Abstract
In transgenic mice overexpressing the major myelin protein of the central nervous system, proteolipid protein, CD8+ T-lymphocytes mediate the primarily genetically caused myelin and axon damage. In the present study, we investigated the cellular and molecular mechanisms underlying this immune-related neural injury. At first, we investigated whether T-cell receptors (TCRs) are involved in these processes. For this purpose, we transferred bone marrow from mutants carrying TCRs with an ectopic specificity to ovalbumin into myelin mutant mice that also lacked normal intrinsic T-cells. T-lymphocytes with ovalbumin-specific TCRs entered the mutant central nervous system to a similar extent as T-lymphocytes from wild-type mice. However, as revealed by histology, electron microscopy and axon- and myelin-related immunocytochemistry, these T-cells did not cause neural damage in the myelin mutants, reflecting the need for specific antigen recognition by cytotoxic CD8+ T-cells. By chimerization with bone marrow from perforin- or granzyme B (Gzmb)-deficient mice, we demonstrated that absence of these cytotoxic molecules resulted in reduced neural damage in myelin mutant mice. Our study strongly suggests that pathogenetically relevant immune reactions in proteolipid protein−overexpressing mice are TCR-dependent and mediated by the classical components of CD8+ T-cell cytotoxicity, perforin, and Gzmb. These findings have high relevance with regard to our understanding of the pathogenesis of disorders primarily caused by genetically mediated oligodendropathy.
Recent studies in myelin mutant mice revealed a substantial pathomechanistic implication of immune cells in both the central nervous system (CNS) and peripheral nervous system (PNS).1,2,3,4,5,6 In a CNS myelin mutant mildly overexpressing the major oligodendrocytic integral membrane protein, proteolipid protein (PLP/DM20), bone marrow chimera experiments unequivocally identified CD8+ T-lymphocytes as mediators of the primarily genetically caused myelin and axon damage, whereas CD4+ T-cells appeared nonrelevant for the demyelinating and axonopathic features.2
The pathogenetically relevant involvement of components of the adaptive immune system in diseases primarily caused by oligodendrocyte injury may be highly relevant for inflammatory disorders of the CNS, including multiple sclerosis (MS). Indeed, pathological studies suggest primary oligodendropathy as one possible cause of MS.7,8 Moreover, primary oligodendrocyte damage may be causally linked to MS as suggested by some clinical reports demonstrating the concurrence of PLP mutations and primary progressive or relapsing-remitting MS.9,10 Thus, identifying pathomechanistic pathways as a consequence of primary oligodendropathy is important for our understanding of neural damage in some forms of MS. In this context, it is of note that CD8+ T-lymphocytes might play a larger role in MS pathology than previously thought.11 It is known that the CD8+ T-cells in PLP/DM20 transgenic mice display the phenotype of CD44+, CD69+, and CD62L− effector cells. Moreover, in the CNS of the mutants, T-lymphocytes are often in tight association with oligodendrocytes, which—as a likely immune target—had up-regulated MHC I molecules.2 Spectratype analysis of T-cell receptors (TCRs) of T-lymphocytes isolated from PLP mutant CNS revealed monoclonal expansions, which serve as a further strong hint for a pathogenetic, antigen-specific role of the CD8+ T-cells.4 However, the functional implication of TCR in the pathogenesis still requires clarification. Furthermore, the molecular agents involved in neural damage are still unknown. Among other established candidates, such as Fas-FasL and interferon-γ, the likely mediators of CD8+ T-cell−mediated cytotoxicity are perforin and granzyme B (Gzmb).12 Together with perforin, the granzyme serine proteases are released from cytotoxic granules and can activate three distinct pathways of cell damage in the interior of target cells.12,13
In the present study, we provide strong evidence that in PLP-overexpressing mice, neural damage is dependent on TCR specificity. Moreover, perforin and—to a lesser extent—Gzmb mediate immune-related cytotoxicity in these mice. Our data have high relevance with regard to understanding pathogenesis of some forms of multiple sclerosis and other leukodystrophies.
Materials and Methods
Animals and Determination of Genotypes
PLP transgenic mice14 were bred and genotyped as described previously.2 PLPtg/RAG-1−/− mice were used as recipients for bone marrow chimerization experiments. The RAG-1 deficiency and the absence of mature B- and T-lymphocytes was confirmed as previously described.2,6,15 Ovalbumin-specific (OT-I) transgenic mice16 and perforin-deficient mice17 were kindly provided by T. Hünig (Würzburg). Granzyme B−/− mice18 were obtained from the Jackson Laboratory (Bar Harbor, ME). Genotyping was performed using PCR according to the provider’s protocols (The Jackson Laboratory, Bar Harbor, Maine 04609). All investigated mice were on a C57BL/6 background. Animal experiments were approved by the Regierung von Unterfranken Wuerzburg.
