Abstract
The mucosal addressin cell adhesion molecule (MAdCAM) and vascular cell adhesion molecule (VCAM) appear to play roles in the recruitment of leukocytes to specialized endothelium lining the gastrointestinal tract. The purpose of this study was to clarify the role of MAdCAM and VCAM in the central nervous system by comparing protein expression in patients with multiple sclerosis (MS) and control subjects by immunohistochemistry. Specific antibodies to human VCAM and MAdCAM were used to confirm expression in control and MS nervous system specimens by immunohistochemistry. VCAM immunoreactivity was detected in endothelial cells, perivascular tissue, and in some cases, leukocytes within the meninges, gray, and white matter, of both controls and MS patients. VCAM immunoreactivity was maximal in a patient with acute active plaques, but of lower intensity and reduced distribution in controls and those with chronic active or inactive MS plaques. In contrast, MAdCAM immunoreactivity could not be detected in brain tissue from unaffected or MS patients. Taken together, these data support a role of VCAM, but not MAdCAM in the development of MS.
In recent years, interest and emergence has increased tremendously surrounding therapeutic approaches that interfere with normal leukocyte trafficking as alternative mechanisms to conventional immunosuppressive agents for inflammatory diseases. These include approaches that block leukocyte homing (such as natalizumab [Tysabri; Biogen Idec and Elan Pharmaceuticals], or sphingosine-1 phosphate-mediated egress from lymphoid tissue (fingolimod [Novartis]), or deplete specific populations of leukocytes (such as rituximab [Rituxan; Genentech and Biogen Idec]). In multiple sclerosis (MS), classically described as a chronic inflammatory disease of the central nervous system (CNS), focal autoreactive T-cell and macrophage infiltrates lead to demyelination and axonal loss.1,2 Blood–brain barrier damage, prominent infiltration by activated CD4+ T cells and clonotypic CD8+ T cells, the presence of macrophages with phagocytosed myelin debris, reactive astrocytes and proliferating oligodendrocytes are characteristic of acute plaques. In chronic plaques, inflammation is less pronounced and generally restricted to the rim of the plaque, which exhibits gliosis, while the hypocellular center exhibits axonal and oligodendrocyte loss, and variable demyelination. In treatment of MS by natalizumab, the CNS restriction of CD4+, CD8+ T cells, CD19+ B lymphocytes, and CD138+ plasma cells by blocking α4β1 and α4β7 integrin mediated binding to endothelial cells expressing vascular cell adhesion molecule (VCAM), fibronectin, and MAdCAM has resulted in observations of improved outcome by magnetic resonance imaging, reductions in disease progression, and relapse in clinical studies. However, while the exaggerated recruitment of activated autoreactive leukocytes is one of the predisposing features that can lead to MS, CNS immune surveillance is also a critically important process. Natalizumab was temporarily withdrawn from the market in 2005 after three patients developed progressive multifocal leukoencephalopathy (PML)3,4 and more recently, multiple cases of PML in efalizumab (anti-CD11a) and rituximab (anti-CD20)-treated individuals have been observed,5,6 leading to the recent withdrawal of efalizumab by Genentech.
PML is a rare, rapidly progressive and often fatal form of demyelinating disease caused by a reactivation of latent polyomavirus JC within a setting of immunosuppression. JC virus does not cause disease in healthy individuals. It was first described in 1958, and up to the early 1980s, reports of PML showed that it mainly occurred in AIDS patients or elderly individuals as a terminal complication of lymphoproliferative disorders.7,8 Given that more than 70% of the adult population are carriers of the JC virus,9 the potential clinical implication of agents that either deplete immune cells or potentially interfere with the leukocyte trafficking in the CNS needs consideration.
PF-00547659 has been recently described as a potent and selective anti-human MAdCAM monoclonal antibody that blocks the ability of α4β7 integrin-bearing leukocytes to home to specialized endothelium.10 Several studies have concluded that MAdCAM expression appears to be restricted to the endothelium of the gastrointestinal tract;10,11 however, the observed expression in brain tissue under certain circumstances, the cloning of the receptor from CNS tissue,12,13,14 as well as the observed effects of blocking anti-β7 integrin or anti-MAdCAM antibodies in models of experimental autoimmune encephalomyelitis have suggested that MAdCAM may have an additional role in CNS immune surveillance in normal, as well as inflamed conditions.15,16,17
In the context of these observations, the purpose of this study was to characterize and compare the pattern as well as intensity of CNS MAdCAM expression with VCAM in control and MS brain tissue.
