Abstract
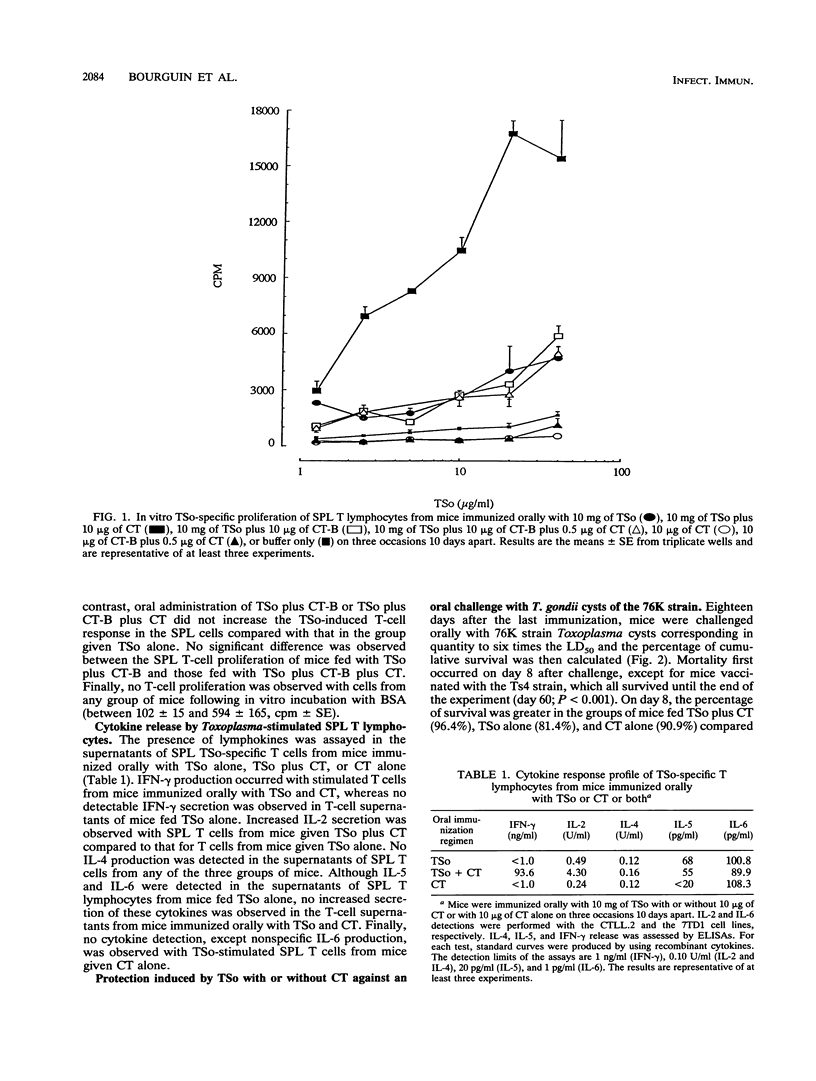
Following oral immunization of C57BL/6 mice with a Toxoplasma gondii sonicate (TSo) in association with either cholera toxin (CT) or CT B subunit, the T. gondii-specific in vitro proliferation of splenic T lymphocytes was determined. Cytokines produced by these T cells were then characterized. After oral challenge with T. gondii 76K cysts, the percentage of cumulative survival was assessed, as was the number of brain cysts in the mice which survived. The TSo-specific proliferation of splenic T lymphocytes was greatly enhanced by the use of CT, whereas CT B subunit alone did not lead to amplification of splenic T-cell proliferation. The use of CT was associated with an increase of interleukin-2 (IL-2) and gamma interferon synthesis by TSo-stimulated splenic T cells, whereas no enhancement of IL-5 and IL-6 production was observed. IL-4 was not detected. A significant protection of mice immunized orally with TSo plus CT was observed in comparison with those immunized with TSo alone. This protection was associated with a large decrease in the number of brain cysts compared with the number found in naive mice infected orally with a sublethal dose of T. gondii 76K cysts. Further studies, using well-defined T. gondii proteins which are known to induce both mucosal and systemic immune responses, are needed to confirm the value of CT in the enhancement of protection against oral toxoplasmosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991 May;59(5):1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguin I., Chardes T., Mevelec M. N., Woodman J. P., Bout D. Amplification of the secretory IgA response to Toxoplasma gondii using cholera toxin. FEMS Microbiol Lett. 1991 Jul 1;65(3):265–271. doi: 10.1016/0378-1097(91)90225-y. [DOI] [PubMed] [Google Scholar]
- Bromander A., Holmgren J., Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991 May 1;146(9):2908–2914. [PubMed] [Google Scholar]
- Bülow R., Boothroyd J. C. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol. 1991 Nov 15;147(10):3496–3500. [PubMed] [Google Scholar]