Bone Marrow Chimerization
PLPtg/RAG-1−/− recipients and PLPwt/RAG-1−/− controls were transplanted with bone marrow from OT-I transgenic, perforin- or Gzmb-deficient mice or wild-type mice at the age of 6 to 8 weeks. Transplantation and control of successful transplantation was performed as described before.2,19 OT-I transgenic cells were identified using Vβ5.1/5.2 antibodies (BD Biosciences Pharmingen, San Jose, CA). All mice were sacrificed and investigated at the age of 10 months, thereby allowing a period of 8 months for engraftment of transplanted bone marrow and production of hematopoietic cells.
Preparation of Splenocytes and CNS Mononuclear Cells
Mononuclear cells were harvested from spleens and CNS as described before.20 Spleens were homogenized through a cell strainer (BD Biosciences Pharmingen), and erythrocyte lysis was performed using a lysis buffer (150 mmol/L NH4Cl2, 10 mmol/L KHCO3, 0.1 mmol/L EDTA in distilled water at pH 7.3). CNS mononuclear cells were obtained from tissue by homogenization and separation by Percoll gradient centrifugation. Cells were harvested from the interface. Afterward, cells were washed and processed for further experiments.
Detection of Perforin and Granzyme by Flow Cytometry and Enzyme-Linked Immunosorbent Spot Analysis
Presence of perforin in the CNS of myelin mutants was assessed by flow cytometry using a phycoerythrin-labeled antibody (clone eBIOMAK-D, ebioscience, San Diego, CA) on CNS cells and splenocytes stimulated with phorbol 12-myristate 13-acetate (20 ng/ml)/ionomycin (500 ng/ml) (both from Sigma) in the in the presence of GolgiStop (1 μl/ml) (BD Biosciences). Stained cells were measured on a FACSCalibur cytometer and analyzed with FlowJo software (TreeStar, Ashland, OR) version 7.2.1. Assessment of granzyme B producing cells was performed by enzyme-linked immunosorbent spot analysis. CNS lymphocytes (2 × 104) or splenocytes (2 × 105) per well were incubated for 24 hours in provided 96-well-plates, unstimulated or stimulated with phorbol 12-myristate 13-acetate/ionomycin as described above. The enzyme-linked immunosorbent spot assay was performed according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Assessment of Immunological Competence
To assess if T-lymphocytes cells obtained from OT-I bone marrow chimeras were immunologically functional, spleens were harvested, homogenized, and labeled with 5 μmol/L carboxyfluorescein succinimidyl ester for 1 minute, followed by addition of fetal calf serum and two washing steps. Afterward, cells were cultured in standard medium for 4 days in the presence and absence of 100 nmol/L or 200 nmol/L SIINFEKL peptide or lipopolysaccharide (5 μg/ml). After 4 days, proliferation rates were detected using flow cytometry.
Antibodies and Immunohistochemistry
Activated macrophage-like cells were detected with rat anti-mouse CD11b (clone M1/70.15, Serotec, Oxford, UK); rat anti-mouse CD4 (clone YTS191.1, Serotec, Oxford, UK) and rat anti-mouse CD8 (IBL-3/25, Chemicon, Temecula, CA) antibodies were used for the identification of T-lymphocytes. Dephosphorylated neurofilament (SMI32) was detected with a SMI32-R mouse anti-mammal antibody (Covance, Princeton, NJ). Myelin basic protein (MBP) labeling, was performed as previously described1,2,3,4,6 on optic nerve cross sections from the region of 1200 to 1400 μm caudal to the pigment epithelium using a rabbit anti-mouse antibody (MBL, Woburn, MA). Detection of primary antibodies was achieved by using biotinylated secondary antibodies to rat (macrophage-like cells, T-cell antibodies), mouse (SMI32) or rabbit (MBP) Igs for 1 hour, followed by avidin/biotin reagent (Dako, Carpinteria, CA) before incubation and staining with diaminobenzidine-HCl and H2O2. The specificity was controlled by omission of the primary antibodies. For tissue preparation and preservation, mice were transcardially perfused with 0.1 mol/L PBS. Tissue was harvested and snap-frozen, and 10-μm thick longitudinal sections of the optic nerve were cut. For CD11b or SMI32 staining, sections from PBS-perfused mice were pre-incubated with 4% paraformaldehyde. For SMI32 staining, sections were also incubated in methanol containing 0.3% H2O2. After washing steps, unspecific binding was blocked for 30 minutes in 5% normal bovine serum and then incubated with the primary antibodies diluted in 1% normal bovine serum, containing 0.3% Triton-X (CD11b, MBP) overnight at 4°C.