Materials and Methods
PF-00547659 and anti-keyhole limpet hemocyanin control monoclonal antibodies were directly biotinylated using EZ-Link Sulfo-NHS-LC-Biotin following the manufacturer’s protocol as described previously.10 An anti-human mouse monoclonal IgG1 VCAM antibody as well as mouse IgG1 control monoclonal antibody were purchased from Abcam (ab58838, ab27479 respectively). Frozen control (n = 4, 2 blocks per donor) and MS brain tissue (n = 10, 4 blocks per donor) were kindly provided by the UK MS Society Tissue Bank (http://www.ukmstissuebank.org.uk/). Patient information and pathology report data provided by the MS Society Tissue bank are summarized in Supplemental Table S1 at http://ajp.amjpathol.org.
Immunohistochemistry
Anti-MAdCAM and VCAM antibody validation was performed on acetone fixed cryosections (10 μm) of human ileum containing gut associated lymphoid tissue. Negative control tissue, and isotype (anti-keyhole limpet hemocyanin IgG2-bio) and method controls (omission of primary) were included to determine the extent of any nonspecific binding. Acetone-fixed cryosections (10 μm) from four separate brain regions from 10 MS sufferers and 2 brain regions from 4 control subjects were then assessed for MAdCAM and VCAM expression. The expression of MAdCAM was assessed using a direct immunoperoxidase procedure (Vector Laboratories) as previously described.10 VCAM expression was detected using an indirect immunoperoxidase method with a biotinylated horse anti-mouse secondary antibody 1:200 for 1 hour at room temperature before diaminobenzidine detection. Both methods included hydrogen peroxide, serum, and avidin/biotin blocking steps. After visualization the slides were washed with distilled water and counterstained with Meyer’s hemalum for 30 seconds before rinsing, dehydration, and mounting. Studies were also performed with additional commercial sources of anti-MAdCAM antibodies, clones 17F5 and 314G8,18 purchased from Abcam to confirm the observations acceded with PF-00547659, see Supplemental Table S2 at http://ajp.amjpathol.org.
Histopathological Assessment
Serial cryosections (10 μm) were stained with H&E, Luxol Fast Blue, MAdCAM or VCAM antibodies for immunohistochemistry. All sections and controls were evaluated. Slides were assessed for the following criteria: autolysis, slide quality, non-MS neuropathology and MS plaques, which were assessed for number, location, margin type, and lesion stage. Grading criteria and MS lesion characterization allowed MS plaques to be staged according to criteria laid out by Raine,19 the basic features of which are summarized in Supplemental Table S3 at http://ajp.amjpathol.org. Lesions were graded in a semiquantitative fashion using the following criteria: negative 0, minimal/slight-grade 1, mild-grade 2, moderate-grade 3, marked-grade 4, very marked-grade 5. MAdCAM and VCAM expression were graded in the following anatomical locations: gray matter, white matter, meninges, choroid plexus, and ependyma; and in the following tissues: endothelium, perivascular tissue (pericytes, smooth muscle, and fibrocytes), leukocytes, large arteries, small arteries, veins of any size, and capillaries. Distribution and intensity of MAdCAM and VCAM expression were assessed with respect to the proximity to, and distribution within, MS plaques.
