Abstract
We recently demonstrated that neointima formation of adult heterozygous apolipoprotein E (apoE+/−) offspring from hypercholesterolemic apoE−/− mothers was significantly increased as compared with genetically identical apoE+/− offspring from normocholesterolemic wild-type mothers. Since atherosclerosis is the consequence of a complex microenvironment and local cellular interactions, the effects of in utero programming and type of postnatal diet on epigenetic histone modifications in the vasculature were studied in both groups of offspring. An immunohistochemical approach was used to detect cell-specific histone methylation modifications and expression of accompanying lysine methyltransferases in the carotid arteries. Differences in histone triple-methylation modifications in vascular endothelial and smooth muscle cells revealed that the offspring from apoE−/− mothers had significantly different responses to a high cholesterol diet when compared with offspring from wild-type mothers. Our results suggest that both in utero programming and postnatal hypercholesterolemia affect epigenetic patterning in the vasculature, thereby providing novel insights regarding initiation and progression of vascular disease in adults.
An increasing amount of epidemiological and pathological evidence has been provided that indicates that an adverse maternal environment during embryonic development is correlated with an increased risk for cardiovascular disease in the offspring during adulthood.1 The “fetal origins hypothesis” postulated by Barker2 proposes that adaptation to an adverse maternal environment is favorable to the developing embryo. However, when the adult surroundings differ from the fetal setting, these fetal adaptations may lead to an increased disease risk. Intrauterine exposure to maternal hypercholesterolemia has been reported to enhance fatty streak formation in the vasculature of adult humans, as well as lesion size in aortas of fetuses from hypercholesterolemic mothers, as compared with fetuses from normocholesterolemic mothers.3 After birth, lesion progression remained accelerated in these children in relation to children from normocholesterolemic mothers.4
In a recent animal study, we were able to demonstrate that maternal hypercholesterolemia and associated risk factors caused by maternal apolipoprotein E-deficiency (apoE−/−) induced susceptibility for neointima formation in the offspring. Placement of a constrictive collar around the left carotid artery of adult heterozygous apoE+/− offspring from apoE−/− mothers resulted in extensive neointima formation, in the presence of high cholesterol feeding.5 ApoE+/− offspring from wild-type mothers were mainly nonresponders. In a separate study, we investigated whether increased neointima formation still occurred when offspring were fed a chow diet. We found that, although less severe than in high cholesterol-fed animals, intrauterine exposure to apoE-deficiency still resulted in neointimal lesions, deposition of extracellular matrix proteins, disorganization of smooth muscle cells (SMCs), and increased adventitial macrophage content after collar placement in chow-fed apoE+/− offspring from apoE−/− mothers (Alkemade et al, unpublished data). This strongly supported our previous findings and hypothesis that atherosclerosis-susceptibility may be programmed within differentiating arteries during embryonic and fetal development in apoE−/− mothers. Subsequently, neointimal lesion development in adults is accelerated and aggravated by a high cholesterol diet. Because the heterozygous offspring used in these studies were 100% genetically homogeneous, the observed differences in atherosclerosis-susceptibility could well be governed by epigenetic mechanisms.
Epigenetic mechanisms affect gene expression by modification of the chromatin organization without changing the nucleotide sequence within a cell and can be maintained during mitotic cell division into daughter cells. The major targets for epigenetic histone modifications are conserved amino acid residues located mainly in the amino-terminal tails of histones H3 and H4. These post-translational modifications include methylation, acetylation, phosphorylation, ubiquitination, and sumoylation, and together they establish the “histone code.” Histone methylation is thought to be the most stable of these modifications (reviewed in 6). Especially lysine methylation of histones along with DNA methylation establishes the framework for long-term epigenetic maintenance of gene transcription programs.7 Triple-methylation of lysine 27 in histone H3 (3Me-K27-H3) by the accompanying lysine methyltransferase EZH2 is an epigenetic modification generally associated with gene silencing. Until recently, triple-methylation of lysine 9 within histone H3 (3Me-K9-H3) by the lysine methyltransferase SUV39H1 was also thought to be a unique hallmark of gene silencing. However, Vakoc and co-workers8 showed that 3Me-K9-H3 is also present in transcribed regions of active genes indicating that 3Me-K9-H3 may have a dual role. In contrast to 3Me-K9-H3 and 3Me-K27-H3, triple-methylation of lysine 4 in histone 3 (by the lysine methyltransferase hSet1) is linked to active transcription.
