Abstract
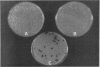
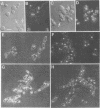
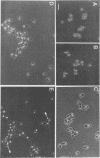
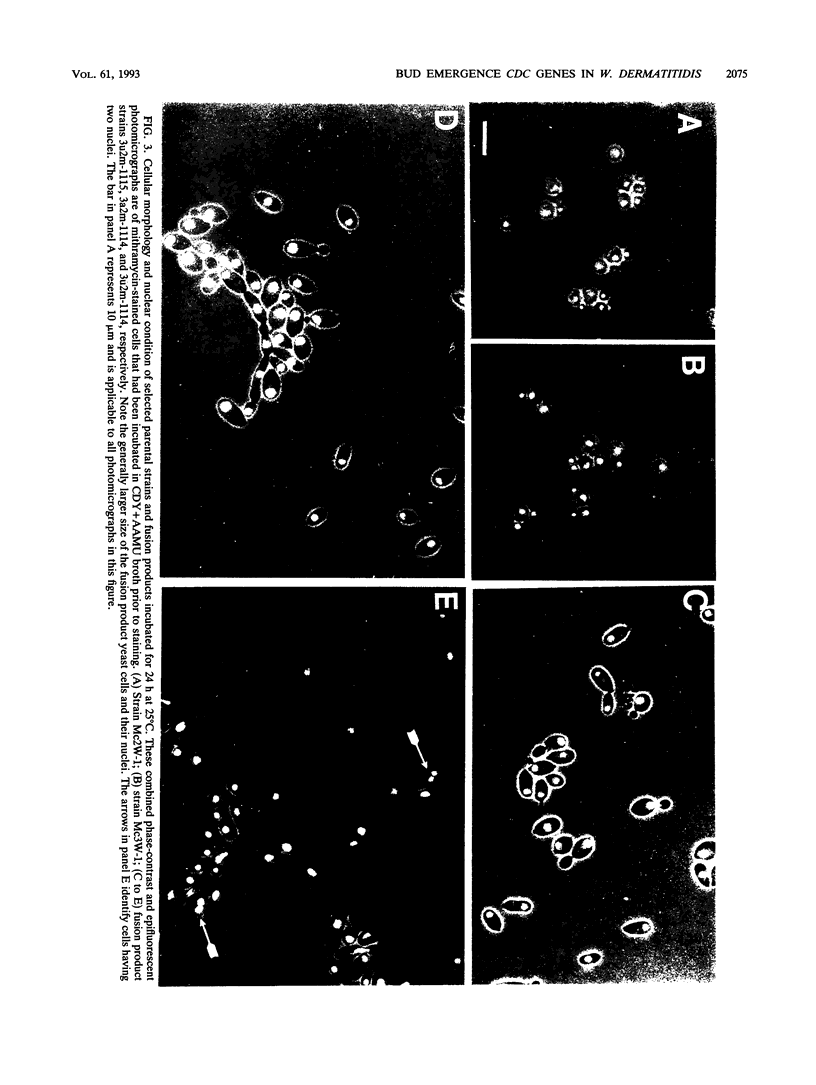
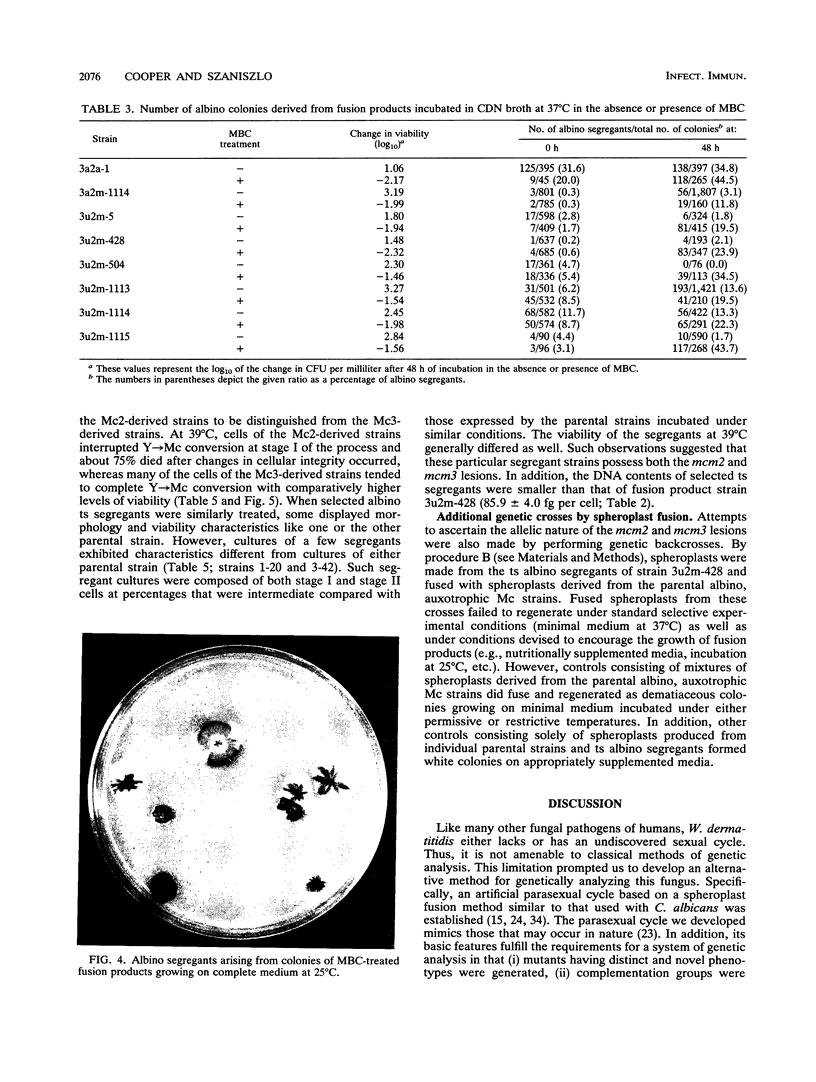
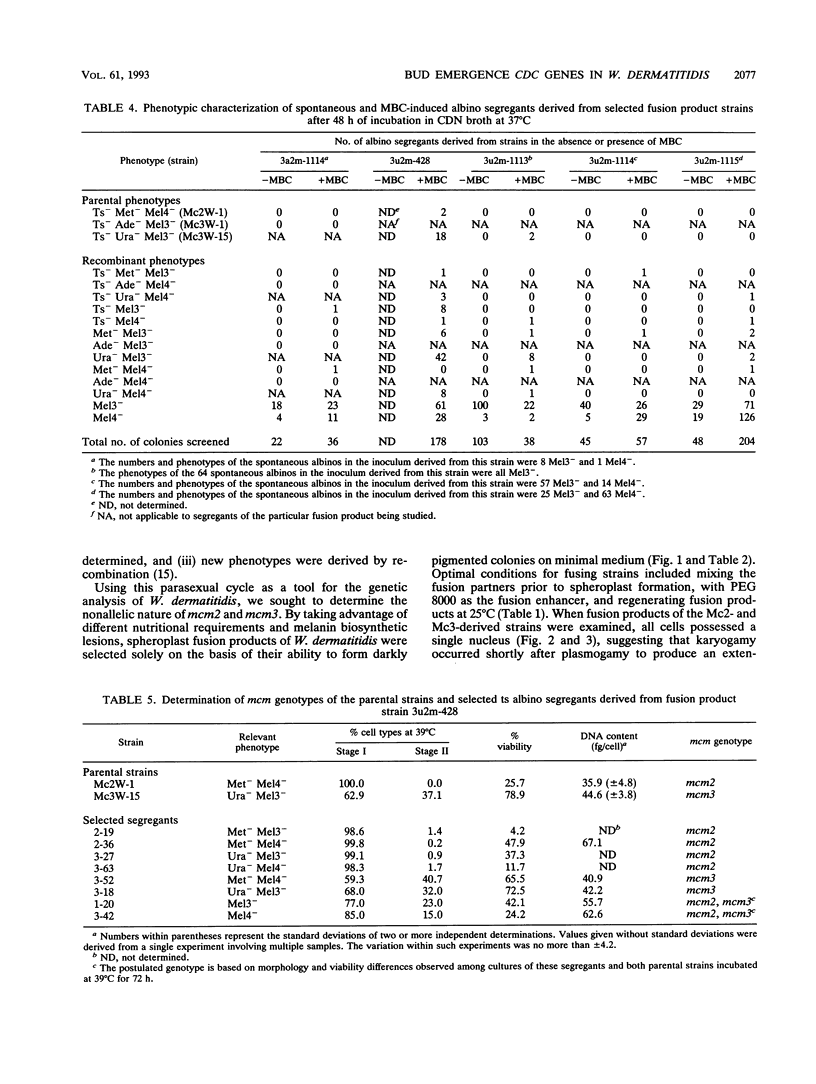
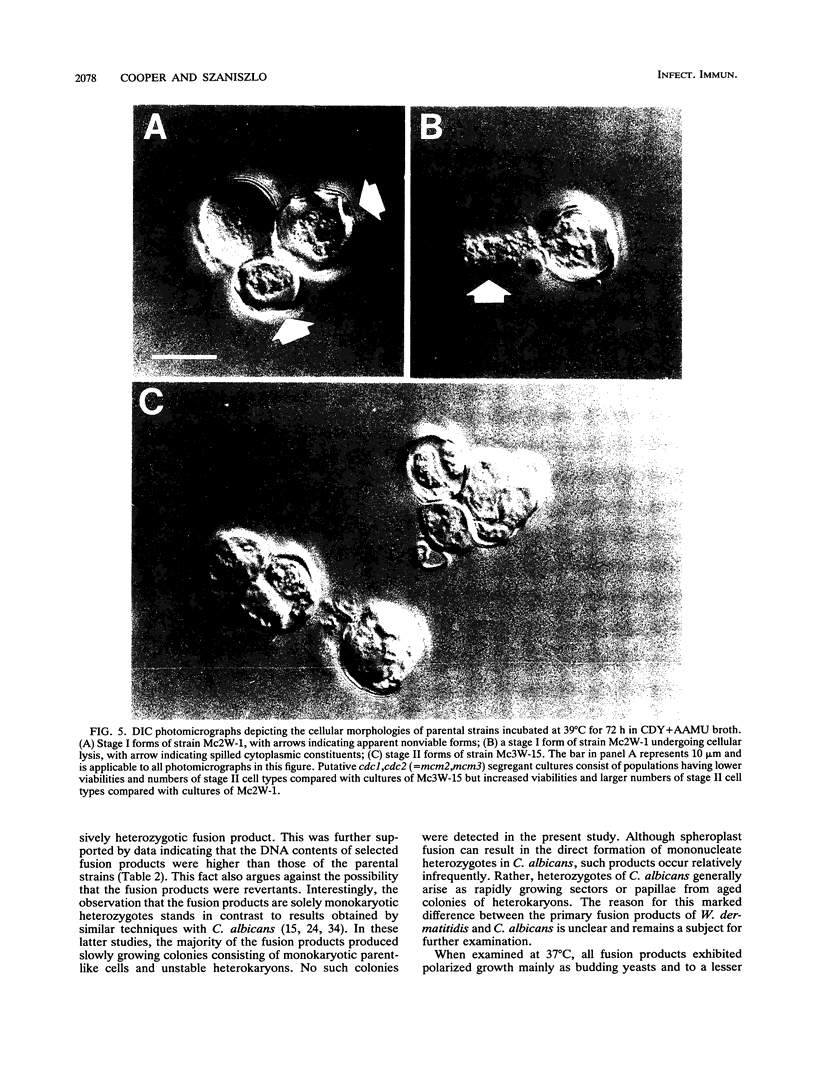
Strains Mc2 and Mc3 are morphological mutants of the melanized, pathogenic fungus Wangiella dermatitidis. These strains possess temperature-sensitive (ts) mutations designated mcm2 and mcm3, respectively. At the restrictive temperature (37 degrees C), uninucleate yeast cells of strains Mc2 and Mc3 cease budding and initiate an isotropic mode of cellular development, which is reflected in the formation of a multicellular and multinucleate morphology. Because W. dermatitidis either lacks or has an undiscovered sexual cycle, parasexual methods of analysis were used to confirm that mcm2 and mcm3 define separate bud emergence control genes in the wild-type strain. Spheroplasts of albino auxotrophs derived from strains Mc2 and Mc3 were fused and then regenerated on minimal medium. The resulting fusion products grew as darkly pigmented, prototrophic colonies. When incubated at 37 degrees C, all fusion products exhibited polarized growth predominantly as uninucleate, budding yeasts and less frequently as pseudohyphae and moniliform hyphae. Subsequent analysis of cultures derived from albino, ts segregants, which were induced from fusion products by using methyl benzimidazole-2-yl-carbamate, revealed three types of cell populations. Two resembled those expressed by strain Mc2 or Mc3. The third consisted of a cell population unlike the former, suggesting the presence of both ts mutations in all