Results
Immunohistochemical Characterization of VCAM and MAdCAM Expression in Human Ileum
The specificity of PF-00547659 and the VCAM antibody (ab58838) was confirmed by immunohistochemical assessment on human ileum (see Supplemental Figures S1 and S2 at http://ajp.amjpathol.org). Consistent with previously reported data,10,11 PF-00547659 produced marked to very marked membranous and cytoplasmic MAdCAM labeling of the vascular endothelium, perivascular tissue, and aggregates of leukocytes in the mucosa, submucosa, and Peyer’s patches. In contrast, PF-00547659 did not bind to the gut epithelium, and the isotype and method controls were negative. These observations are also supported by similar data obtained with the anti-human MAdCAM antibody clone 314G8,18 (see Supplemental Figure S3 at http://ajp.amjpathol.org). Ab58838 produced mild to moderate membranous and cytoplasmic VCAM labeling of vascular endothelial cells and aggregates of leukocytes within Peyer’s patches in human ileum, consistent with published data.20,21 In contrast, the gut epithelium, isotype, and method controls were all negative. Collectively the pattern of immunoreactivity observed with PF-00547659 and Ab58838 correlates closely with the expected expression profile for MAdCAM and VCAM respectively.
MAdCAM and VCAM Expression in Normal Brain and Non-Plaque Tissues from Patients with Multiple Sclerosis
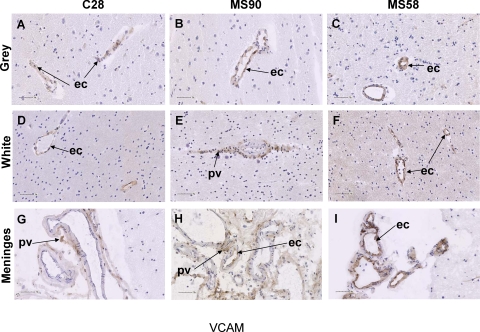
Case control human brain sections (n = 4, 2 sections per case) and multiple sclerosis patients (n = 10, 4 sections per case) were assessed for VCAM (Figure 1) and MAdCAM (Figure 2) expression using PF-00547659 and Ab58838 antibodies as described above as well as by quantitative real time-PCR (data summarized in Supplemental Figure S4 at http://ajp.amjpathol.org). MAdCAM expression in all brain tissues examined, including gray matter (Figure 2, A−C) and white matter (Figure 2D−F), meninges (Figure 2, G−I), and ependyma and choroid plexus (data summarized in Supplemental Table S2 at http://ajp.amjpathol.org), was negative as assessed with PF-00547659 and clone 314G8 (see Supplemental Figure S3 at http://ajp.amjpathol.org), consistent with previous observation.10 In contrast, VCAM immunoreactivity was observed in all cases, both MS and control (Figure 1). VCAM staining was specifically localized to vascular endothelial cells, perivascular tissue and in some cases leukocytes (1/4 controls, 2/10 MS patients) (see Supplemental Table S2 at http://ajp.amjpathol.org), within the gray matter (Figure 1, A−C), white matter (Figure 1, D−F), and meninges (Figure 1, G−I). The VCAM staining was membranous and cytoplasmic, finely stippled to diffuse in distribution and variable in intensity. VCAM expression on ependyma (present in 8/14 cases) and choroid plexus (present in 1/14 cases) was negative (summarized in Supplemental Table S2 at http://ajp.amjpathol.org). VCAM expression was generally similar in white and gray matter, except 1/4 controls and 1/10 MS cases where white matter staining was marginally increased (see Supplemental Table S2 at http://ajp.amjpathol.org). Meningeal staining was more prominent than in gray or white matter in 1/4 controls and 6/10 MS cases (see Supplemental Table S2 at http://ajp.amjpathol.org).
Figure 1.
Immunohistochemical staining of VCAM in non-plaque brain tissues in MS and control patients. VCAM labeling of non-plaque brain region vascular endothelium and perivascular tissue, in the gray matter (A, B, and C), white matter (D, E, and F) and meninges (G, H, and I) in a control brain (C28), and MS suffers who exhibited chronic active (MS90) and acute active (MS58) MS plaques. Arrows indicate ec- endothelial cell, or pv-perivascular tissue as noted. Scale bar = 50 μm.
Figure 2.
Immunohistochemical staining MAdCAM in non-plaque brain tissues in MS and control patients. MAdCAM labeling was absent from non-plaque brain regions including gray matter (A, B, and C), white matter (D, E, and F), and meninges (G, H, and I) in a control brain (C28), and MS suffers who exhibited chronic active (MS90) and acute active (MS58) MS plaques. Scale bar = 50 μm.
