages of 25 -30 cells from a single experiment, representative of 3 experiments. B) For control and Orai2 shRNA- transfected cells, summarized data are shown for the change in the peak Ca2+ entry amplitude.
We also carried out Western Blot analysis and an immunostain to verify reduction by RNAi of the STIM1 protein (See Supplementary Figure 2). Unfortunately, none of several currently available antibodies for Orai proteins that we tested had sufficient sensitivity to detect endogenous levels of Orai in hepatocytes.
Effect of STIM2 overexpression or knockdown on SOCE
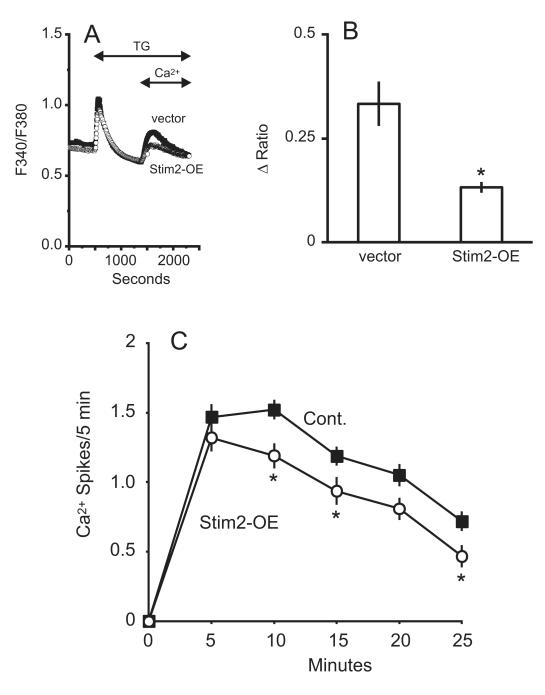
In a previous report, overexpression of STIM2 significantly attenuated store-operated entry, an effect attributed to a dominant negative effect of overexpressed STIM2 (34). Hepatocytes transfected with 5 μg of STIM2 cDNA exhibited a 60% decrease in Ca2+ entry (Figure 8a-b). With the exception of the initial 5-minute period following vasopressin addition, there was a significant decrease in calcium oscillation frequency (Figure 8c). Transfecting hepatocytes with shRNA against STIM2 had no effect on either the thapsigargin-induced entry or the frequency of oscillations (data not shown).
Figure 8. Effect of STIM2 Overexpression on thapsigargin (TG) induced calcium entry and vasopressin-induced Ca2+ oscillations.
A) Transfection with STIM2 cDNA (open symbols) attenuates the thapsigargin-stimulated calcium entry compared to vector transfected cells (filled symbols). The traces are averages of 25-30 cells from a single experiment, representative of 3 experiments. B) For control and STIM2-transfected cells, summarized data are shown for the change in the peak Ca2+ entry amplitude. Student’s t-test revealed a significant decrease in the thapsigargin-induced entry in STIM2-transfected cells, denoted by “*” (p < 0.05). C) The average frequency of oscillations is shown for STIM2-transfected and vector cells, where N=50 and 96 cells, respectively. Student’s t-test revealed a significant change in the number of Ca2+ spikes for the 10, 15, and 25 minute time points, denoted by “*” (p < 0.05).
Discussion
Hepatocytes, which respond to a variety of hormones, have been widely used to study IP3-mediated calcium signals, specifically those involving intracellular Ca2+ oscillations (23, 35). While most of the studies have focused on the receptor-activated signaling pathway, only a few have provided direct evidence supporting a role for SOCE in hepatocytes (32, 36). Yet, the fact that vasopressin-induced oscillations ceased in hepatocytes after several minutes in Ca2+-free media suggests that some mechanism of Ca2+ entry is necessary for normal signaling under physiological conditions. These oscillations in Ca2+-free media likely run down as stores fall below a critical level necessary for generating all-or-none release events. A primary focus of the current study was to gain information on the nature of the entry responsible for maintaining the stores. We have currently focused on the SOCE mechanism, bearing in mind that other pathways have been proposed in hepatocytes (21, 22) and in other cell types (37). In previous studies, we utilized a combined pharmacological and molecular approach to establish that SOCE is the major, although perhaps not exclusive Ca2+ entry mechanism supporting Ca2+ oscillations in a kidney (HEK-293) cell line (25, 28). The inhibition of oscillations by Gd3+ and 2APB is key, because one non-store-operated pathway, involving arachidonic acid-activated channels, similarly depends upon STIM and Orai proteins (38, 39), but is not inhibited by Gd3+ or 2APB in the concentrations used in the current study (25, 40). In contrast to the findings shown here, an earlier study investigating the effects of 2APB and Gd3+ on oscillations in hepatocytes found that these two inhibitors of SOCE blocked oscillations in a manner similar to that observed in calcium-free conditions (26). It should be noted that there are some differences in both the experimental methods that we used to conduct our work as well as the manner in which the results are reported that may account for these discrepancies. In the paper by Gregory and Barritt (26), Fura-2AM was used in the calcium signaling experiments, while Fura5F-AM was used for all calcium measurements in our studies. Fura5-F has a greater capacity to capture calcium transients compared to Fura-2 because it has a higher Kd. Fura-2 has been known to buffer cytoplasmic calcium, and may thus interfere with the measurement of calcium oscillations. Moreover, we have reported statistical summaries from populations of cells because of the considerable variability among individual hepatocytes. In a large proportion of the cells, we did observe clear cessation of calcium spikes after 10-15 minutes following agonist stimulation in the presence of either 2APB or Gd3+. In other instances, the initiation of the first spike was delayed, and was not observed in these cells until 10 minutes or later following stimulation. However, significantly, the SOCE inhibitors did not produce the same degree of reduction in oscillation frequency as removal of extracellular Ca2+, nor did they inhibit oscillations as completely as they inhibited SOCE activated by thapsigargin or agonist at a high concentration. This was the first indication that although SOCE may contribute in a significant way to the maintenance of Ca2+ oscillations, it may not be the only pathway in the rat hepatocyte.