cells. These results imply that yeast development in the fusion products resulted from intergenic complementation of mcm2 and mcm3, i.e., they are nonallelic. Because mcm2 and mcm3 are equivalent to certain cdc lesions in the yeast Saccharomyces cerevisiae, we have renamed the analogous genes defined by the mutations in W. dermatitidis as CDC1 and CDC2. To our knowledge, these are the first CDC genes identified in a dematiaceous fungus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock C. J. DNA synthesis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1970 Apr;60(1):16–26. doi: 10.1016/0014-4827(70)90484-2. [DOI] [PubMed] [Google Scholar]
- Dixon D. M., Migliozzi J., Cooper C. R., Jr, Solis O., Breslin B., Szaniszlo P. J. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidis in mice: mortality and histopathology studies. Mycoses. 1992 Jan-Feb;35(1-2):17–21. doi: 10.1111/j.1439-0507.1992.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Drubin D. G. Development of cell polarity in budding yeast. Cell. 1991 Jun 28;65(7):1093–1096. doi: 10.1016/0092-8674(91)90001-f. [DOI] [PubMed] [Google Scholar]
- Geis P. A., Wheeler M. H., Szaniszlo P. J. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol. 1984 Apr;137(4):324–328. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Oujezdsky K. B., Szaniszlo P. J. Budding in the dimorphic fungus Phialophora dermatitidis. J Bacteriol. 1973 Jul;115(1):323–329. doi: 10.1128/jb.115.1.323-329.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. W., Szaniszlo P. J. Microtubule function and its relation to cellular development and the yeast cell cycle in Wangiella dermatitidis. Arch Microbiol. 1982 Dec;133(2):155–161. doi: 10.1007/BF00413531. [DOI] [PubMed] [Google Scholar]