Immunohistochemical Characterization of VCAM and MAdCAM Expression in Plaque Regions from MS Patients
MS brain tissues from 10 different donors were assessed for VCAM and MAdCAM expression, intensity and colocalization with MS plaques relative to white matter expression in controls. MAdCAM was negative as assessed with PF-00547659 in all cases (Figure 3B, D, F). Maximal intensity VCAM immunoreactivity correlated with the presence of acute active disease (Figure 3A, patient MS58), in contrast MS patients with chronic active (Figure 3C, patient MS90) or inactive disease (see Supplemental Table S2 at http://ajp.amjpathol.org) and controls (Figure 3E, patient C16) showed a lower intensity (see Supplemental Table S2 at http://ajp.amjpathol.org). Vessels within MS plaques typically exhibited VCAM intensity equal or less than vessels in surrounding normal appearing white matter. VCAM distribution did not colocalize with MS plaques of any type, however the endothelium of vessels with perivascular leukocytic cuffs was typically positive (Figure 3A). However, the distribution and intensity of VCAM immunoreactivity were related, with grade 1–2 intensity typically being distributed multifocally in a lower percentage of vessels, whereas grade 2–4 immunoreactivity was more diffuse and present in a greater percentage of vessels (see Supplemental Table S2 at http://ajp.amjpathol.org), suggesting generalized vascular activation.
Figure 3.
Immunoreactivity for VCAM and MAdCAM in multiple sclerosis plaques and control white matter. Comparison of vessels in an acute active plaque (A, B) MS58, chronic active plaque (C, D) MS90, and control white matter (E, F) C16. MAdCAM immunoreactivity (B, D, F) was not observed in MS plaques of any type or control white matter. VCAM immunoreactivity (A, C, E) did not specifically colocalize with MS plaques, and intensity was similar to or less than surrounding normal appearing white matter. Arrows indicate ec- endothelial cell, or le-leukocyte as noted. Scale bar = 50 μm.
VCAM immunoreactivity did not correlate with patient age, sex, clinical MS status, concurrent neuropathology (degenerative conditions) or systemic pathology, which included non-inflammatory conditions like myocardial infarction, congestive heart failure, and cancer, as well as inflammatory conditions like pneumonia, and urinary tract infection. Likewise, the quality of sections, amount of autolysis, and postmortem interval did not affect the intensity of VCAM immunoreactivity (see Supplemental Table S2 at http://ajp.amjpathol.org).
VCAM and MAdCAM expression were also assessed by quantitative RT-PCR on purified RNA from the same tissue samples that were subjected to immunohistochemical detection. Both VCAM and MAdCAM mRNA transcripts were detected; the level of VCAM mRNA expression was four- to five-fold greater than MAdCAM and MAdCAM expression was low compared with glyceraldehyde-3-phosphate dehydrogenase (see Supplemental Figure S4 at http://ajp.amjpathol.org). The level of VCAM mRNA transcript was significantly elevated in MS tissue, as compared with control tissue. While demonstration of mRNA expression does not necessarily correlate or prove protein expression, the confirmation of CNS VCAM expression by immunohistochemical expression is consistent with this observation.
Discussion
The widely held view that CNS immune surveillance is principally mediated by interactions between α4β1-integrin bearing lymphocytes and VCAM-expressing endothelium is supported by several observations in preclinical models of experimental autoimmune encephalomyelitis;22,23,24,25 and the clinical efficacy demonstrated by natalizumab in MS patients.26 However, the introduction of immunosuppressive agents for inflammation that directly or indirectly impair leukocyte trafficking (such as a natalizumab, efalizumab, and rituximab), have also been associated with rare instances of fatal progressive multifocal leukoencephalopathy,4,5,6,26 suggesting that substantial impairment of normal CNS immune trafficking is undesirable, given the high proportion of individuals with latent JC virus infection.
