Abstract
The first chemical synthesis of MeO-3-GlcUAβ(1→3)GlcNAc-UDP to elucidate the catalytic mechanism of hyaluronic acid synthases (HASs) is described. Construction of the desired β(1→3)-linked disaccharide 10 was achieved very efficiently by coupling MeO-3-GlcUA donor 3 with the suitable protected GlcNTroc acceptor 4 using BF3.Et2O as Lewis acid. Chemoselective removal of anomeric NAP, phosphorylation, hydrogenation, coupling with UMP-morpholidate and finally complete deprotection gave the target compound 1 in good yield.
Keywords: MeO-3-GlcUA, hyaluronic acid synthases (HASs), oligosaccharides, phosphorylation
1. Introduction
Hyaluronic acid (HA), a vital extracellular matrix component of vertebrate tissues, has been implicated in numerous physiological and biological phenomena.1 HA is a linear biopolymer composed of thousands of alternating repeating disaccharide units of glucuronic acid [GlcUAβ(1→3)] and N-acetylglucosamine [GlcNAcβ(1→4)].2 The enzymes that catalyze the polymerization of HA, the hyaluronan synthases (HASs), are unique glycosyltransferases that have both selective GlcNAcβ(1→4) transferase and GlcUAβ(1→3) transferase activities.3 There is an ambiguity regarding the directionality of chain elongation of HA biosynthesis i.e. whether the addition of monosaccharide units occurs at the reducing or non-reducing terminus of growing polysaccharide chains. Although, many groups have investigated this problem extensively, till date there is no accurate knowledge regarding the mechanism of HA biosynthesis and hence only minimal information is available on the molecular basis for HASs catalytic action. Consequently, our understanding of the detailed mechanisms whereby hyaluronan influences cell behavior is still very incomplete.3
It was identified recently that the small HA fragments behave as potent activators of immunocompetent cells which play a decisive role in the development of T cell-mediated immune responses.4 Also, studies such as NMR spectroscopic investigations and the mechanism of degradation, performed to understand the chemical properties of HA, are preferably executed using low-molecular-weight HA fragments rather than polymeric HA.5 Thus, syntheses of well-defined HA fragments is required to provide adequate material for carrying out such investigations. Consequently, several strategies have been reported for the construction of HA fragments containing either a GlcUA or a GlcNAc at the reducing end.6
Moreover, it was observed that the deoxy analogs obtained by the substitution of one of the -OH groups of basic enzyme acceptor with -F, -OMe, -N3, -NH2 etc. sometimes results in highly specific acceptors or inhibitors for the individual enzymes.7 For example, Scott and Viola found that 3-fluoro and 4-fluoro analogues of D-glucose were higher affinity substrates than D-glucose for aldolase reductase but 2-fluoro and as well as 4-fluoro analogues of D-glucitol were inactive acceptors for sorbitol dehydrogenase.7a It was noticed by us that the activities of only Gal 3-O-sulfotransferases and not sialyltransferases (Sia-T) were adversely affected by Galβ(1→4)GlcNAcβ(1→6)[F-3-Galβ(1→3)]GalNAcα-OBn. Amazingly, F-4-Galβ(1→4)GlcNAcβ(1→6)[Galβ(1→3)]GalNAcα-OBn was found to be an inhibitor of α2,6(N)Sia-T activity but not α2,3(N)Sia-T activity.7b The deoxy analogs of disaccharide peracetylated GlcNAcβ1–3Galβ-O-naphthalenemethanol containing -H, -F, -N3, -NH2, or -OCH3 at C-3' and C-4' positions of the terminal N-acetylglucosamine residue presumably inhibits one or more galactosyltransferases in vivo, thereby blocking sLeX formation and experimental tumor cell metastasis.7c Thus, functionalization of hydroxyl group of an HA fragment structure by substituting with -F, -OMe, -N3, -NH2 etc. may result in highly specific acceptors or inhibitors for the individual HAS. In this context, we have reported very recently the first chemical synthesis of F-4-GlcUAβ(1→3)GlcNAc-UDP for investigating the catalytic mechanism of HASs.8 In general, OMe derivatives have similar potency and their synthesis requires cheap and less hazardous reagents than their respective fluoro derivatives. Therefore, we became interested in developing a facile synthesis of these OMe derivatives and report herein the first chemical synthesis of MeO-3-GlcUAβ(1→3)GlcNAc-UDP (1) which may have the potential to be a specific donor for the HASs.