Tissue Preservation for Light and Electron Microscopy
For the preservation of tissue for semithin and ultrathin sections, mice were transcardially perfused with 4% paraformaldehyde/2% glutaraldehyde. Optic nerves were dissected and processed for light and electron microscopy as reported.2,20 Tissue damage was assessed by quantification of axonopathic vacuoles >6 μm in semithin sections. Electron microscopy was performed by using a Leo 906 E electron microscope (Zeiss, Oberkochen, Germany), equipped with a ProScan Slow Scan CCD (Proscan, Lagerlechfeld, Germany) camera and the corresponding software iTEM (Olympus Soft Imaging Solutions, Muenster, Germany).
Quantification of Immune Cells in the CNS
Longitudinal sections of the optic nerve of PLPtg/ RAG-1−/− or PLPwt/RAG-1−/− mice transplanted with either wild-type, OT-I transgenic, perforin- or Gzmb-deficient bone marrow were investigated at the age of 10 months. Quantification of CD11b+, CD4+, and CD8+ cells was performed as previously described.2,20 Similarly, SMI32+ profiles were quantified on complete longitudinal sections. Only profiles with visible axonal staining were counted. All quantifications were performed using a light microscope (Olympus BH2, Olympus, Hamburg, Germany) or a Zeiss Axiophot2 (Zeiss, Jena, Germany) microscope.
Quantification of Demyelination
As previously described2,20 myelin damage characterized by lack of MBP staining in optic nerve cross sections was quantified by measuring MBP negative areas. Data are shown as percentage of the total area. As wild-type mice do not show any myelin damage, the percentage was defined as 0 in these mice. The area was measured using digital images acquired via a CCD-camera and ImagePro 4.0 software on a Zeiss Axiophot2. In addition, MBP+ and MBP− areas were quantified using ImageJ 1.410 software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Statistical Analysis
Quantifications were tested with two-tailed student’s t-test. P values <0.05 were considered significant.
Results
CD8+ T-Lymphocytes with Irrelevant Ectopic TCR Enter the Mutant CNS but Are Not Competent to Mediate Neural Damage
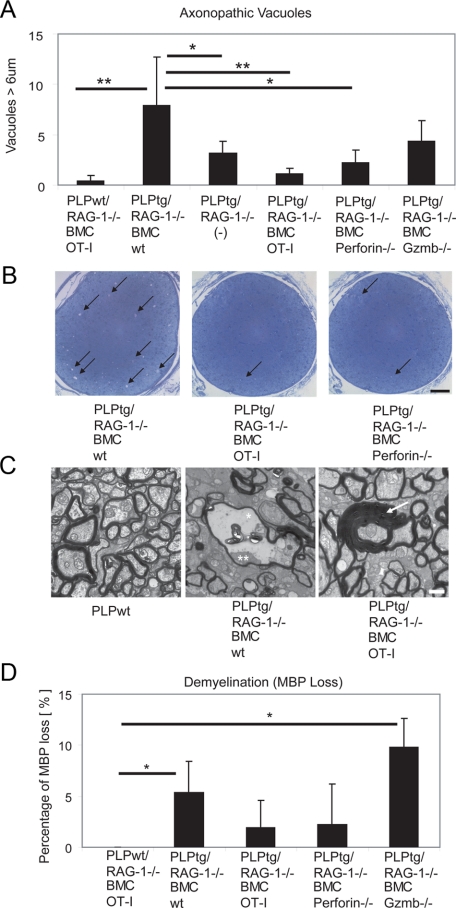
To investigate whether functional TCRs are necessary for neural damage in PLPtg mice, we used PLP overexpressing mice devoid of RAG-1 (PLPtg/RAG-1−/−) and reconstituted them with either bone marrow from wild-type mice or from TCR-mutants (OT-I mice; see below). PLPwt/RAG-1−/− mice with bone marrow reconstitution from wild-type or OT-I mice showed no pathology in optic nerves and served as a control. PLPtg/RAG-1−/− mice that had received bone marrow from wild-type mice (PLPtg/RAG-1−/− BMCwt) showed neural damage in form of demyelination and periaxonal vacuoles as described,2,20 (Figure 1A−D), and as seen in single transgenic mice.14,21 This neural damage is particularly prominent in electron micrographs, showing myelin pathology including periaxonal vacuoles, occasionally containing axonal remnants, in myelin mutant mice that received wild-type bone marrow (Figure 1C). As a next step, we quantified loss of myelin by calculating reduction of MBP immunoreactivity in optic nerves, with a robust reduction of myelin in PLPtg/RAG-1−/− BMCwt mice (Figure 1D), as described.2,20
Figure 1.