Work from several different laboratories has established the key roles of STIM1 (9, 10) and Orai (or CRACM) (12-14) proteins as key mediators of SOCE. Studies utilizing specific mutations in these proteins have provided strong evidence that STIM1 functions as the endoplasmic reticulum Ca2+ sensor (10, 11) while Orai appears to constitute a pore-forming subunit of at least one type of store-operated channel, underlying the extensively studied calcium-release activated calcium current, or Icrac. Much attention has been directed to the roles of STIM and Orai homologues in immune cells, but prior to the current study there have been no reports on the function of these proteins in other primary mammalian cells. Furthermore, the majority of studies implicate Orai1 as the predominant mediator in SOCE, although evidence suggests potential involvement for each of the three homologues (41-43). Additionally, the role of these proteins in the oscillatory mode of signaling is necessary in some cells (28) but not obligatory in other cells (44).
Knockdown of STIM1 resulted in partial inhibition of Ca2+ oscillations. Furthermore, overexpression of STIM2, which when overexpressed has been shown to have dominant negative activity against STIM1 (34), also reduced oscillation frequency. Thus, STIM1 likely serves as the initiator of SOCE in hepatocytes and contributes to the maintenance of Ca2+ oscillations. We found that Orai3 and Orai1 messages are present in roughly equal amounts in rat hepatocytes. RNAi against either Orai1 or 3 significantly reduced their respective message levels as well as oscillation frequency. However, the role of Orai3 in SOCE-mediated signaling in hepatocytes remains unclear due to the fact that the Orai1 message was also significantly down-regulated by shRNA constructs targeted against Orai3. This does not seem to result from off-target effects of the shRNA constructs, since several different constructs all produced the same effect. Furthermore, the reduction in thapsigargin-induced entry obtained by knocking down Orai3 was not greater than that achieved with Orai1 knockdown, nor was there a greater reduction in the frequency of vasopressin-induced oscillations. We therefore conclude that Orai1 has a major role in SOCE-mediated signaling in hepatocytes. Partial knockdown of Orai1, achieved directly by RNAi against Orai1 or indirectly by RNAi against Orai3, led to a significant reduction in the number of calcium spikes within the initial 5 minute period following agonist application. The functional specificity for the response of a particular cell to a Ca2+-mobilizing stimulus may be encoded by the frequency, amplitude and spatial localization of intracellular Ca2+ oscillations (45-47). Thus, Orai1 might play a significant role in a hepatocyte’s ability to regulate cellular processes such as glycogenolysis and gene expression.
The current study has clearly established a role for store-operated channels, involving STIM1 and Orai1, in the maintenance of Ca2+ oscillations in hepatocytes. However, as was observed for the pharmacological inhibitors, Gd3+ and 2APB, knockdown of STIM1 or Orai1 did not result in complete abrogation of Ca2+ oscillations. This suggests that the Ca2+ entry that supports Ca2+ oscillations in hepatocytes comprises both a store-operated and a non-store-operated component. Hepatocytes express members of the TRPC family of non-store-operated channels ((48) and unpublished observations) which may be involved. Alternatively, the non-store-operated component could represent the arachidonic acid activated Iarc (37). Future studies will focus on the contribution and molecular nature of the non-store-operated entry pathway.
