Abstract
Inadequate invasion of the uterus by cytotrophoblasts is speculated to result in pregnancy-induced disorders such as preeclampsia. However, the molecular mechanisms that govern appropriate invasion of cytotrophoblasts are unknown. Here, we demonstrate that under low-oxygen conditions (2.5% oxygen), 2-methoxyestradiol (2-ME), which is a metabolite of estradiol and is generated by catechol-o-methyltransferase (COMT), induces invasion of cytotrophoblasts into a naturally-derived, extracellular matrix. Neither low-oxygen conditions nor 2-ME alone induces the invasion of cytotrophoblasts in this system; however, low-oxygen conditions combined with 2-ME result in the appropriate invasion of cytotrophoblasts into the extracellular matrix. Cytotrophoblast invasion under these conditions is also associated with a decrease in the expression of hypoxia-inducible factor-1α (HIF-1α), transforming growth factor-β3 (TGF-β3), and tissue inhibitor of metalloproteinases-2 (TIMP-2). Pregnant COMT-deficient mice with hypoxic placentas and preeclampsia-like features demonstrate an up-regulation of HIF-1α, TGF-β3, and TIMP-2 when compared with wild-type mice; normal levels are restored on administration of 2-ME, which also results in the resolution of preeclampsia-like features in these mice. Indeed, placentas from patients with preeclampsia reveal lower levels of COMT and higher levels of HIF-1α, TGF-β3, and TIMP-2 when compared with those from normal pregnant women. We demonstrate that low-oxygen conditions of the placenta are a critical co-stimulator along with 2-ME for the proper invasion of cytotrophoblasts to facilitate appropriate vascular development and oxygenation during pregnancy.
Appropriate organization of the placenta involves the differentiation of cytotrophoblasts into multinuclear syncytiotrophoblast or extravillous trophoblasts (EVTs).1,2 EVTs play a crucial role in the establishment of utero-placental circulation. EVTs initiate invasion into the decidua/endometrium at 2 weeks after implantation (interstitial invasion),3 and this invasion by EVTs reaches the proximal third of uterine wall.4 In addition, EVTs invade the uterine vasculature (endovascular invasion) and make direct contact with maternal blood,5,6 a critical step for fetal viability.
The concentration of oxygen in the early placenta is ∼3% O2 (∼18 mmHg).7 This hypoxic environment of the first trimester is believed to facilitate trophoblast invasion.4,5,6,8,9,10,11,12,13,14,15 However, there is conflicting evidence regarding the effect of low-oxygen levels on the invasion of trophoblasts. Some authors have suggested that low-oxygen levels may also inhibit trophoblast invasion.16,17,18,19,20,21,22 In this report, we employ bioengineering, cell biology, and molecular genetics to demonstrate the effect of 2-methoxyestradiol (2-ME) on cytotrophoblast invasion in the placenta.
2-ME is a natural metabolite of estradiol and is generated via catechol-o-methyltransferase (COMT); 2-ME is suppressed in humans with preeclampsia.23 In fact, COMT-deficient mice exhibit placental hypoxia and a preeclampsia-like phenotype.23 2-ME suppresses abnormal levels of hypoxia within the placenta and prevents the emergence of a preeclamptic phenotype in COMT−/− mice.23 During pregnancy, the serum concentration of 2-ME increases from 2 to 15 nmol/L during the 11th to 16th week of gestation to a peak of roughly 18 to 96 nmol/L during the 37th to 40th week of gestation24; this increase during pregnancy demonstrates that 2-ME is naturally present within the placenta at relatively high levels. 2-ME inhibits hypoxia-inducible factor-1α (HIF-1α),23,25,26,27 a transcription factor that regulates the expression of hypoxia-responsive genes28 and has been shown to have an inhibitory effect on the trans-differentiation of trophoblasts via induction of transforming growth factor-β3 (TGF-β3).18 This evidence suggests that 2-ME may play a role in the regulation of hypoxia-induced events within cytotrophoblasts via inhibition of HIF-1α; however, a clear mechanism has not yet been established.