Neural damage in PLPtg mice depends on an intact TCR repertoire and molecules typical for cytotoxic T-lymphocytes. A: Quantification of periaxonal vacuoles in semithin sections of optic nerves from PLPwt mice and PLPtg mutants with normal or molecularly altered T-lymphocytes. Note reduction in vacuole numbers when T-lymphocytes lack relevant TCR (OT-I) or the cytotoxic molecules perforin and Gzmb. B: Representative semithin sections from PLPtg mutants with wild-type immune system, with irrelevant TCR (OT-I) and with lymphocytes lacking perforin (perforin−/−). Note reduced vacuole numbers when T-lymphocytes lack relevant TCR (OT-I) or the cytotoxic molecule perforin. Arrows point to periaxonal vacuoles. Scale bar = 50 μm. C: Representative electron micrographs from optic nerves of PLPwt mice, PLPtg mutants with wild-type immune system (PLPtg/RAG-1−/− BMCwt) and of PLPtg mutants with irrelevant TCR (PLPtg/RAG-1−/− OT-I). Note normal appearance of myelin in the PLPwt mice (left) and occurrence of myelin vacuoles in PLPtg mutants with wild-type immune system (middle; asterisk). Remnant of an axon is indicated by a double asterisk. In PLPtg mutants with irrelevant TCR (right), demyelination and formation of vacuoles is substantially reduced. Occasionally, formation of redundant myelin loops (arrow) is seen. Scale bar = 1 μm. D: Reduction in MBP immunoreactivity in optic nerves from PLPtg mutants with normal or molecularly altered T-lymphocytes indicative of demyelination. Note that loss of MBP is minimized in the absence of relevant TCR or perforin, whereas absence of Gzmb apparently does not reduce demyelination. PLPwt mice do not show demyelination. *P < 0.05, **P ≤ 0.01, n = 3 to 6 per group.
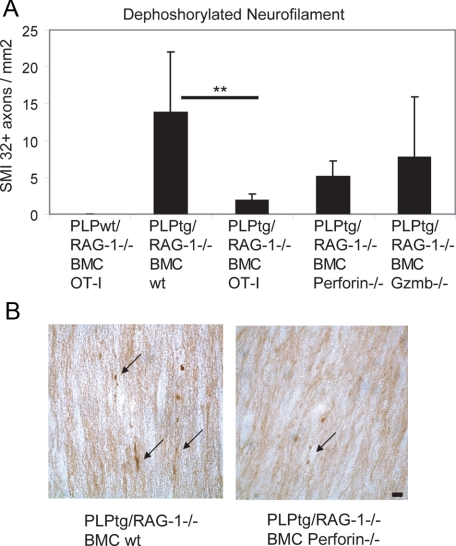
As an additional marker for neural damage, we quantified in optic nerves the axonal profiles with SMI32-immunoreactivity, identifying dephosphorylated neurofilament. Interestingly, in the PLPtg/RAG-1−/− BMC wild-type mice, SMI32+ profiles were abundant (Figure 2A) and were mostly characterized by varicosities typical for degenerating axons (Figure 2B).
Figure 2.
Axonal damage in optic nerves from PLPwt and PLPtg mutants with normal or molecularly altered T-lymphocytes. A: Quantification of dephosphorylated neurofilament (SMI32) in optic nerve longitudinal sections. Note reduction of numbers of profiles with dephosphorylated neurofilaments in PLPtg mutants with irrelevant TCR (OT-I) or in the absence of cytotoxic molecules, while PLPwt mice do not display SMI32+ profiles. **P ≤ 0.01. B: Representative examples of SMI32+ profiles (arrows) in optic nerves of PLPtg mutants with wild-type immune system or in the absence of perforin. Note reduced SMI32+ profiles in the latter. Scale bar = 10 μm.
Next, we investigated PLPtg/RAG-1−/− mice transplanted with bone marrow from transgenic mice that carry a TCR specific for the ovalbumin-derived peptide SIINFEKL presented by H-2Kb on approximately 90% of their CD8+ T-cells (OT-I16,22,23). Importantly, other lymphocyte populations, like CD4+ T-cells and B-cells, are present in these mice.