2. Results and discussion
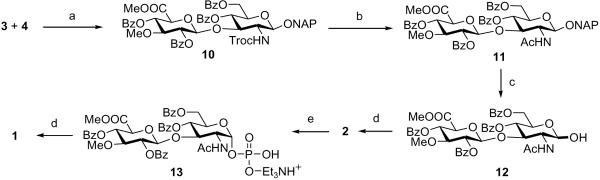
It was envisaged that MeO-3-GlcUAβ(1→3)GlcNAc-1-phosphate derivative 2 would be an ideal intermediate to generate target molecule 1 (Figure 1). The phosphate derivative 2 could in turn be obtained by the glycosylation of known GlcNTroc acceptor 48 by MeO-3-GlcUA imidate donor 3. To avoid the possible formation of side product due to transacetylation during O-glycosylation, benzoyl group was preferred over acetyl group for protection of the 2-OH position of GlcUA in donor 3.9 We preferred to utilize 2-naphthylmethyl (NAP) as an anomeric protecting group in 3 and 4, because of its ease in removal by DDQ oxidation.10 Due to the straightforward transformation of N-Troc into N-Ac, Troc group was chosen as a temporary protecting group for nitrogen in the glycosyl acceptor 4.11
Figure 1.

Retrosynthesis of target molecule MeO-3-GlcUAβ(1→3)GlcNAc-UDP (1).
Scheme 1 outlines the synthesis of donor 3. Synthesis of imidate 3 was initiated with known GlcUA donor 5.12 Induction of anomeric NAP protection in 5 was executed by the glycosylation of NAP-OH with donor 5 in presence of TMSOTf at 0 °C to afford 6 in 80% yield. Deacetylation of 6 with NaOMe-MeOH followed by saponification of the methyl ester using LiOH gave 7 in good yield. 2,4-di-O-acylated derivative 8 was prepared from 7 following a 3-steps procedure. Lactonisation of 7 with benzoic anhydride in DMF, followed by complete benzoylation using DMAP-pyridine and finally methanolysis of the lactone ring in presence of anhydrous NaOAc afforded the desired 2,4-di-O-acylated derivative 8 in 76% yield for three steps.13 O-Methylation of the 3-hydroxyl group of 8 with CH3I and freshly prepared Ag2O provided 9 in 92% yield. Removal of anomeric NAP protection of 9 was carried out using DDQ,10 followed by imidation14 afforded the MeO-3-GlcUA donor 3 in high yield (Scheme 1).
Scheme 1.

Preparation of donor 3. Reagents and conditions: (a) NAPOH, TMSOTf, CH2Cl2, 4Å MS, 0 °C, 2 h, 80%; (b) (i) NaOMe, MeOH, r.t., 6 h, 90%; (ii) LiOH,THF, H2O, 0 °C, 4 h, 95%; (c) (i) Bz2O, DMF, 85 °C., 4 h, 86%; (ii) Bz2O, Py, DMAP, DMF, r.t., 40 h, 96%; (iii) NaOAc, MeOH, DMF, r.t., 10 h, 92%; (d) MeI, Ag2O, CH2Cl2, 4Å molecular sieves, r.t., 20 h, 92%; (e) (i) DDQ, CH2Cl2-MeOH (4:1), r.t., 12 h, 89%; (ii) CCl3CN, DBU, CH2Cl2, 0 °C, 2 h, 78%.