We, therefore, hypothesized that (1) low-oxygen levels are required for the invasion of cytotrophoblasts and (2) 2-ME counteracts the deleterious effects of HIF-1α-mediated gene expression and enables cytotrophoblasts to differentiate into an invasive phenotype to establish appropriate utero-placental circulation. Here, we used HTR-8 cells (human, cytotrophoblast cell line), a model system containing naturally-derived extracellular matrix, COMT−/− mice, and human placentas from patients with and without preeclampsia to determine the mechanistic significance of 2-ME on cytotrophoblast function during pregnancy.
Materials and Methods
Chamber for in Vitro Hypoxia Studies
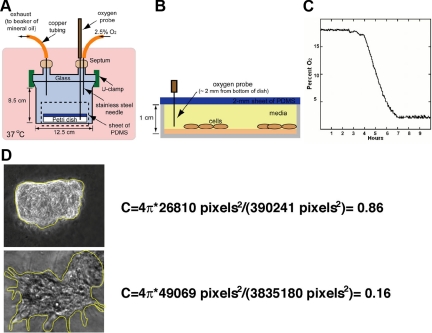
We created a glass chamber to minimize random diffusion of oxygen within the chamber (Figure 1A). The mixture of gas contains 5% CO2, 2.5% O2, and 92.5% N2. Cultured cells were placed into the glass chamber, which was set at 37°C in an incubator. With a ruthenium-based probe29,30,31,32,33 to measure the oxygen content within the media in the glass chamber (Figure 1B), we demonstrated a drop in oxygen content in the medium to ∼2.5% after ∼8 hours (Figure 1C). When the level of oxygen reached 2.5%, we began the experiments (t = 0 hours).
Figure 1.
Hypoxic exposure and imaging. A: Schematic of the controlled-atmosphere and glass chamber we used to culture cells in hypoxia. B: Enlarged view of the area enclosed by the dotted line in A. C: Concentration of oxygen within the media. We used an oxygen probe to track the concentration of oxygen and plotted the depletion as a function of time. The concentration of oxygen in the media in which trophoblasts were cultured decreased with time over 7 hours until it equilibrated with the mixture of gas that was injected into the chamber. D: Examples of the circularity ratio and the tracing by using Image J software are shown.
Culture of Cytotrophoblasts
We used the human trophoblast cell line, HTR-8, which was generated as previously described.34 The procedure for the culture of cytotrophoblasts on the extracellular matrix surrogate, Matrigel (MG) (BD Biosciences, San Jose, CA), was as follows: we (1) added 200 μl of the liquid form of MG (at 4°C) to each well of a six-well Petri dish (Falcon, BD, Franklin Lakes, NJ), (2) used a cell scraper (Falcon) to spread the MG uniformly across the bottom of the well, (3) placed the coated Petri dish in a 37°C incubator for 10 minutes to allow the layer of MG to solidify, (4) seeded ∼80,000 cells (630,000 cells/ml) onto the layer of MG, and (5) returned the dish to the incubator for 10 minutes to allow the cells to settle on the MG. After 10 minutes, we added 8 ml-aliquots of culturing medium, containing 0, 0.2, or 0.5 μmol/L of 2-ME (Sigma-Aldrich, St. Louis, MO). We then placed a 2 mm-thick sheet of polydimethylsiloxane (PDMS; Dow Corning, Midland, MI), which formed a conformal seal with the Petri dish (Figure 1B). PDMS is a highly gas-permeable polymer that allows an oxygen exchange from the media and prevents evaporation of the medium during the experiment. This sheet of PDMS was formed from curing a mixture of a prepolymer of PDMS and a curing agent at a ratio of 10:1 (w/w) in a large, flat plastic surface at 70°C for at least 8 hours. To sterilize the sheet of PDMS, we sprayed the sheet with 70% ethanol in water (v/v) and subsequently washed the sheet with sterile PBS. To track the concentration of oxygen (in the culturing media and in its gas phase), we punctured the septa on the lid of the chamber with an 18-gauge, ruthenium-based probe (Ocean Optics, Dunedin, FL), then punctured the sheet of PDMS, and brought the probe in contact with the culturing medium. We used the software provided with the probe to record oxygen measurements.