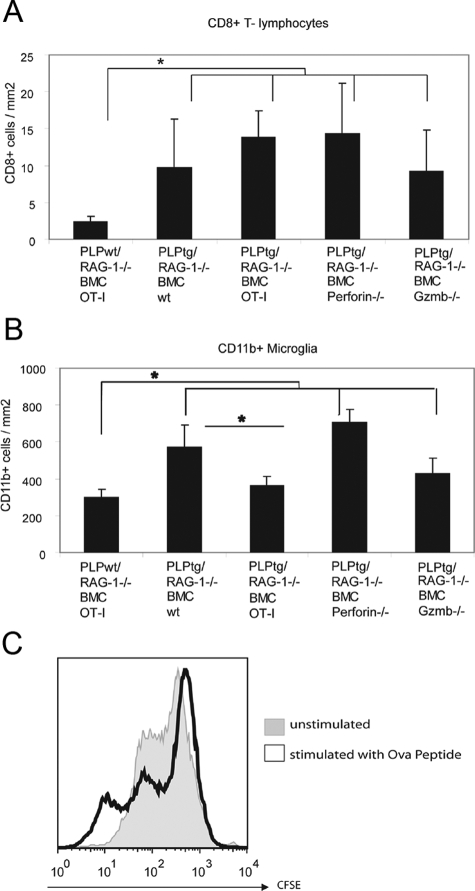
In comparison with PLPtg/RAG-1−/− BMCwt mice, PLPtg/RAG-1−/− mice with bone marrow from OT-I mice (PLPtg/RAG-1−/− BMC OT-I) showed a robust amelioration of the pathological alterations, including periaxonal vacuoles (Figure 1, A−C), dephosphorylated neurofilament (Figure 2A), and loss of myelin immunoreactivity (Figure 1D). PLPtg mice that were chimerized with OT-I transgenic bone marrow, rarely show axonopathic vacuoles, but occasionally show some redundant myelin loops that are also present in PLPtg mice with wild-type TCR (Figure 1C). To rule out that the reduced pathological alterations in PLPtg/RAG-1−/− OT-I mice are caused by an incompetence of the corresponding T-lymphocytes to enter the CNS, we quantified the number of CD8+ T-cells in CNS tissue of both wild-type- and the OT-I-transplanted mutants. Interestingly, the number of CD8+ cells of the OT-I-transplanted mutants was similar to the number of CD8+ T-cells in CNS tissue from PLPtg/RAG-1−/− BMCwt mice (Figure 3A). Thus, OT-I cells are able to enter the mutant CNS tissue with a similar efficiency as CD8+ T-cells from immune competent mice, but appear to be less competent to cause neural damage. We also quantified CD4+ T-lymphocytes in optic nerves of these mice. As reported earlier, numbers of CD4+ T-lymphocytes were not elevated in PLPtg, compared with PLPwt mice.2,20 Chimerization with either type of bone marrow did not alter CD4+ cell counts (data not shown). Interestingly, numbers of CD11b+ microglial cells were reduced in PLPtg/RAG-1−/− BMC OT-I mice, compared with PLPtg/RAG-1−/− BMC wild-type mice (Figure 3B).
Figure 3.
Number of immune cells in optic nerves from PLPwt and PLPtg mutants with normal or molecularly altered T-lymphocytes. A: As shown previously, CD8+T-lymphocytes are more frequent in PLPtg mice compared with PLPwt mice. Note that in all types of PLPtg mutants, numbers of CD8+T-lymphocytes are similar, reflecting their unchanged ability to enter the mutant CNS. B: Numbers of CD11b+ microglial cells are higher in PLPtg as compared with PLPwt mice. PLPtg mutants with irrelevant TCR show a reduced number of microglial cells. C: Histogram overlay of carboxyfluorescein succinimidyl ester-labeled splenocytes stimulated with Ova peptide (black) or unstimulated (gray). Reduction of carboxyfluorescein succinimidyl ester intensity reflects proliferation. *P < 0.05, n = 3 to 6 per group.
To test the general immunological competence of the OT-I cells, we isolated these cells from spleens of OT-I-transplanted PLPtg/RAG-1−/− mice and challenged them with the corresponding antigen ovalbumin peptide SIINFEKL. Corroborating previous studies from other groups,22,23 T-lymphocytes isolated from the mutants proliferated when challenged with SIINFEKL, as revealed by carboxyfluorescein succinimidyl ester labeling in vitro (Figure 3C).