We have recently described PF-00547659, a fully human blocking anti-MAdCAM monoclonal antibody,10 as a potentially new biotherapeutic in the treatment of inflammatory bowel disease and other diseases of the gastrointestinal tract where the exaggerated expression of MAdCAM drives the recruitment of activated lineages of α4β7+ leukocytes. The expression of MAdCAM in gut-associated lymphoid tissue (eg, Peyer’s patches and high endothelial venules) is widely reported,10,11 whether there is broader tissue expression and a functional role in leukocyte homing, such as in the CNS, is more contentious. MAdCAM mRNA message has been detected in brain tissue13 and pro-inflammatory cytokines can increase the expression of MAdCAM on brain-derived endothelial cells,12 but most studies have not observed MAdCAM protein expression in the CNS.10,11 In preclinical models, including experimental autoimmune encephalomyelitis, CNS MAdCAM expression has been observed14 or reported to be absent22,27,28 and the effects of blocking MAdCAM/α4β7 interactions on encephalomyelitis lesions have either been reported to be beneficial,15,16,17 or without effect22,23 and dependent on α4β1/VCAM interactions. In order, to address these contentions described in the preclinical literature and understand the potential role for MAdCAM in control and inflamed CNS tissue, we have characterized the protein expression of MAdCAM in human unaffected and MS brain tissue and compared this with that of VCAM.
In this study, while we able to detect presence of mRNA transcript in brain tissue, as reported by others,13 MAdCAM protein expression by immunohistochemistry was not detectable in meninges, white or gray matter, choroid plexus, and ependyma CNS samples taken from either unaffected or MS patients. In contrast, VCAM expression was detected in brain specimens from unaffected as well MS patients, and although VCAM expression did not co-localize with MS plaques, it was maximal in the presence of acute active MS lesions, with the caveat that only 1/10 samples from MS patients in this study had acute plaques. The distribution and intensity of VCAM immunoreactivity were related, with grade 1–2 intensity typically being distributed multifocally in a lower percentage of vessels, whereas grade 2–4 intensity was more diffuse and present in a greater percentage of vessels. The maximal intensity of VCAM protein expression observed in the patient with acute MS plaques was associated with a diffuse, as opposed to a multifocal, distribution, suggesting acute active disease is accompanied by generalized vascular activation. Supporting this theory, it has been noted that genes associated with anti-inflammatory functions are down-regulated in both MS lesions and normal appearing white matter from the same patient, consistent with widespread dysregulation of CNS inflammatory response.1
Constitutive expression of ICAM and VCAM, and de novo expression of MAdCAM has been previously reported on epithelial cells, but not the fenestrated capillaries of the choroid plexus, in a murine model of experimental autoimmune encephalomyelitis.14 In contrast to other observations29 we were not able to detect VCAM expression on choroid plexus and MAdCAM expression was additionally negative, but only 1/14 of the examined cases had choroids plexus that could be examined (case MS94).
Taken together, the data presented here underwrite previous observations that VCAM plays a central role in the recruitment and homing of leukocytes to the CNS under normal and inflammatory conditions. By comparison, MAdCAM protein does not appear to be expressed in the human brain under control or inflammatory conditions, arguing that MAdCAM does not play a role in the development of MS and control of CNS immune surveillance. The emergence of PML in individuals that experience chronic immune suppression either by immune depletion (eg, HIV infection, rituximab) or prevention of normal leukocyte homing (eg, efalizumab, natalizumab) has underscored the importance of CNS immune surveillance of latent JC virus infection. If, as a hypothesis, one of the potential consequences of VCAM blockade by natalizumab or CD11a ligation by efalizumab is to impair CNS immune surveillance and allow the re-emergence of JC virus infection, then the absence of observed MAdCAM expression in the CNS would suggest that blockade of MAdCAM-dependent processes would not adversely affect CNS immune surveillance and the risk of PML is greatly reduced.
Acknowledgments
We thank the UK MS Society Tissue Bank for providing the brain tissue specimens used in the immunohistochemistry studies performed in this manuscript.
Footnotes
Address reprint requests to Dr. Nick Pullen, Pfizer PGRD, Ramsgate Road, Sandwich, Kent, CT13 9NJ, UK. E-mail: nick.pullen@pfizer.com.
Supported by Pfizer Global Research & Development; R.A., S.N., M.A. and N.P. are employees of Pfizer Global Research & Development; PF-00547659 is currently in clinical development for inflammatory bowel disease.