Western Blot Analysis
To harvest and lyse the cells after completion of an experiment, we removed the culturing medium from the MG-coated Petri-dish wells and immediately added 200 μl of cold lysis buffer to each well (50 mmol/L Tris pH 7.5, 0.15 M NaCl, 0.1% SDS, 1% Triton X-100, and 1% deoxycholate and protease inhibitor cocktail [Roche, Basel, Switzerland]). The samples were incubated for 5 minutes over ice and then scraped from the well. After measuring the protein concentration by bicinchoninic acid protein assay method (Thermo Fisher Scientific, Rockford, IL), we transferred 50 μg of protein lysate to Eppendorf test tubes and boiled it in the presence of 2X SDS sample buffer. After centrifugation at 17,000 g for 5 minutes at 4°C, we separated the supernatant on 8% or 12% SDS-polyacrylamide gels, and we blotted the gels onto polyvinylidene difluoride membranes (Immobilon, Bedford, MA) by using a semidry transfer method. The transferred protein on the polyvinylidene difluoride membrane was visualized with Coomassie Brilliant Blue. After blocking the membrane with Tris-buffered saline-T (0.05% Tween-20) containing 5% nonfat milk, we incubated the membranes with antibodies against HIF-1α (1:500 dilution; Novusbio, Littleton, CO), CD31 (1:500 dilution; Dako, Carpinteria, CA), E-cadherin (1:500 dilution; BD Biosciences, Franklin Lakes, NJ), or tissue inhibitor of metalloproteinases-2 (TIMP-2; 1:1000 dilution; Chemicon International, Temecula, CA) in Tris-buffered saline-T with 5% bovine serum albumin at 4°C overnight. The membranes were washed three times with Tris-buffered saline-T and incubated with horseradish peroxidase-conjugated, anti-rabbit secondary antibody (1:10,000 dilution; Promega, Madison, WI) or anti-mouse IgG-peroxidase antibody (1:1000; Sigma-Aldrich) at room temperature for 1 hour. The membranes were then rinsed, and immunoreactive bands were detected with an enhanced chemiluminescence detection system (Pierce Biotechnology, Rockford, IL). The equal amount of loading was confirmed with Coomassie brilliant blue staining.
RT-PCR
RNA was isolated from the trophoblast and 0.5 μg of total RNA was used for making complementary DNA. RT–PCR was performed with SuperScript II (Invitrogen, Carlsbad, CA) by using oligo-dT primer (Invitrogen) following the manufacturer’s instruction. After generating the complementary DNA, PCR was performed with specific primer for human TGF-β3 (forward primer, 5′-TTGCAAAGGGCTCTGGTGGTCCTGG-3′; reverse primer, 5′-ACTCGGTGTTTTCCTGGGTGCAGCC-3′). Conditions for the PCR were as follows: 95°C for 4 minutes; 95°C for 30 seconds, 61°C for 30 seconds, and 72°C for 30 seconds (50 cycles); 72°C for 7 minutes followed by 4°C. PCR product was confirmed by gel electrophoresis.
Imaging Clusters of Cells
An epifluorescent microscope (Leica DM IRB; Leica Microsystems GmbH, Wetzlar, Germany) with a charge-coupled camera (Hamamatsu ORCA-ER, Hamamatsu, Japan) served to image the cells. To determine the area and perimeter of the projection of the clusters of cells (for the circularity ratio), we uploaded images into Image J (NIH). We drew a line to surround the cluster of cells and used Image J to export the area and perimeter values of the shape enclosed by the line (Figure 1D).