CD8+ T-Lymphocytes Devoid of Perforin or Granzyme B Are Impaired in Transmitting Neural Damage in PLP Mutants
After having shown that CD8+ cells require TCR receptors for neural damage in PLPtg mice, we investigated the role of the classical cytotoxic agents perforin and granzyme B (Gzmb) in neural damage. Both perforin and Gzmb were detectable by flow cytometry and enzyme-linked immunosorbent spot assays, respectively, from PLPtg-mutant CNS tissue (not shown). PLPtg/RAG-1−/− mice transplanted with bone marrow from perforin-deficient mice showed a significantly ameliorated neural damage, comparable with PLPtg/RAG-1−/− mice transplanted with bone marrow from OT-I mice (Figures 1, A, B, and D; Figure 2, A and B). Interestingly, in Gzmb-deficient PLPtg mice, only axons, but not myelin was protected from damage (Figure 1, A and D; Figure 2A). In both perforin- and Gzmb- deficient chimeras, neither the number of CD8+ cells nor the number of CD11b+ cells was significantly changed in comparison with PLPtg/RAG-1−/− mice transplanted with bone marrow from wild-type mice (Figure 3, A and B). These combined observations strongly suggest that in PLP overexpressing mice, neural damage is strongly dependent on relevant TCRs and the cytotoxic components perforin and Gzmb.
Discussion
We have previously shown that a transgenic mouse mutant overexpressing the major CNS myelin component PLP/DM20 develops a low-grade inflammatory reaction comprising CD8+/CD44+/CD69+ T-lymphocytes that showed substantial pathogenic relevance with regard to myelin and axonal damage.2 By contrast, CD4+ cells did not play a pathogenic role as revealed by bone marrow chimeric myelin mutants.2 Also in MS, the presence of clonally expanded CD8+ cells in lesions and their possible role in pathogenesis shifted interest onto this lymphocyte population.11,24 To gain further insights into possible antigen-specific reactions in the mutant mice, we previously performed spectratype analyses of TCR from systemic and CNS-derived immune cells from both PLPtg and wild-type mice. Exclusively in PLPtg mice, we found mono- or oligoclonal expansions of the TCR.4 Although these results can be viewed as a hint in favor of an antigen-specific autoimmune response,25,26,27 the findings by Leder and colleagues4 cannot be viewed as a strict proof for an antigen-specific response.
We now chimerized PLPtg mice with MHC I-restricted T-lymphocytes that carry a monospecific, neural tissue-irrelevant TCR against the ectopic antigen ovalbumin.16 In the context of our study, two issues are important to mention. Firstly, the TCR-transgenic T-lymphocytes are able to enter the myelin mutant CNS at a similar degree as T-lymphocytes from wild-type mice. Secondly, the OT-I cells are immunologically highly competent. This is reflected by our present observation that isolated OT-I cells proliferate when challenged with their specific antigen. In line with these observations was a series of earlier experiments focusing on the cytotoxicity of the OT-I cells in situ. Na and colleagues28 tested the encephalitogenic potential of OT-I cells from exactly the same transgenic line as ours in a mouse model specifically expressing ovalbumin in oligodendrocytes. These studies demonstrated a full-blown, demyelinating, inflammatory reaction in mice doubly transgenic for OT-I and oligodendrocytic ovalbumin. Despite of the high affinity OT-I transgenic TCR, these lymphocytes are unable to induce damage in PLPtg chimeras, reflecting the strong dependency of the OT-I cells on their corresponding antigen. Based on these combined observations, the highly reduced demyelinating phenotype in PLPtg/OT-I mice cannot be explained by a generally reduced capacity of OT-I cells to transmit cytotoxicity to target cells. Rather, the absence of neural damage in PLPtg/OT-I mice strongly suggests lack of antigen recognition in the mutants with transgenic T-lymphocytes. Thus, antigen recognition appears to be pivotal for neural damage in PLPtg mice.
Interestingly, chimerization of PLPtg mice with OT-I transgenic bone marrow resulted in a reduced neural damage, not only compared with chimeras that received wild-type bone marrow, but also to nontransplanted, PLPtg/RAG-1−/− mice of the same age. This might be indicative of a potentially neuroprotective influence of a lymphocytic cell subset being absent in RAG-1−/− mice. Protective effects of immune cells have been postulated.29,30 In mice doubly transgenic for OT-I and oligodendrocytic ovalbumin, RAG-1 deficiency resulted in additional severity of disease, suggesting the influence of a neuroprotective or regulatory cell subset in RAG-1+ mice.28
As a next step, we investigated the molecular mechanisms involved in neural damage in PLPtg mice. Since CD8+ T-lymphocytes have unequivocally been identified as mediators of neural damage in PLPtg mice,2 we focused our attention on the possible impact of two typical damage-related agents of cytotoxic T-cells, perforin and granzymes. Both are constituents of cytotoxic granules that become—on antigen recognition—concentrated and eventually released in the region of the immunological synapse.12,31 In this context, perforin plays a pivotal role by “trafficking” the granzymes. It either forms pores in the plasma membrane that help granzymes to enter the cytosol of the target cells or leads to release of granzymes from endosomes that have been formed during granzyme uptake by the target cells.12,31,32 In any case, the presence of perforin is essential for neural damage in our model as reflected by the robust beneficial effect in perforin-deficient myelin mutants. It is of note that this effect is more pronounced than the effect of Gzmb inactivation, particularly when parameters for demyelination are considered. It is plausible to assume that other perforin-dependent members of the granzyme family mediate myelin damage in PLPtg mutants.