We next investigated multistep synthesis of the crucial phosphate intermediate 2 (Scheme 2). Coupling of the MeO-3-GlcUA donor 3 and the known acceptor 48 was carried out with BF3.Et2O in presence of 4Å molecular sieves in toluene to afford the desired disaccharide 10 with complete β1→3 stereoselectivity in 83% yield.6e,8,12b,15 Detrimental effect on the yield of above-mentioned glycosylation reaction was observed upon substitution of dichloromethane as a solvent. Also, TMSOTf was found ineffective for the same glycosylation as a promoter in comparison with BF3.Et2O. It is worth mentioning that the glycosylation reaction reported here is one among the few procedures available for efficient synthesis of β1→3 linkage with high β-stereoselectivity and high yield.8,15d Most of the literature available on synthesis of β1→3 linkage suffers from certain drawbacks such as poor β-selectivity and low yield.6e,k,n,12b,15b,c Following above glycosylation reaction, MeO-3-GlcUAβ1→3 linked disaccharide 10 could be accessed with complete β1→3 stereoselectivity and in high yield. Next, replacement of the N-Troc group in 10 by an N-acetyl group was effected with Zn-Ac2O and the subsequent acetylation of the free amine offered 11 in 88% yield.11 Chemoselective removal of NAP in 11 with DDQ in CH2Cl2-MeOH (4:1)10 provided anomeric free hydroxyl disaccharide 12 in 89% yield. Phosphorylation of 12 with tetrabenzyl pyrophospgate16 gave the benzyl-protected anomeric phosphate 2 as the desired α anomor in high yield. Prominent signals in the 1H NMR spectrum of 2 at δ = 5.68 (dd, J 1,2 = 3.4 Hz, J 1,P = 6.4 Hz, 1 H, H-1 of GlcNAc), and the signal in the 13P NMR of 2 at δ = −1.90 (s, 1 P) were observed, confirming the linkage between the disaccharide and phosphoric acid moieties. Deprotection of the benzyl group in 2 by hydrogenation over palladium catalyst gave 13 in 90% yield (Scheme 2). Coupling of 13 with UMP-morpholidate in presence of 1H-tetrazole in DMF-pyridine (3:1)16 followed by deprotection of the benzoyl groups and the methyl ester using 3M NaOH afforded the target compound 117 in 45% yield over two steps after isolation and purification by reverse-phase column HPLC and gel-filtration column HPLC.
Scheme 2.
Synthesis of MeO-3-GlcUAβ(1→3)GlcNAc-UDP 1. Reagents and conditions: (a) BF3.Et2O, toluene, 4Å MS, 0 °C, 2 h, 83%; (b) Zn, Ac2O, AcOH, THF, r.t., 4 h, 88%; (c) DDQ, CH2Cl2-MeOH (4:1), r.t., 12 h, 89%; (d) LHMDS, [(BnO)2P]2O, THF, −78 to 0 °C, 3 h, 81%; (e) H2, Pd/C, EtOAc-MeOH (1:1), Et3N, r.t., 9 h, 90%; (f) (i) UMP-morpholidate, 1H-tetrazole, DMF-py (3:1), r.t., 2 d; (ii) 3M NaOH, MeOH, r.t., 10 h; (iii) RP column HPLC; gel-filtration column HPLC, 45% from 13.
3. Conclusions
In summary, we have successfully accomplished the first chemical synthesis of MeO-3-GlcUAβ(1→3)GlcNAc-UDP following a very efficient glycosylation reaction which proceeded with complete stereoselectivity and high yield. We speculate that this compound would serve as a novel substrate to investigate the catalytic mechanism of HASs.