We hypothesized that adverse factors associated with maternal apoE deficiency alter histone lysine methylation modification patterns in embryonic vascular endothelial cells (ECs) and SMCs, and that these differences persist into adulthood. An immunohistochemical approach was used to detect histone modification profiles and the presence of accompanying lysine methyltransferases in carotid arteries of adult apoE+/− offspring from apoE−/− mothers and apoE+/− offspring from wild-type mothers. Furthermore, to compare these profiles in normocholesterolemic and hypercholesterolemic offspring, we investigated histone modification expression in the absence (chow) and presence of a high cholesterol diet. We show that in utero programming highly influenced the response of adult atherosclerosis-susceptible offspring to a postnatal chow versus high cholesterol diet, as reflected by distinct changes in histone methylation modifications in vascular ECs and SMCs. These changes in histone methylation modifications within vascular cells point toward underlying epigenetic mechanisms associated with increased atherosclerosis-susceptibility and the effect of hypercholesterolemia on neointima formation.
Materials and Methods
Mice
ApoE−/− and wild-type C57Bl/6J mice were purchased from Charles River Laboratories, the Netherlands (import agency for Jackson Laboratories). The apoE−/− and wild-type mice were crossbred to generate genetically identical, female apoE+/− offspring from apoE−/− as well as from wild-type mothers. From weaning onwards, the female offspring received a regular (chow) or a high cholesterol, Western-type diet (1% cholesterol, Hope Farms). Diet and water were provided ad libitum. The Committee on Animal Welfare, Leiden University Medical Center, Leiden, the Netherlands, approved all animal experiments.
Tissue Harvesting
Non-collared 20-week-old female apoE+/− offspring (n = 5 each group) from apoE−/− and wild-type mothers were anesthetized and the thoraces were opened.5 The carotid arteries were dissected and perfused with PBS for 5 minutes and subsequently fixed for 6 hours in 4% paraformaldehyde in 0.1 mol/L sodium phosphate buffer. After fixation, the tissues were dehydrated in graded ethanol and xylene and paraffin-embedded. Transverse 5 μm sections were cut and serially mounted.
Immunohistochemistry
Unless indicated otherwise, immunohistochemistry was performed as described earlier.5,9 Overnight incubation at room temperature was performed with rabbit anti-trimethyl-histone H3 (Lys 27) (1:1000, Upstate, Lake Placid, NY, Cat no. 07-449) and the accompanying lysine methyltransferase mouse anti-EZH2 (1:250, BD Biosciences, Breda, The Netherlands, Cat no. 612267), rabbit anti-trimethyl-histone H3 (Lys 9) (1:1000, Upstate, Cat no. 07-442) and its accompanying lysine methyltransferase rabbit anti-SUV39H1 (1:1000, Abcam, Cambridge, UK, ab38637), or rabbit anti-trimethyl-histone H3 (Lys 4) (1:1000, Upstate, Cat no. 07-473) and its accompanying lysine methyltransferase rabbit anti-hSet1 (1:500, Bethyl Laboratories, Montgomery, AL, Cat no. IHC-00171).
Goat anti-rabbit biotin conjugate (1:200, Vector Laboratories) with normal goat serum diluted in PBS was used as secondary antibody. For EZH2, the primary antibody was coupled overnight to polyclonal rabbit anti-mouse peroxidase conjugate (1:200, Dako, Denmark) secondary antibody.10 Sections were incubated for two hours at room temperature. Biotin labeling was followed by incubation with Vectastain ABC (Vector Laboratories). The SUV39H1 signal was enhanced with a CSA kit (Dako), 3-3′ diaminobenzidine tetrahydrochloride was used for visualization, and counterstaining was performed with Mayer’s hematoxylin.