Supplementary Material
Figure S1. Presence of messenger RNA for Stim and Orai homologues in primary hepatocytes. ) RT-PCR reveals mRNA message for Stim1, Stim2, Orai1, Orai2, and Orai3 from RNA extracted immediately after isolation of cells, 24, and 48 hours after isolation. There is a significant decrease in each gene message from 0 hrs to 24 hours, but the message levels do not change significantly for any of the genes from 24 hours to 48 hours post-isolation. Real-time PCR measurements were made from RNA extracted from 3 independent isolations, each in triplicate.
Figure S2. A. Western blot verification of Stim1 protein knockdown. Protein extracted from hepatocytes transfected with control shRNA or shRNA against Stim1 shows there was a significant reduction of the protein 48 hours after transfection. Actin was used as the loading control. B. Quantification of immunostaining intensity reveals knockdown of Stim1. The intensity of control shRNA-transfected, Stim1 shRNA-transfected or overexpressing wt Stim1 immunostaining was measured using several regions of interest from each sample. The regions were quantified by an image analyzer equipped with Zeiss LSM Image software and averaged to yield a mean. The average intensity for the control group was 81.29 ± 3.05 from 55 ROIs compared to 69.68 ± 2.86 from 44 ROIs. Hepatocytes transfected with the wt Stim1 protein exhibited a marked increase in intensity, 165.9 ± 22.38.
Figure S3. Effect of 2APB on high vasopressin IP3-mediated release and store-operated entry. A) Treatment of hepatocytes with 30 μM 2APB before addition of 1 μM vasopressin significantly attenuates the calcium release from 1.86 ± .05 (n=123 cells) for the control condition to 1.41 ± .06 (n=120 cells). B) Treatment of hepatocytes with 30 μM 2APB reduces the percentage of cells that respond to a high vasopressin dose from 96.63% ± 1.63 for the control condition to 73.25% ± 10.96. This reduction is not statistically significant however, and represents data taken from 4 independent calcium imaging experiments.
Acknowledgements
Dr. David Miller and Dr. Jae Choi read the manuscript and provided helpful comments.
Financial Support: This work was supported by the Intramural Program of NIH, National Institute of Environmental Health Sciences.
Abbreviations
- ER
endoplasmic reticulum
- SOCE
store-operated calcium entry
- CRAC
Ca2+-release-activated-Ca2+
- STIM
stromal interacting molecule
- 2APB
2-aminoethyldiphenyl borate
- RNAi
RNA interference
- shRNA
small hairpin RNA
- GFP
green fluorescent protein
- [Ca2+]i
intracellular calcium concentration
- HBSS
hepes-buffered saline solution
- PCR
polymerase chain reaction
- IP3
inositol trisphosphate
Footnotes
In one recent study, effects of knocking down Stim1 in a cell line derived from the liver, H411E, was reported (49).
Reference List
- 1.Woods NM, Cuthbertson KS, Cobbold PH. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- 2.Rooney TA, Sass EJ, Thomas AP. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989;264:17131–17141. [PubMed] [Google Scholar]
- 3.Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- 4.Tordjmann T, Berthon B, Claret M, Combettes L. Coordinated intercellular calcium waves induced by noradrenaline in rat hepatocytes: dual control by gap junction permeability and agonist. EMBO J. 1997;16:5389–5407. doi: 10.1093/emboj/16.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 7.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 8.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 9.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 16.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuttleworth TJ, Thompson JL. Evidence for a non-capacitative Ca2+ entry during [Ca2+] oscillations. Biochem J. 1996;316:819–824. doi: 10.1042/bj3160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J Biol Chem. 1997;271:21720–21725. doi: 10.1074/jbc.271.36.21720. [DOI] [PubMed] [Google Scholar]
- 20.Sneyd J, Tsaneva-Atanasova K, Yule DI, Thompson JL, Shuttleworth TJ. Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci U S A. 2004;101:1392–1396. doi: 10.1073/pnas.0303472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llopis J, Kass GEN, Gahm A, Orrenius S. Evidence for two pathways of receptor-mediated Ca2+ entry in hepatocytes. Biochem J. 1992;284:243–247. doi: 10.1042/bj2840243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kass GEN, Chow SC, Gahm A, Webb D-L, Berggren P-O, Llopis J, et al. Two separate plasma membrane Ca2+ carriers participate in receptor-mediated Ca2+ influx in rat hepatocytes. Biochim Biophys Acta. 1994;1223:226–233. doi: 10.1016/0167-4889(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AP, Bird GStJ, Hajnóczky G, Robb-Gaspers LD, Putney JW. Spatial and temporal aspects of cellular calcium signalling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 24.Bootman MD, Young KW, Young JM, Moreton RB, Berridge MJ. Extracellular calcium concentration controls the frequency of intracellular calcium spiking independently of inositol 1,4,5-trisphosphate production. Biochem J. 1996;314:347–354. doi: 10.1042/bj3140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird GS, Putney JW. Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory RB, Barritt GJ. Evidence that Ca2+-release-activated Ca2+ channels in rat hepatocytes are required for the maintenance of hormone-induced Ca2+ oscillations. Biochem J. 2003;370:695–702. doi: 10.1042/BJ20021671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedel B, Boyles RR, Putney JW, Bird GS. Role of the Store-operated Calcium Entry Proteins, Stim1 and Orai1, in Muscarinic-Cholinergic Receptor Stimulated Calcium Oscillations in Human Embryonic Kidney Cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawanishi T, Blank LM, Harootunian AT, Smith MT, Tsien RY. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989;264:12859–12866. [PubMed] [Google Scholar]