Acknowledgments
We acknowledge grant support from DOD (W81XWH-06-1-0013) and support, in part, by the NCI Cancer Center Support Grant to the Roswell Park Cancer Institute (P30 -CA016056).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Laurent TC, Fraser JRE. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]; (b) Knudson CB, Knudson W. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]; (c) Zeng C, Toole BP, Kinney SD, Kuo J, Stamenkovic I. Int. J. Cancer. 1998;77:396–401. doi: 10.1002/(sici)1097-0215(19980729)77:3<396::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]; (d) DeAngelis PL. Cell. Mol. Life, Sci. 1999;56:670. doi: 10.1007/s000180050461. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Toole BP, Wight TN, Tammi MI. J. Biol. Chem. 2002;277:4593. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]; (f) Toole BP. Nat. Rev. Cancer. 2004;4:528. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]; (g) Williams KJ, Halkes KM, Kamerling JP, DeAngelis PL. J. Biol. Chem. 2006;281:5391. doi: 10.1074/jbc.M510439200. [DOI] [PubMed] [Google Scholar]
- 2.Meyer K, Palmer JW. J. Biol. Chem. 1934;107:629–634. [Google Scholar]
- 3.(a) Prehm P. Biochem. J. 2006;398:469. doi: 10.1042/BJ20060431. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weigel PH, DeAngelis PL. J. Biol. Chem. 2007;282:36777. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 4.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. J. Exp. Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Slaghek TM, Nakahara Y, Ogawa T, Kamerling JP, Vliegenthart JFG. Carbohydr. Res. 1994;255:61–85. doi: 10.1016/s0008-6215(00)90971-6. [DOI] [PubMed] [Google Scholar]; (b) Sičinska W, Adams B, Lerner L. Carbohydr. Res. 1993;242:29–51. doi: 10.1016/0008-6215(93)80020-f. [DOI] [PubMed] [Google Scholar]
- 6.(a) Fowers HM, Jeanloz RW. Biochemistry. 1964;3:123–125. doi: 10.1021/bi00889a020. [DOI] [PubMed] [Google Scholar]; (b) Slaghek TM, Hyppönen TK, Kruiskamp PH, Ogawa T, Kamerling JP, Vliegenthart JFG. Tetrahedron Lett. 1993;34:7939–7942. [Google Scholar]; (c) Blatter G, Jacquinet J-C. Carbohydr. Res. 1996;288:109–125. doi: 10.1016/s0008-6215(96)90785-5. [DOI] [PubMed] [Google Scholar]; (d) Garcia BA, Poole JL, Gin DY. J. Am. Chem. Soc. 1997;119:7597–7598. [Google Scholar]; (e) Yeung BKS, Hill DC, Janicka M, Peuntilo PA. Org. Lett. 2000;2:1279–1282. doi: 10.1021/ol0057075. [DOI] [PubMed] [Google Scholar]; (f) Crich D, Smith M. J. Am. Chem. Soc. 2001;123:9015–9020. doi: 10.1021/ja0111481. [DOI] [PubMed] [Google Scholar]; (g) Yeung BKS, Chong PYC, Peuntilo PA. Carbohydr. Res. 2002;21:779–865. [Google Scholar]; (h) Codée JDC, Litjens REJN, Den Heeten R, Overkleeft HS, Van Boom JH, Van der Marel GA. Org. Lett. 2003;5:1519–1522. doi: 10.1021/ol034312t. [DOI] [PubMed] [Google Scholar]; (i) Iyer SS, Rele SM, Baskaran S, Chaikof EL. Tetrahedron. 