Statistical Analysis
In randomly selected sections (5 to 10 per carotid artery), the number of ECs and SMCs that stained positively or negatively for histone modifications or their associated enzymes were counted for each antibody. The relative numbers of positively stained cells are represented as average ± SEM. The data were analyzed using a linear mixed model with a diet effect, maternal apoE-deficiency effect and interaction effect, and a random intercept per mouse. The analysis was performed using the lme4 package (http://cran.r-project.org/web/packages/lme4, version 0.999375-32)11 in R (R, http://www.r-project.org). With this approach we were able to take into account the possible variation between individual mice. Tests for all contrasts were performed using a χ2 likelihood ratio test. To correct each test for multiple testing of several histone modification sites we used Holm’s method.12 Test results were considered to be significant if P < 0.05 after multiple testing correction.
Results
Effects of Maternal Genotype on Epigenetic Patterning in the Offspring Vasculature
To study the influence of maternal genotype on athero-susceptibility in female chow-fed offspring on an epigenetic level, we stained for histone methylation modifications (Figure 1, A–B, E–F, and I–J) or associated lysine methyltransferase expression (see Supplemental Figure S1, A–B and E–F at http://ajp.amjpathol.org). We subsequently quantified the relative number of intimal ECs or medial SMCs that were positive for a specific modification. This revealed that there were no statistically significant differences in ECs or SMCs from chow-fed apoE+/− offspring from apoE−/− mothers versus apoE+/− offspring from wild-type mothers (compare bars 1 vs. 2 in Figure 2, A–F and Supplemental Figure S2, A–F at http://ajp.amjpathol.org). This indicated that in utero programming was not significantly reflected by differential histone methylation patterning in the vasculature of adult chow-fed offspring.
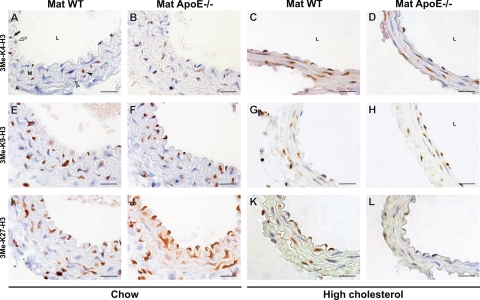
Figure 1.
Histone methylation patterning after intrauterine exposure to maternal apoE-deficiency and postnatal normal versus high cholesterol diet. Staining for 3Me-K4-H3 (A–D), 3Me-K9-H3 (E–H), and 3Me-K27-H3 (I–L) in intimal ECs (between lumen and media) and medial SMCs from chow-fed (A, B, E, F, I, J) and high cholesterol-fed (C, D, G, H, K, L) apoE+/− offspring from wild-type (Mat WT, A, C, E, G, I, K) and apoE−/− mothers (Mat ApoE−/−, B, D, F, H, J, L). Figure 1K shows partial nuclear staining in the SMCs. The apparent differences in lumen and media size between chow- and high cholesterol-fed mice were caused by different pressures during fixation and did not affect quantification and comparison of positive and negative ECs and SMCs between experimental and control conditions. Black and white arrows respectively indicate positively and negatively stained ECs. Black and white arrowheads respectively indicate positively and negatively stained SMCs. L = lumen, M = media, A = adventitia. Scale bars = 20 μm.
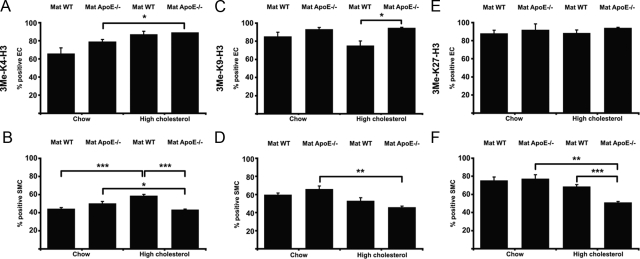
Figure 2.