- 30.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 31.Bootman MD, Collins TJ, Mackenzie L, Roderick HJ, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 32.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lievremont JP, Bird GS, Putney JW. Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Mol Pharmacol. 2005;68:758–762. doi: 10.1124/mol.105.012856. [DOI] [PubMed] [Google Scholar]
- 34.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 35.Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium. 2005;38:329–342. doi: 10.1016/j.ceca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Rychkov GY, Litjens T, Roberts ML, Barritt GJ. ATP and vasopressin activate a single type of store-operated Ca2+ channel, identified by patch-clamp recording, in rat hepatocytes. Cell Calcium. 2005;37:183–191. doi: 10.1016/j.ceca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 38.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol (Lond) 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol (Lond) 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 41.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, et al. Biochemical and Functional Characterization of Orai Proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 42.Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalie A, Flockerzi V. Murine ORAI2 Splice Variants Form Functional Ca2+ Release-activated Ca2+ (CRAC) Channels. J Biol Chem. 2007;282:19375–19384. doi: 10.1074/jbc.M701962200. [DOI] [PubMed] [Google Scholar]
- 43.DeHaven WI, Smyth JT, Boyles RR, Putney JW. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 44.Lorin-Nebel C, Xing J, Yan X, Strange K. CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologs and is essential for ovulation and fertility. J Physiol. 2007;580:67–85. doi: 10.1113/jphysiol.2006.124883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolmetsch RE, Xu KL, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 46.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 47.Adachi Y, Kindzelskii AL, Ohno N, Yadomae T, Petty HR. Amplitude and frequency modulation of metabolic signals in leukocytes: synergistic role of IFN-gamma in IL-6- and IL-2-mediated cell activation. J Immunol. 1999;163:4367–4374. [PubMed] [Google Scholar]
- 48.Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, et al. Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J. 2002;364:641–648. doi: 10.1042/BJ20020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litjens T, Nguyen T, Castro J, Aromataris EC, Jones L, Barritt GJ, et al. Phospholipase C-gamma1 is required for the activation of store-operated Ca2+ channels in liver cells. Biochem J. 2007;405:269–276. doi: 10.1042/BJ20061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Presence of messenger RNA for Stim and Orai homologues in primary hepatocytes. ) RT-PCR reveals mRNA message for Stim1, Stim2, Orai1, Orai2, and Orai3 from RNA extracted immediately after isolation of cells, 24, and 48 hours after isolation. There is a significant decrease in each gene message from 0 hrs to 24 hours, but the message levels do not change significantly for any of the genes from 24 hours to 48 hours post-isolation. Real-time PCR measurements were made from RNA extracted from 3 independent isolations, each in triplicate.
Figure S2. A. Western blot verification of Stim1 protein knockdown. Protein extracted from hepatocytes transfected with control shRNA or shRNA against Stim1 shows there was a significant reduction of the protein 48 hours after transfection. Actin was used as the loading control. B. Quantification of immunostaining intensity reveals knockdown of Stim1. The intensity of control shRNA-transfected, Stim1 shRNA-transfected or overexpressing wt Stim1 immunostaining was measured using several regions of interest from each sample. The regions were quantified by an image analyzer equipped with Zeiss LSM Image software and averaged to yield a mean. The average intensity for the control group was 81.29 ± 3.05 from 55 ROIs compared to 69.68 ± 2.86 from 44 ROIs. Hepatocytes transfected with the wt Stim1 protein exhibited a marked increase in intensity, 165.9 ± 22.38.
Figure S3. Effect of 2APB on high vasopressin IP3-mediated release and store-operated entry. A) Treatment of hepatocytes with 30 μM 2APB before addition of 1 μM vasopressin significantly attenuates the calcium release from 1.86 ± .05 (n=123 cells) for the control condition to 1.41 ± .06 (n=120 cells). B) Treatment of hepatocytes with 30 μM 2APB reduces the percentage of cells that respond to a high vasopressin dose from 96.63% ± 1.63 for the control condition to 73.25% ± 10.96. This reduction is not statistically significant however, and represents data taken from 4 independent calcium imaging experiments.