- Chardès T., Bourguin I., Mevelec M. N., Dubremetz J. F., Bout D. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect Immun. 1990 May;58(5):1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardès T., Velge-Roussel F., Mevelec P., Mevelec M. N., Buzoni-Gatel D., Bout D. Mucosal and systemic cellular immune responses induced by Toxoplasma gondii antigens in cyst orally infected mice. Immunology. 1993 Mar;78(3):421–429. [PMC free article] [PubMed] [Google Scholar]
- Clarke C. J., Wilson A. D., Williams N. A., Stokes C. R. Mucosal priming of T-lymphocyte responses to fed protein antigens using cholera toxin as an adjuvant. Immunology. 1991 Mar;72(3):323–328. [PMC free article] [PubMed] [Google Scholar]
- Couzineau P., Baufine-Ducrocq H. Etude des possibilités d'utilisation du sarcome TG 180 de la souris. Application à la toxoplasmose. Ann Parasitol Hum Comp. 1969 May-Jun;44(3):217–224. [PubMed] [Google Scholar]
- Czinn S. J., Nedrud J. G. Oral immunization against Helicobacter pylori. Infect Immun. 1991 Jul;59(7):2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J., Underdown B. J. Cognate T-cell help in the induction of IgA responses in vivo. Immunology. 1990 Sep;71(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O. Cholera toxin as a mucosal adjuvant: effects of H-2 major histocompatibility complex and lps genes. Infect Immun. 1992 Jul;60(7):2874–2879. doi: 10.1128/iai.60.7.2874-2879.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
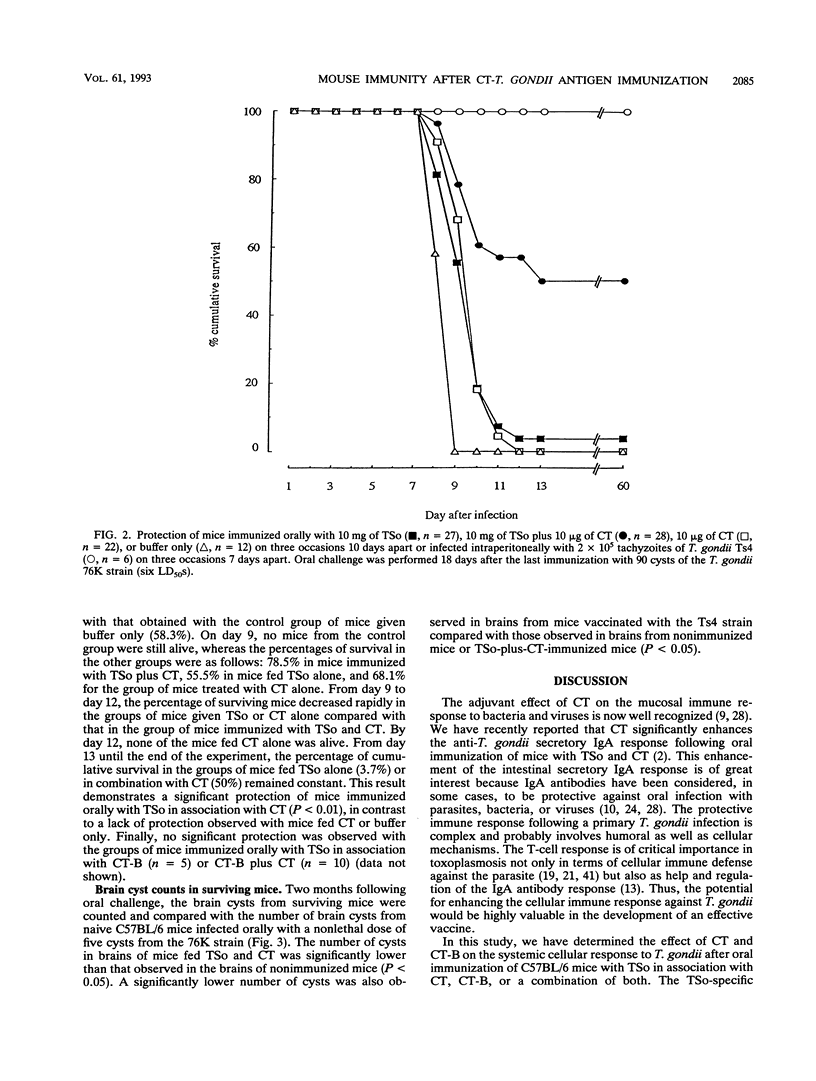
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Frenkel J. K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hakim F. T., Gazzinelli R. T., Denkers E., Hieny S., Shearer G. M., Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991 Oct 1;147(7):2310–2316. [PubMed] [Google Scholar]
- Johnson A. M. Strain-dependent, route of challenge-dependent, murine susceptibility to toxoplasmosis. Z Parasitenkd. 1984;70(3):303–309. doi: 10.1007/BF00927816. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Scott P. J., Rosner A. M., Strubel E. Effect of antigen form on local immunoglobulin A memory response of intestinal secretions to Shigella flexneri. Infect Immun. 1985 Jan;47(1):123–128. doi: 10.1128/iai.47.1.123-128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I. A., Ely K. H., Kasper L. H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991 Nov 15;147(10):3501–3506. [PubMed] [Google Scholar]