R.A., S.N., M.A. and N.P. are employees of Pfizer Global Research & Development.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Khalili K, White MK, Lublin F, Ferrante P, Berger JR. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology. 2007;68:985–990. doi: 10.1212/01.wnl.0000257832.38943.2b. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- Freim Wahl SG, Folvik MR, Torp SH. Progressive multifocal leukoencephalopathy in a lymphoma patient with complete remission after treatment with cytostatics and rituximab: case report and review of the literature. Clin Neuropathol. 2007;26:68–73. doi: 10.5414/npp26068. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Watanabe T, Maruyama D, Kim SW, Kobayashi Y, Tobinai K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: case report and review of the literature. Int J Hematol. 2008;88:443–447. doi: 10.1007/s12185-008-0168-2. [DOI] [PubMed] [Google Scholar]
- Berger JR, Houff S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol Res. 2006;28:299–305. doi: 10.1179/016164106X98198. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984;2:299–313. [PubMed] [Google Scholar]
- Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26:1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Pullen N, Molloy E, Carter D, Syntin P, Clemo F, Finco-Kent D, Reagan W, Zhao S, Kawabata T, Sreckovic S. Pharmacological characterisation of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281–293. doi: 10.1111/j.1476-5381.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Pavlick KP, Laroux FS, Verma SK, Jordan P, Grisham MB, Williams L, Alexander JS. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol. 2001;281:C1096–C1105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- Leung E, Greene J, Ni J, Raymond LG, Lehnert K, Langley R, Krissansen GW. Cloning of the mucosal addressin MAdCAM-1 from human brain: identification of novel alternatively spliced transcripts. Immunol Cell Biol. 1996;74:490–496. doi: 10.1038/icb.1996.81. [DOI] [PubMed] [Google Scholar]
- Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1. VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- Kanwar JR, Harrison JE, Wang D, Leung E, Mueller W, Wagner N, Krissansen GW. Beta7 integrins contribute to demyelinating disease of the central nervous system. J Neuroimmunol. 2000;103:146–152. doi: 10.1016/s0165-5728(99)00245-3. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Kanwar RK, Krissansen GW. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain. 2004;127:1313–1331. doi: 10.1093/brain/awh156. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Kanwar RK, Wang D, Krissansen GW. Prevention of a chronic progressive form of experimental autoimmune encephalomyelitis by an antibody against mucosal addressin cell adhesion molecule-1, given early in the course of disease progression. Immunol Cell Biol. 2000;78:641–645. doi: 10.1046/j.1440-1711.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- Leung ELKB, Kanwar JR, Yang YMYMH, Patrick, Krissansen GW. Bioassay detects soluble MAdCAM-1 in body fluids. Immunol Cell Biol. 2004;82:400–409. doi: 10.1111/j.0818-9641.2004.01247.x. [DOI] [PubMed] [Google Scholar]
- Raine CS. Davis RL, Robertson DM, editors. London,: Williams and Wilkins,; Demyelinating diseases. 1985:pp. 468–547. [Google Scholar]
- Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992;103:840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Tsuzuki Y, Matsunaga H, Inoue T, Miyazaki J, Hokari R, Okada Y, Kawaguchi A, Nagao S, Itoh K, Matsumoto S, Miura S. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Conley FK, Kilshaw PJ, Butcher EC. Lymphocytes infiltrating the CNS during inflammation display a distinctive phenotype and bind to VCAM-1 but not to MAdCAM-1. Int Immunol. 1995;7:481–491. doi: 10.1093/intimm/7.3.481. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Roytta M, Salmi A, Salonen R. Therapy with antibody against leukocyte integrin VLA-4 (CD49d) is effective and safe in virus-facilitated experimental allergic encephalomyelitis. J Neuroimmunol. 1997;72:95–105. doi: 10.1016/s0165-5728(96)00158-0. [DOI] [PubMed] [Google Scholar]
- Theien BE, Vanderlugt CL, Eagar TN, Nickerson-Nutter C, Nazareno R, Kuchroo VK, Miller SD. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:995–1006. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- Wang X, Michie SA, Xu B, Suzuki Y. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J Interferon Cytokine Res. 2007;27:329–338. doi: 10.1089/jir.2006.0154. [DOI] [PubMed] [Google Scholar]
- Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199:133–141. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]