Quantitative analysis of histone methylation patterns in endothelial and smooth muscle cells. Figure shows the relative number of ECs and SMCs positive for 3Me-K4-H3 (A–B), 3Me-K9-H3 (C–D), and 3Me-K27-H3 (E–F), as compared with total cell number. Data are average ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
We then determined the effects of distinct maternal genotypes in ECs and SMCs from offspring that were subjected to diet-induced hypercholesterolemia (Figure 1, C–D, G–H, and K–L, and Supplemental Figure S1, C–D, G–H, and I–J at http://ajp.amjpathol.org). Quantitative comparison of these groups (bars 3 vs. 4 in Figure 2, A–F and Supplemental Figure S2, A–F at http://ajp.amjpathol.org) showed a significantly increased relative number of EC positive for 3Me-K9-H3 (Figure 2C) or EZH2 (Supplemental Figure S2E at http://ajp.amjpathol.org), and a decreased relative number of SMCs positive for 3Me-K4-H3 (Figure 2B), 3Me-K27-H3 (Figure 2F), or hSet1 (Supplemental Figure S2B at http://ajp.amjpathol.org) in high cholesterol-fed apoE+/− offspring from apoE−/− mothers versus wild-type mothers. Thus, in utero programming and postnatal diet both influence epigenetic patterning in the vasculature of female offspring.
Effects of Diet on Epigenetic Patterning in the Offspring Vasculature
To further investigate the effect of postnatal diet on histone modifications in vascular cells, we compared chow versus high cholesterol-fed offspring from wild-type mothers (bars 1 vs. 3 in Figure 2, A–F and Supplemental Figure S2, A–F at http://ajp.amjpathol.org) as well as chow versus high cholesterol-fed offspring from apoE−/− mothers (bars 2 vs. 4).
A significantly increased number of hSet1-positive ECs was found in high cholesterol- versus chow-fed apoE+/− offspring from wild-type mothers (Supplemental Figure S2A at http://ajp.amjpathol.org). Additionally, the number of SMCs positive for 3Me-K4-H3 was increased by postnatal hypercholesterolemia (Figure 2B). In contrast, the number of SUV39H1-positive SMCs was decreased (Supplemental Figure S2D at http://ajp.amjpathol.org). No other statistically significant differences in the response to diet were noted in ECs and SMCs in female apoE+/− offspring from wild-type mothers.
The effect of diet became even more pronounced in apoE+/− offspring from apoE−/− mothers. The relative number of ECs positive for 3Me-K4-H3 significantly increased in high cholesterol-fed versus chow-fed offspring (Figure 2A), while the number of positive SMCs decreased (Figure 2B). Similarly, the number of hSet1-positive ECs increased (Supplemental Figure S2A at http://ajp.amjpathol.org), while hSet1-positive SMCs decreased (Supplemental Figure S2B at http://ajp.amjpathol.org). The change from chow to high cholesterol diet further resulted in a significantly decreased number of SMCs positive for 3Me-K9-H3 (Figure 2D), 3Me-K27-H3 (Figure 2F), or SUV39H1 (Supplemental Figure S2D at http://ajp.amjpathol.org) in offspring from apoE−/− mothers.
Together, this shows that changes in diet have a substantial influence on histone methylation modifications and lysine methyltransferase expression in the vasculature, especially in offspring susceptible for neointima formation.
In Utero Epigenetic Programming Affects the Response to Diet
During our quantitative analyses for postnatal diet-induced effects on histone modification patterning in the offspring vasculature, we observed that offspring from wild-type mothers (bars 1 vs. 3 in Figure 2, A–F and Supplemental Figure S2, A–F at http://ajp.amjpathol.org) often responded differently to diet-induced hypercholesterolemia than offspring from apoE−/− mothers (bars 2 vs. 4). Depending on the type of cell and modification, these responses were often characterized by increases or decreases in the relative number of positively stained cells (summarized in Table 1). Especially in SMCs from apoE+/− offspring from apoE−/− mothers (Table 1, Mat KO), a consistent decrease in the number of positively stained cells was observed in response to hypercholesterolemia. On the other hand, SMC from the offspring from wild-type mothers (Table 1, Mat WT) showed a more varied response. This was shown by an increase in the number of SMCs positive for 3Me-K4-H3, a decreased number of SMCs positive for SUV39H1, and a lack of response regarding the number of SMCs positive for 3Me-K9-H3, 3Me-K27-H3, or hSet1 (Table 1). Statistical comparisons of the increases versus decreases versus lack thereof in ECs or SMCs showed that these distinct responses in maternal wild-type versus maternal apoE−/− offspring were significantly different. This indicated that the reaction to hypercholesterolemia in apoE+/− offspring is highly influenced by the maternal genotype and in utero programming. In contrast, diet-induced hypercholesterolemia resulted in highly similar responses in apoE+/− offspring from wild-type and apoE−/− mothers with regard to the number of ECs positive for 3Me-K27-H3 or hSet1 (P > 0.999, Table 1). This suggests that these specific patterns in ECs are highly representative for a maternal genotype-independent response to hypercholesterolemia in the apoE+/− offspring.