Circularity Ratio
Shape is an intrinsic property of all objects; we quantified the shape of these clusters of trophoblasts in terms of the “circularity ratio,” as a means to differentiate between those clusters of cells that had long dendritic extensions and those that did not.35 The circularity ratio (C) of a particle (or cluster of cells) is defined as the ratio of the area of the projection of the particle onto the surface of a plane to that of a circle with the same perimeter (equation 1).35,36,37 A circle has a ratio of one; a square has a ratio of π/4, and a very long and narrow shape, or a nearly circular shape with a very convoluted periphery has a ratio of nearly zero.35,38
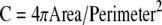 |
1 |
We examined the projection of the clusters in two dimensions and measured their circularity.39 Clusters of trophoblasts that remained compact and circular had values of C approaching 1; however, clusters of trophoblasts that did not remain compact and had dendrites extending from the cluster into the Matrigel had values of C near 0.2 (Figure 1D, Table 1). We repeated the experiments four independent times and measured a total of 10 clusters for circularity ratio assessment.
Table 1.
Circularity Ratios of the Clusters of Trophoblasts
| C = 4π (Area/Perimeter2) | 2-ME, μm | % O2 | Invasive phenotype |
|---|---|---|---|
| 0.88 ± 0.0096 | 0 | 2.5 | No |
| 0.96 ± 0.0048 | 0 | 17.0 | No |
| 0.16 ± 0.0072* | 0.2 | 2.5 | Yes |
| 0.95 ± 0.0081 | 0.2 | 17.0 | No |
| 0.15 ± 0.0070* | 0.5 | 2.5 | Yes |
| 0.89 ± 0.0141 | 0.5 | 17.0 | No |
A comparison of the circularity ratios of trophoblasts cultured in 2.5% O2 in the presence or absence of 2-ME suggests that the trophoblasts have adopted a morphology indicative of differentiation to an invasive phenotype. We repeated experiments four independent times and measured a total of 10 clusters for circularity ratio assessment. The results are shown as mean ± SE.
P < 0.01 compared with all 17% O2or no 2-ME groups.
Cell Migration Assay
Cell-migration assays were performed in a Boyden chamber as previously described.40 Polyvinyl-pyrrolidone-free polycarbonate membranes with 10-μm pores (Neuro Probes, Inc., Gaithersburg, MD) were coated with Matrigel. The bottom wells of a 12-well Boyden chamber were filled with RPMI1640 medium containing 10% fetal calf serum. Wells were covered with the coated membrane sheet and 2000 cells/well were added into the upper chamber. Twenty-four hours after the cells were passaged, the chamber was incubated with or without 2-ME under 2.5% or 17% O2 for 48 hours. Membranes were stained with Wright-Giemsa, and the number of cells migrating to the lower chamber was counted.
Animals for in Vivo Experiments
COMT−/− mice were developed by Karayiorgou and colleagues41 and were provided as a gift to our laboratory under an interinstitutional Material Transfer Agreement. Animal experiments were performed as previously described.23 Briefly, at 8 weeks of age, mating cages were set up (COMT+/+/COMT+/+ or COMT−/−/COMT−/−). The vaginal plug—confirmed mice were separated to other cages until sacrifice. From day 10 of pregnancy, mice were injected everyday with 10 ng of 2-ME (COMT−/− mice) or placebo (olive oil) subcutaneously. Mouse studies followed the Institutional Animal Care and Use Guidelines of the Beth Israel Deaconess Medical Center.
Human Placentas
Human placental samples were obtained from pregnant women at Brigham and Women’s Hospital (Boston, MA). All of the samples were collected via an approved institutional review board protocol. Two groups of women were studied: women with pregnancies complicated by severe preeclampsia (as per the definition of the American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics42; n = 3) and normal pregnancies as control (n = 3). All cases were nulliparous and unlabored. Preeclampsia was defined as pregnancy-induced hypertension (blood pressure ≥140/90 mmHg) and proteinuria (≥300 mg/24 hours) in women who were normotensive before pregnancy and had no other underlying clinical problems such as renal disease.