- Laugier M., Quilici M. Intérêt expérimental d'une souche de Toxoplasme peu pathogène pour la souris. Ann Parasitol Hum Comp. 1970 Jul-Aug;45(4):389–403. [PubMed] [Google Scholar]
- Lautenslager J. P. Toxoplasmosis as a significant disease in man and animals with special reference to preventive measures by the farm community. Can Vet J. 1987 May;28(5):261–264. [PMC free article] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Lycke N., Karlsson U., Sjölander A., Magnusson K. E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991 Jun;33(6):691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989 Jun 1;142(11):3781–3787. [PubMed] [Google Scholar]
- McLeod R., Frenkel J. K., Estes R. G., Mack D. G., Eisenhauer P. B., Gibori G. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital toxoplasma challenge. J Immunol. 1988 Mar 1;140(5):1632–1637. [PubMed] [Google Scholar]
- McLeod R., Mack D. G. Secretory IgA specific for Toxoplasma gondii. J Immunol. 1986 Apr 1;136(7):2640–2643. [PubMed] [Google Scholar]
- Mevelec M. N., Chardès T., Mercereau-Puijalon O., Bourguin I., Achbarou A., Dubremetz J. F., Bout D. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol Biochem Parasitol. 1992 Dec;56(2):227–238. doi: 10.1016/0166-6851(92)90172-g. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976 Jun;39(3):365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Mullenax J., Araujo F. G., Erlich H. A., Remington J. S. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983 Aug;131(2):977–983. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]
- Tamura S., Ito Y., Asanuma H., Hirabayashi Y., Suzuki Y., Nagamine T., Aizawa C., Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992 Aug 1;149(3):981–988. [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Bailey M., Williams N. A., Stokes C. R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991 Oct;21(10):2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- Wilson A. D., Clarke C. J., Stokes C. R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990 Apr;31(4):443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Woodman J. P., Dimier I. H., Bout D. T. Human endothelial cells are activated by IFN-gamma to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991 Sep 15;147(6):2019–2023. [PubMed] [Google Scholar]