Table 1.
Maternal Wild-Type and Maternal apoE−/− Offspring Show Different Responses to Diet-Induced Hypercholesterolemia
| EC
|
SMC
|
|||||
|---|---|---|---|---|---|---|
| Mat WT | Mat KO | Sign. | Mat WT | Mat KO | Sign. | |
| 3Me-K4-H3 | = | + | + | − | *** | |
| 3Me-K9-H3 | = | = | = | − | * | |
| 3Me-K27-H3 | = | = | # | = | − | * |
| hSet1 | + | + | # | = | − | * |
| SUV39H1 | = | = | − | − | ||
| EZH2 | N.D. | N.D. | N.D. | N.D. | ||
Overview showing the responses to diet-induced hypercholesterolemia in maternal wild-type (Mat WT) and maternal apoE−/ − (Mat KO) offspring, based on the relative number of vascular endothelial (EC) and smooth muscle cells (SMC) positive for a specific histone triple-methylation modification or expression of a lysine methyltransferase.
+ significant up-regulation of the relative number of positively stained cells in response to diet-induced hypercholesterolemia.
− significant down-regulation.
= not significantly up- or down-regulated.
N.D. not determined.
Significant differences between the types of diet-induced responses in Mat WT versus Mat KO offspring are indicated with *(P < 0.05) and ***(P < 0.001). Significantly similar responses are indicated with #(P > 0.999).
Epigenetic Programming of Gene Expression in Endothelial versus Smooth Muscle Cells
Since ECs and SMCs play different roles in the vasculature and neointima formation, we also analyzed whether the effects of maternal genotype or postnatal diet were cell type-specific. We observed that the maternal genotype-dependent histone modification patterns in ECs were significantly different compared with the maternal genotype-dependent patterns in SMCs (P < 0.001 for each modification or enzyme described above). This was also the case when the diet-dependent patterns were compared in ECs versus SMCs (P < 0.001). This indicates that distinct cell types obtain different histone modifications in response to in utero programming or postnatal diet and that the effects of these epigenetic modifications should thus be studied for each cell type separately.
Discussion
Epigenetic Regulation in Endothelial and Smooth Muscle Cells
Since the epigenetic histone methylation modifications that were studied here control gene transcription, identification of the affected genes may reveal the nature of the pathways involved. In this respect, several interesting candidate genes have been described of which the transcription is affected by epigenetic histone acetylation and methylation modifications. For example, endothelial nitric oxide synthase (eNOS) catalyzes the production of nitric oxide in mammalian vascular endothelial cells and is important for cellular homeostasis and vasomotor tone.13 Expression of the anti-atherogenic eNOS is severely compromised in advanced human atherosclerotic plaques and atherosclerosis-prone regions of the mouse aorta.14,15 Interestingly, eNOS expression in endothelial and non-endothelial cells depends on acetylation of histones H3 and H4, and triple-methylation of K4-H3.16,17 In this study we show that in particular the 3Me-K4-H3 patterning in ECs and SMCs was affected by in utero programming and diet-induced hypercholesterolemia. Because eNOS expression depends on the level of 3Me-K4-H3,16,17 the observed alterations in 3Me-K4-H3 in our study may therefore have resulted in differential eNOS expression and, subsequently, altered functionality of the vascular wall in apoE+/− offspring from apoE−/− mothers. Further investigation will reveal whether these altered levels of 3Me-K4-H3 indeed affect eNOS expression and whether this contributes to the development of atherosclerosis susceptibility in these ApoE+/− mice.