The placenta was retrieved from the operating room immediately on its removal from the uterus. It was placed fetal side up in a sterile pan that was taken for immediate processing. The amniotic membrane was pealed back from the fetal surface in an area one half of the way between the cord insertion and the farthest lateral edge. This was to exclude any contamination from maternal blood. The exposed surface was then tented with sterile forceps and a 1-cubic cm section of tissue was cut free in such a fashion so as not to penetrate through to the maternal surface and risk contamination. This portion was then placed in a 1-cubic cm cryovile and was snap frozen immediately in liquid nitrogen then stored at −80°C. Total processing time was 2 to 5 minutes between delivery and freezing.
Immunohistochemistry
Formalin fixed-deparaffinized (2 minutes in xylene, four times; 1 minute in 100% ethanol, twice; 30 seconds in 95% ethanol; 45 seconds in 70% ethanol; and 1 minute in distilled water) human or mouse placental/decidual sections were used for COMT, HIF-1α, TGF-β3, or TIMP-2 labeling. Immunohistochemistry was performed by using a Vectastain avidin-biotin complex kit (Vector Laboratories, Burlingame, CA) or a Vector M.O.M peroxidase kit (Vector Laboratories). The dilutions of primary antibody are COMT (1:200; Chemicon International), HIF-1α (1:100; Novusbio), TGF-β3 (1:200; Abcam, Cambridge, MA), and TIMP-2 (1:100; Chemicon International). In negative control, primary antibody was omitted and replaced by blocking solution. We also confirmed that nonspecific isotype IgG showed no staining. We used a scoring system to quantify the relative levels of immunoreactivity in human placentas as previously described.43 Intensity of immunostaining was scored on an arbitrary scale of 0 to 3 where 0 represents no or faint staining, 1 represents mild staining, 2 represents moderate staining, and 3 represents dark and strong staining. At least six high-power fields (×200) per each human placenta were scored. The scoring of the samples was performed by two separate observers blinded to the tissue identity.
Statistical Analysis
The data are expressed as mean ± SE. Statistical significance was determined by using the nonparametric Mann-Whitney test for comparison of data. Statistical significance was defined as P < 0.05. Graphpad Prism (La Jolla, CA) was used for statistical analysis.
Results
Trophoblast Migration and Clustering on the Extracellular Matrix (Matrigel)
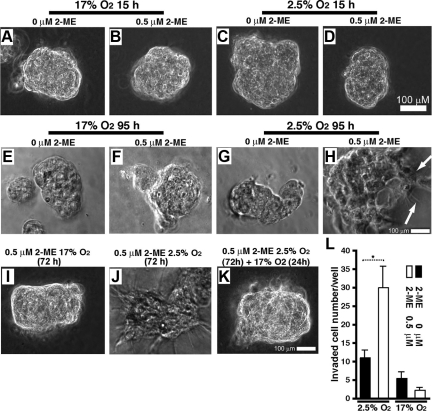
Cytotrophoblasts that were seeded on MG formed a monolayer on the surface of MG within 3 hours (data not shown). Within 24 hours of seeding the cells onto the MG, we observed that the cells had migrated along the surface of the MG, had found neighbors, and had clustered (Figure 2, A–D).
Figure 2.