We show the necessity of functional TCRs for neural damage, which is eventually mediated by the release and cytotoxicity of perforin and—to a lesser degree—Gzmb. Notably, perforin has recently been implicated in damage of larger caliber axons after infection with Theiler’s murine encephalomyelitis virus.33 These results were confirmed in a recent study, showing that the absence of perforin in Theiler’s virus-infected mice resulted in axonal protection independent of demyelination.34 Moreover, there is increasing evidence that, in multiple sclerosis, CD8+ T-lymphocytes11,35,36,37 and perforin/granzymes are involved in pathogenesis.38,39 These results provide emerging evidence for the functional implication of cytotoxic, granule-dependent neural damage in oligodendropathy-induced neural inflammation and may help to understand the pathogenesis of many inflammatory diseases of the nervous system including multiple sclerosis.
Acknowledgments
We thank Heinrich Blazyca, Silke Loserth, and Bettina Meyer for skillful technical assistance and Helga Brünner and Karl-Heinz Aulenbach for animal care. We are grateful to Thomas Hünig for providing OT-I transgenic and perforin-deficient mice and valuable comments on the manuscript. Valuable discussions with Heinz Wiendl are gratefully acknowledged.
Footnotes
Address reprint requests to Prof. Rudolf Martini, Department of Neurology, Section of Developmental Neurobiology, University of Wuerzburg, Josef-Schneider Str. 11, D-97080 Wuerzburg, Germany, E-mail: rudolf.martini@mail.uni-wuerzburg.de.
Supported by Gemeinnützige Hertie Foundation (1.01.1/07/12), Fritz-Thyssen Foundation (10.07.2.152), and DFG (SFB581, TP A3).
A.K. and C.W.I. contributed equally to this work.
Current address of A.K.: Center for Research in Neuroscience, Research Institute of the McGill University Health Center, Montreal, Quebec, Canada H3G 1A4.
References
- Ip CW, Kroner A, Fischer S, Berghoff M, Kobsar I, Mäurer M, Martini R. Role of immune cells in animal models for inherited peripheral neuropathies. Neuromol Med. 2006;8:175–189. doi: 10.1385/nmm:8:1-2:175. [DOI] [PubMed] [Google Scholar]
- Ip CW, Kroner A, Bendszus M, Leder C, Kobsar I, Fischer S, Wiendl H, Nave KA, Martini R. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J Neurosci. 2006;26:8206–8216. doi: 10.1523/JNEUROSCI.1921-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip CW, Kroner A, Crocker PR, Nave KA, Martini R. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol Dis. 2007;25:105–111. doi: 10.1016/j.nbd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Leder C, Schwab N, Ip CW, Kroner A, Nave KA, Dornmair K, Martini R, Wiendl H. Clonal expansions of pathogenic CD8+ effector cells in the CNS of myelin mutant mice. Mol Cell Neurosci. 2007;36:416–424. doi: 10.1016/j.mcn.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kassmann CM, Lappe-Siefke C, Baes M, Brugger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J, Nave KA. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- Kroner A, Schwab N, Ip CW, Sommer C, Wessig C, Wiendl H, Martini R. The co-inhibitory molecule PD-1 modulates disease severity in a model for an inherited, demyelinating neuropathy. Neurobiol Dis. 2009;33:96–103. doi: 10.1016/j.nbd.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–714. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Warshawsky I, Rudick RA, Staugaitis SM, Natowicz MR. Primary progressive multiple sclerosis as a phenotype of a PLP1 gene mutation. Ann Neurol. 2005;58:470–473. doi: 10.1002/ana.20601. [DOI] [PubMed] [Google Scholar]
- Gorman MP, Golomb MR, Walsh LE, Hobson GM, Garbern JY, Kinkel RP, Darras BT, Urion DK, Eksioglu YZ. Steroid-responsive neurologic relapses in a child with a proteolipid protein-1 mutation. Neurology. 2007;68:1305–1307. doi: 10.1212/01.wnl.0000259522.49388.53. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Keefe D, Durand E, Feng H, Zhang D, Lieberman J. Granzyme B binds to target cells mostly by charge and must be added at the same time as perforin to trigger apoptosis. J Immunol. 2005;174:5456–5461. doi: 10.4049/jimmunol.174.9.5456. [DOI] [PubMed] [Google Scholar]