Vascular SMCs are responsible for vessel contraction/relaxation in response to altered blood pressure or flow and can undergo rapid phenotype switching after changes in the local environment, like vessel injury or neointima formation. Tight molecular control of the SMC phenotype is therefore required. SMC-specific gene expression is directed by several factors, including platelet-derived growth factors BB18 and DD19 and their downstream targets serum response factor and Krüppel-like factor-4.20 Binding of the transcriptional activator serum response factor and repressor Krüppel-like factor-4 to the promoters of their target genes is respectively associated with activating and repressing histone modifications, thereby maintaining a differentiated phenotype in SMCs. This indicates that control of the SMC phenotype is regulated by epigenetic mechanisms (comprehensively reviewed in 21). This is supported by other studies that showed that the enhanced pro-atherogenic and inflammatory phenotype of vascular SMCs from type 2 diabetic db/db mice versus those from db/+ mice was associated with differential histone modification patterning.22,23 In these studies, transcriptionally activating histone methylation modifications on the promoters of inflammatory genes resulted in enhanced expression of these genes in db/db SMCs compared with db/+ SMCs in response to pro-atherogenic stimuli. To elucidate the molecular mechanisms that contribute to enhanced athero-susceptibility in apoE+/− offspring from apoE−/− mothers compared with those from wild-type mothers (Alkemade et al, unpublished data and5), identification of the genes that are affected by alterations in specific histone methylation modifications, as revealed in this study, will be required.
Possible Mechanisms that Induce Differential Epigenetic Patterning in Vascular Cells
Although the current study points toward several underlying mechanisms that may cause differential epigenetic patterning in the vasculature, the physiological factors that may have affected susceptibility for neointima formation remained unknown. Comparisons between our and other models may reveal which specific elements regulate this athero-susceptibility and subsequently enhanced progression of atherosclerosis. For instance, gene silencing histone modifications were increased in a monocyte-macrophage cell line after exposure to atherogenic lipoproteins in vitro.24 This may suggest that factors circulating in the blood could affect gene transcription via epigenetic patterning in cells involved in inflammation, which is an important feature of atherosclerosis development. Similarly, athero-prone shear stress levels, an important risk factor of atherosclerosis and characterized by low or oscillatory flow profiles, affected histone H3 and H4 acetylation in cultured human ECs and SMCs.10,25 In vivo, an increase in histone acetylation resulted in exacerbation of atherosclerosis in low-density lipoprotein receptor (LDLR)-deficient mice.26 This suggests that alterations of the histone acetylation status in the LDLR-deficient environment could affect the expression of pro-artherogenic genes and the oxLDL receptor, and as such contributes to atherosclerosis progression.26 Notably, in preliminary experiments of our own, the acetylation of histone H4 was significantly up-regulated in ECs and slightly decreased in SMCs from apoE+/− offspring from apoE−/− versus wild-type mothers (data not shown). This is comparable with the observed patterns in the studies discussed above and suggests that similar factors may be involved in athero-susceptibility in our model. No significant differences were detected in the acetylation pattern of histone H3 in ECs or SMCs from non-collared, high cholesterol-fed apoE+/− offspring (data not shown).
The gene activation versus silencing patterns in our and other studies may have been affected by the different settings (in vitro versus in vivo) and models (monocyte-macrophage cell line versus SMCs, LDLR−/− versus apoE−/−). Careful interpretation of gene expression profiles and histone modifications in the vasculature that are associated with maternal apoE-deficiency will therefore be required.
Conclusions
In summary, intrauterine exposure to maternal apoE-deficiency plays a key role in the susceptibility for neointimal lesion formation in adult mouse offspring. Subsequent postnatal induction of hypercholesterolemia induces changes in the epigenetic profiles that modulate gene expression patterns of the vasculature, thereby enhancing progression of vascular disease in atherosclerosis-susceptible animals. Our results demonstrate that both in utero programming and diet-induced hypercholesterolemia affect histone methylation modifications and expression of accompanying lysine methyltransferases in vascular ECs and SMCs. However, changes in histone modifications between chow-fed apoE+/− offspring from wild-type versus apoE−/− mothers did not reach statistical significance. In line with this, no pathology was detected in (non-compromised) carotid arteries of 20-week-old apoE+/− mice from apoE−/− and wild-type mothers (this study and Alkemade et al, unpublished data), while collar placement induced a more severe pathology (Alkemade et al, unpublished data). The nonsignificant changes in histone methylation modifications therefore provide an essential first step to increased susceptibility for neointima formation, which is further amplified by high-cholesterol diet or restrictive collar placement. Similar observations have been made for epigenetic DNA methylation modifications.24
The observations in this study may help to explain why some people are more prone to develop atherosclerosis than others even when these groups have similar feeding habits. Elucidation of the exact signaling pathways that are affected by these epigenetic mechanisms may provide us with early indicators of atherosclerosis susceptibility (reviewed in1). This will contribute to understanding why and how pre- and postnatal atherosclerotic risk factors influence development of adult vascular disease and may help to improve lifestyle management programs of both parents and their children.