- Kakar S. N., Magee P. T. Genetic analysis of Candida albicans: identification of different isoleucine-valine, methionine, and arginine alleles by complementation. J Bacteriol. 1982 Sep;151(3):1247–1252. doi: 10.1128/jb.151.3.1247-1252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K., Costigan C., Snyder M. Cell polarity and morphogenesis in Saccharomyces cerevisiae. Trends Cell Biol. 1992 Jan;2(1):22–29. doi: 10.1016/0962-8924(92)90140-i. [DOI] [PubMed] [Google Scholar]
- McGinnis M. R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983 Jan;8(1):1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- Oujezdsky K. B., Szaniszlo P. J. Conidial ontogeny in Phialophora dermatitidis. Mycologia. 1974 May-Jun;66(3):537–542. [PubMed] [Google Scholar]
- PONTECORVO G. The parasexual cycle in fungi. Annu Rev Microbiol. 1956;10:393–400. doi: 10.1146/annurev.mi.10.100156.002141. [DOI] [PubMed] [Google Scholar]
- Poulter R. T., Rikkerink E. H. Genetic analysis of red, adenine-requiring mutants of Candida albicans. J Bacteriol. 1983 Dec;156(3):1066–1077. doi: 10.1128/jb.156.3.1066-1077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Jeffery K., Hubbard M. J., Shepherd M. G., Sullivan P. A. Parasexual genetic analysis of Candida albicans by spheroplast fusion. J Bacteriol. 1981 Jun;146(3):833–840. doi: 10.1128/jb.146.3.833-840.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Pogson C. I., Gull K. The influence of the microtubule inhibitor, methyl benzimidazol-2-yl-carbamate (MBC) on nuclear division and the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 1980 Dec;46:341–352. doi: 10.1242/jcs.46.1.341. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Lo R. J., Szaniszlo P. J. Nuclear division in temperature-sensitive multicellular mutants of Wangiella dermatitidis. J Bacteriol. 1979 Mar;137(3):1456–1458. doi: 10.1128/jb.137.3.1456-1458.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Szaniszlo P. J. Temperature-sensitive multicellular mutants of Wangiella dermatitidis. J Bacteriol. 1978 Aug;135(2):622–632. doi: 10.1128/jb.135.2.622-632.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Szaniszlo P. J. Yeast-phase cell cycle of the polymorphic fungus Wangiella dermatitidis. J Bacteriol. 1980 Nov;144(2):721–731. doi: 10.1128/jb.144.2.721-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachek A., Rhoads D. D., Schwarzhoff R. H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981 Mar;129(1):1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- Slater M. L. Rapid nuclear staining method for Saccharomyces cerevisiae. J Bacteriol. 1976 Jun;126(3):1339–1341. doi: 10.1128/jb.126.3.1339-1341.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaniszlo P. J., Hsieh P. H., Marlowe J. D. Induction and ultrastructure of the multicellular (sclerotic) morphology in Phialophora dermatitidis. Mycologia. 1976 Jan-Feb;68(1):117–130. [PubMed] [Google Scholar]
- Wood J. S. Genetic effects of methyl benzimidazole-2-yl-carbamate on Saccharomyces cerevisiae. Mol Cell Biol. 1982 Sep;2(9):1064–1079. doi: 10.1128/mcb.2.9.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. S. Mitotic chromosome loss induced by methyl benzimidazole-2-yl-carbamate as a rapid mapping method in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Sep;2(9):1080–1087. doi: 10.1128/mcb.2.9.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]