2003;59:631–638. [Google Scholar]; (j) Lu XA, Chou CH, Wang CC, Hung SC. Synlett. 2003;9:1364–1366. [Google Scholar]; (k) Palmacci ER, Seeberger PH. Tetrahedron. 2004;60:7755–7766. [Google Scholar]; (l) Codée JDC, Stubba B, Schiattarella M, Overkleeft HS, Van Boeckel CAA, Van Boom JH, Van der Marel GA. J. Am. Chem. Soc. 2005;127:3767–3773. doi: 10.1021/ja045613g. [DOI] [PubMed] [Google Scholar]; (m) Huang L, Huang X. Chem.-Eur. J. 2007;13:529–540. doi: 10.1002/chem.200601090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Dinkelaar J, Codée JDC, Van den Bos LJ, Overkleeft HS, Van der Marel GA. J. Org. Chem. 2007;72:5737–5742. doi: 10.1021/jo070704s. [DOI] [PubMed] [Google Scholar]
- 7.(a) Scott ME, Viola RE. Carbohydr. Res. 1998;313:247–253. doi: 10.1016/s0008-6215(98)00266-3. [DOI] [PubMed] [Google Scholar]; (b) Xia J, Xue J, Locke RD, Chandrasekaran EV, Srikrishnan T, Matta KL. J. Org. Chem. 2006;71:3696–3706. doi: 10.1021/jo052626j. [DOI] [PubMed] [Google Scholar]; (c) Brown JR, Yang F, Sinha A, Ramakrishnan B, Tor Y, Qasba PK, Esko JD. J. Biol. Chem. 2009;284:4952–4959. doi: 10.1074/jbc.M805782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei G, Kumar V, Xue J, Locke RD, Matta KL. Tetrahedron Lett. 2009 doi: 10.1016/j.tetlet.2009.09.042. In Press, doi:10.1016/j.tetlet.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RT, Carter NE, Mayalrap SP, Scheinmann F. Tetrahedron. 2000;56:7591. [Google Scholar]
- 10.Xue J, Khaja SD, Locke RD, Matta KL. Synlett. 2004;5:861. [Google Scholar]
- 11.Hancock G, Galpin IJ. Tetrahedron Lett. 1982;23:249. [Google Scholar]
- 12.(a) Yeung BKS, Hill DC, Janicka M, Petillo PA. Org. Lett. 2000;2:1279. doi: 10.1021/ol0057075. [DOI] [PubMed] [Google Scholar]; (b) Chen L, Kong F. Carbohydr. Res. 2002;337:1373. doi: 10.1016/s0008-6215(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 13.Kornilov AV, Sukhova EV, Nifantiev NE. Carbohydr. Res. 2001;336:309. doi: 10.1016/s0008-6215(01)00272-5. [DOI] [PubMed] [Google Scholar]
- 14.(a) Dullenkopf W, Castro-Palomino JC, Manzoni L, Schmidt RR. Carbohydr. Res. 1996;296:135. doi: 10.1016/s0008-6215(96)00237-6. [DOI] [PubMed] [Google Scholar]; (c) Schmidt RR, Michel J. Angew. Chem., Int. Ed. 1980;19:731. [Google Scholar]
- 15.(a) Schmidt RR. Angew. Chem., Int. Ed. 1986;25:212. [Google Scholar]; (b) Allen JG, Fraser-Reid B. J. Am. Chem. Soc. 1999;121:468. [Google Scholar]; (c) Rye CS, Withers SG. J. Am. Chem. Soc. 2002;124:9756. doi: 10.1021/ja020627c. [DOI] [PubMed] [Google Scholar]; (d) Rele SM, Iyera SS, Chaikof EL. Tetrahedron Lett. 2007;48:5055. doi: 10.1016/j.tetlet.2007.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takaku H, Ishida H.-k., Fujita M, Inazu T, Ishida H, Kiso M. Synlett. 2007:818. [Google Scholar]