Acknowledgments
We thank Professor Rob Poelmann for helpful discussions, Simone van de Pas for technical assistance, and Jan Lens for help with the preparation of the artwork of this manuscript. We also thank Bert Wisse for his assistance with the morphometric analyses and the animal caretakers for animal care and breeding.
Footnotes
Address reprint requests to Marco C. DeRuiter, Ph.D., Department of Anatomy and Embryology, Leiden University Medical Center, PO BOX 9600, 2300 RC Leiden, The Netherlands. E-mail: m.c.deruiter@lumc.nl.
Supported by grants from the Netherlands Heart Foundation (F.E.A. and P.v.V., by 2003B241 and 2006B074, respectively). J.J.G. was supported by a NWO Veni grant 91676122. L.M.H. and K.W.v.D. were supported by the Center for Medical Systems Biology and Nutrigenomics Consortium in the framework of the Netherlands Genomics Initiative and P.J.v.d.E. by the Macropa Foundation.
F.E.A. and P.v.V. made equal contributions to this work; and P.J.v.d.E. and M.C.D. made equal contributions to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- DeRuiter MC, Alkemade FE, Gittenberger-de Groot AC, Poelmann RE, Havekes LM, van Dijk KW. Maternal transmission of risk for atherosclerosis. Curr Opin Lipidol. 2008;19:333–337. doi: 10.1097/MOL.0b013e328304b670. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- Alkemade FE, Gittenberger-de Groot AC, Schiel AE, VanMunsteren JC, Hogers B, van Vliet LS, Poelmann RE, Havekes LM, Willems van Dijk K, DeRuiter MC. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler Thromb Vasc Biol. 2007;27:2228–2235. doi: 10.1161/01.ATV.0000282193.31936.fd. [DOI] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Jenuwein T. The epigenetic magic of histone lysine methylation. FEBS J. 2006;273:3121–3135. doi: 10.1111/j.1742-4658.2006.05343.x. [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- Doran H, Bates D, Bliese P, Dowling M. Estimating the multilevel rasch model: with the lme4 package. J Stat Software. 2007;20:1–18. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Luscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 1998;97:2494–2498. doi: 10.1161/01.cir.97.25.2494. [DOI] [PubMed] [Google Scholar]
- Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Matouk CC, Rachlis A, Lin S, Tai SC, D'Abreo C, Marsden PA. The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem. 2005;280:24824–24838. doi: 10.1074/jbc.M502115200. [DOI] [PubMed] [Google Scholar]
- Gan Y, Shen YH, Wang J, Wang X, Utama B, Wang J, Wang XL. Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. J Biol Chem. 2005;280:16467–16475. doi: 10.1074/jbc.M412960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1027–H1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, Topouzis S, Wamhoff BR, Blackman BR, Owens GK. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is up-regulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol. 2009;296:H442–H452. doi: 10.1152/ajpheart.00165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–1441. doi: 10.1161/01.RES.0000266448.30370.a0. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103:615–623. doi: 10.1161/CIRCRESAHA.108.175190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA: 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF, Villar-Garea A, Ballestar E, Esteller M, Zaina S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem. 2004;279:29147–29154. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res. 2003;93:155–161. doi: 10.1161/01.RES.0000080933.82105.29. [DOI] [PubMed] [Google Scholar]
- Choi JH, Nam KH, Kim J, Baek MW, Park JE, Park HY, Kwon HJ, Kwon OS, Kim DY, Oh GT. Trichostatin A exacerbates atherosclerosis in low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2404–2409. doi: 10.1161/01.ATV.0000184758.07257.88. [DOI] [PubMed] [Google Scholar]