- Readhead C, Schneider A, Griffiths I, Nave KA. Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron. 1994;12:583–595. doi: 10.1016/0896-6273(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Kobsar I, Berghoff M, Samsam M, Wessig C, Maurer M, Toyka KV, Martini R. Preserved myelin integrity and reduced axonopathy in connexin32-deficient mice lacking the recombination activating gene-1. Brain. 2003;126:804–813. doi: 10.1093/brain/awg072. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Mäurer M, Schmid CD, Bootz F, Zielasek J, Toyka KV, Oehen S, Martini R. Bone marrow transfer from wild-type mice reverts the beneficial effect of genetically mediated immune deficiency in myelin mutants. Mol Cell Neurosci. 2001;17:1094–1101. doi: 10.1006/mcne.2001.0990. [DOI] [PubMed] [Google Scholar]
- Kroner A, Schwab N, Ip CW, Leder C, Nave KA, Maurer M, Wiendl H, Martini R. PD-1 regulates neural damage in oligodendroglia-induced inflammation. PLoS ONE. 2009;4:e4405. doi: 10.1371/journal.pone.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TJ, Schneider A, Barrie BA, Klugmann M, McCulloch MC, Kirkham D, Kyriakides E, Nave K-A, Griffiths IR. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J Comp Neurol. 1998;394:506–519. doi: 10.1002/(sici)1096-9861(19980518)394:4<506::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Toben C, Na SY, Stark K, Nitschke L, Peterson A, Gold R, Schimpl A, Hunig T. Induction of experimental autoimmune encephalomyelitis in transgenic mice expressing ovalbumin in oligodendrocytes. Eur J Immunol. 2006;36:207–215. doi: 10.1002/eji.200535211. [DOI] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K, Goebels N, Weltzien HU, Wekerle H, Hohlfeld R. T-cell-mediated autoimmunity: novel techniques to characterize autoreactive T-cell receptors. Am J Pathol. 2003;163:1215–1226. doi: 10.1016/S0002-9440(10)63481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundtner R, Dornmair K, Dahm R, Flugel A, Kawakami N, Zeitelhofer M, Schoderboeck L, Nosov M, Selzer E, Willheim M, Kiebler M, Wekerle H, Lassmann H, Bradl M. Transition from enhanced T cell infiltration to inflammation in the myelin-degenerative central nervous system. Neurobiol Dis. 2007;28:261–275. doi: 10.1016/j.nbd.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA. 2004;101:2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na SY, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, Schimpl A, Hunig T. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- Berghoff M, Samsam M, Muller M, Kobsar I, Toyka KV, Kiefer R, Maurer M, Martini R. Neuroprotective effect of the immune system in a mouse model of severe dysmyelinating hereditary neuropathy: enhanced axonal degeneration following disruption of the RAG-1 gene. Mol Cell Neurosci. 2005;28:118–127. doi: 10.1016/j.mcn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells. B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Howe CL, Adelson JD, Rodriguez M. Absence of perforin expression confers axonal protection despite demyelination. Neurobiol Dis. 2007;25:354–359. doi: 10.1016/j.nbd.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb C, Lafrance-Corey RG, Zoecklein L, Papke L, Rodriguez M, Howe CL. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:1037–1048. doi: 10.1097/NEN.0b013e3181b5417e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Wiendl H. The role of CD8 suppressors versus destructors in autoimmune central nervous system inflammation. Hum Immunol. 2008;69:797–804. doi: 10.1016/j.humimm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Weiss HA, Millward JM, Owens T. CD8+ T cells in inflammatory demyelinating disease. J Neuroimmunol. 2007;191:79–85. doi: 10.1016/j.jneuroim.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Malmestrom C, Lycke J, Haghighi S, Andersen O, Carlsson L, Wadenvik H, Olsson B. Relapses in multiple sclerosis are associated with increased CD8+ T-cell mediated cytotoxicity in CSF. J Neuroimmunol. 2008;196:159–165. doi: 10.1016/j.jneuroim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Wang T, Allie R, Conant K, Haughey N, Turchan-Chelowo J, Hahn K, Rosen A, Steiner J, Keswani S, Jones M, Calabresi PA, Nath A. Granzyme B mediates neurotoxicity through a G-protein-coupled receptor. FASEB J. 2006;20:1209–1211. doi: 10.1096/fj.05-5022fje. [DOI] [PubMed] [Google Scholar]