- 17.The selected physical data of key compounds is listed: Compound 3: [α]D25 −12.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.75 (s, 3 H), 3.82 (s, 3 H), 4.22 (t, 1 H, J = 9.6 Hz), 4.41 (d, 1 H, J = 9.6 Hz), 5.10 (dd, 1 H, J = 3.6, 9.6 Hz), 6.07 (t, 1 H, J = 10.2 Hz), 6.36 (d, 1 H, J = 3.6 Hz), 7.35–8.01 (m, 10 H, Ph), 8.74 (s, 1 H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 52.5, 57.6, 69.4, 70.2, 72.8, 79.8, 90.1, 92.3, 159.7, 167.5, 168.8, 169.1. ESI-HRMS: calcd for C24H22Cl3NO9Na [M + Na]+ 596.0253, found: 596.0250. Compound 10: 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.36 (s, 3 H), 3.40 (s, 3 H), 3.91–3.93 (m, 1 H), 4.32–4.34 (m, 2 H), 4.38–4.42 (m, 2 H), 4.48–4.52 (m, 2 H), 4.55–4.57 (m, 1 H), 4.82 (d, 1 H, J = 8.2 Hz, H-1'), 5.02 (d, 1 H, J = 8.0 Hz, H-1), 5.06–5.13 (m, 3 H), 5.17 (t, 1 H, J = 9.6 Hz), 5.24 (t, 1 H, J = 9.6 Hz), 5.34 (bs, 1 H), 5.50 (t, 1 H, J = 9.6 Hz), 7.20–8.20 (m, 27 H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 53.5, 57.2, 58.7, 63.5, 70.5, 70.9, 71.3, 71.8, 72.1 (2 C), 72.7, 74.6, 80.8, 95.6, 99.8, 100.3, 153.2, 165.2, 166.8, 167.1, 169.0, 169.7; ESI-HRMS: calcd for C56H50Cl3NO17Na [M+Na]+ 1136.2037, found: 1136.2035. Compound 2: 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.83 (s, 3 H), 3.31 (s, 3 H), 3.51 (s, 3 H), 3.82 (d, J = 10.0 Hz, 1 H), 4.17 (t, J = 9.8 Hz, 1 H), 4.21–4.26 (m, 2 H), 4.39–4.49 (m, 2 H), 4.67–4.74 (m, 2 H), 4.97–5.10 (m, 6 H), 5.40 (t, J = 8.8 Hz, 1 H), 5.68 (dd, J 1,2 = 3.4 Hz, J 1,P = 6.4 Hz, 1 H, H-1), 5.76 (d, J = 9.2 Hz, NHAc), 7.26–8.20 (m, 30 H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 20.1, 51.7, 52.3, 57.8 (J C-2,P = 8.1 Hz, C-2), 62.1, 68.2, 68.7, 69.4 (J CH2Ph,P = 4.9 Hz, POCH2Ph), 69.7 (J CH2Ph,P = 4.9 Hz, POCH2Ph), 71.6, 72.4, 73.8, 74.2, 79.8, 96.4 (J C-1,P = 6.9 Hz, C-1), 99.4 (C-1'), 164.4, 165.5, 166.4, 168.6, 168.9, 169.4. 31P NMR (202 MHz, CDCl3): δ (ppm) = −1.90 (s, 1 P). ESI-HRMS: calcd for C58H56NO19PNa [M + Na]+ 1124.3077, found: 1124.3080. Compound 1: 1H NMR (400 MHz, D2O): δ (ppm) = 1.93 (s, 3 H), 3.53 (s, 3 H), 3.56 (m, 1 H), 3.60 (t, J = 9.6 Hz, 1 H), 3.63–3.88 (m, 6 H), 3.93 (d, J = 9.2 Hz, 1 H), 4.07 (ddd, J2,1 = 3.4 Hz, J2,3 = 10.2 Hz, J2,P = 3.2 Hz, 1 H, H-2), 4.10–4.13 (m, 2 H), 4.15–4.18 (m, 1 H), 4.20–4.24 (m, 2 H), 4.31 (t, J = 9.6 Hz, 1 H), 4.43 (d, J1',2' = 8.6 Hz, 1 H, H-1'), 5.40 (dd, J1,2 = 3.2 Hz, J1,P = 7.8 Hz, 1 H, H-1), 5.79 (d, 1 H), 5.83 (d, 1 H), 7.83 (d, 1 H). 13C NMR (100 MHz, D2O): δ (ppm) = 23.4, 54.5, 57.6 (J C-2,P = 8.6 Hz, C-2), 62.8, 66.0 (d, J = 3.8 Hz), 70.0, 71.1, 71.6, 72.5, 74.2, 74.8, 75.5, 77.2, 81.2, 84.8 (d, J = 9.8 Hz), 89.5, 96.5 (J C-1,P = 6.0 Hz, C-1), 101.3, 104.1, 141.8, 153.4, 167.4, 175.5, 176.1. 31P NMR (202 MHz, D2O): δ (ppm) = −11.4 (d, J = 22.4 Hz), −13.1 (d, J = 22.6 Hz). ESI-HRMS: calcd for C24H36N3O23P2 [M - H]− 796.1210, found: 796.1214.