Differentiation of trophoblasts to an invasive phenotype by 2-ME and hypoxia. Phase contrast images (A–K) of trophoblasts cultured on a layer of MG, in 17% O2 or 2.5% O2, and in the presence or absence of 2-ME, are shown. A–D: After 15 hours of culture, the trophoblasts adopted a spherical morphology where the cells have clustered. E and F: In 17% O2 for 95 hours, HTR-8 cells with or without 2-ME are shown. G and H: 2.5% O2 for 95 hours, trophoblasts with or without 2-ME are shown. In the presence of 2-ME under hypoxia, trophoblasts exhibit dendrites (highlighted by arrows in H) extended into the MG. We repeated experiments four independent times and measured a total of 10 clusters for circularity ratio assessment. I–K: Loss of invasive phenotype after returning trophoblasts to 17% O2. Trophoblasts cultured with 2-ME under 17% O2 (I) or 2.5% O2 (J) for 72 hours are shown. Similar to H, 72 hours incubation under 2.5% O2 with 2-ME showed extension of dendrites, but after an additional 24 hours of culture under 17% O2, the cells lost the invasive phenotype (K). L: The results of the Boyden chamber assay are shown. Incubation that is longer than 48 hours (72 hours), results in the death of most cells in this system. The results are shown as mean ± SE. n = 5 in each group. *P < 0.05.
Emergence of an Invasive Phenotype in 2-ME Treated Cytotrophoblasts under Low Oxygen Conditions
We evaluated the effect of 2-ME on HTR-8 cells under low-oxygen levels. At 3 hours, the cells remained in a monolayer (data not shown). After 15 hours of culture, the trophoblasts clustered and did not demonstrate a morphological difference between all groups (Figure 2, A–D). At 95 hours in the chamber, cells clustered in high-oxygen conditions (17% O2, 5% CO2, and 75% N2 in an incubator) and in low-oxygen levels (in the glass chamber) without 2-ME (Figure 2, E–G, Table 1). Unlike those cells cultured in high-oxygen conditions, trophoblasts cultured for 95 hours under low-oxygen levels in the presence of 2-ME switched to an invasive phenotype (Figure 2H, Table 1) that is illustrated by long dendrites extending into the MG. This change in morphology corresponded to a lower value of the circularity ratio (Table 1). Trophoblasts cultured in 17% oxygen, or without 2-ME in 2.5% oxygen, had larger values of C (ranging from ∼0.8 to 0.9); this range corresponded with rounder clusters of cells, without dendritic extensions penetrating the MG.
This invasive phenotype of trophoblast incubated in 2.5% oxygen with 2-ME was already observed at 72 hours after incubation in this system (Figure 2, I and J); however, this phenotype was reversible when cells were cultured for an additional 24 hours in 17% O2 conditions (Figure 2K), suggesting that both low-oxygen levels (2.5% O2) and 2-ME play critical roles in changing the phenotype of the trophoblast from non-invasive to invasive.
Cell Migration Under Low-Oxygen with 2-ME
To confirm the role of low-oxygen levels and 2-ME on the development of an invasive phenotype in HTR-8 cytotrophoblasts, we tested the migratory behavior of cells by using the Boyden chamber cell migration analysis. HTR-8 cells migrated into the lower chamber in the presence of 2-ME (0.5 μmol/L) under 2.5% oxygen (Figure 2L); however, such migration was significantly lower in the absence of 2-ME even under 2.5% oxygen. The 17% oxygen condition induced minimal migration of HTR-8 cells even in the presence of 2-ME (Figure 2L), suggesting that both low-oxygen levels (2.5% O2) and 2-ME play critical roles in the phenotypic change of trophoblast migration.
Endothelial Marker CD31 Expression Is Induced by 2-ME under Low-Oxygen
Endovascular invasion by trophoblasts is dependent on the expression of endothelial adhesion molecules, such as CD31.44 The cells under low-oxygen levels increased the expression levels of CD31 (135 kDa) either in the presence or absence of 2-ME (albeit at different levels) when compared with cells in 17% O2 (Figure 3, A and B). Other endothelial markers, such as Tie-2 and VE-cadherin, were not detected by the Western blot analysis (data not shown). Despite the invasive phenotype and expression of CD31 on cytotrophoblasts cultured under low-oxygen levels with 2-ME, the expression of a epithelial marker protein E-cadherin was unaltered by 2-ME under 2.5% oxygen in our analysis (Figure 3C); this result strongly suggests that the expression of endothelial marker CD31 is not prevented by a sustained expression of epithelium-associated cell surface proteins (Figure 3C).
Figure 3.
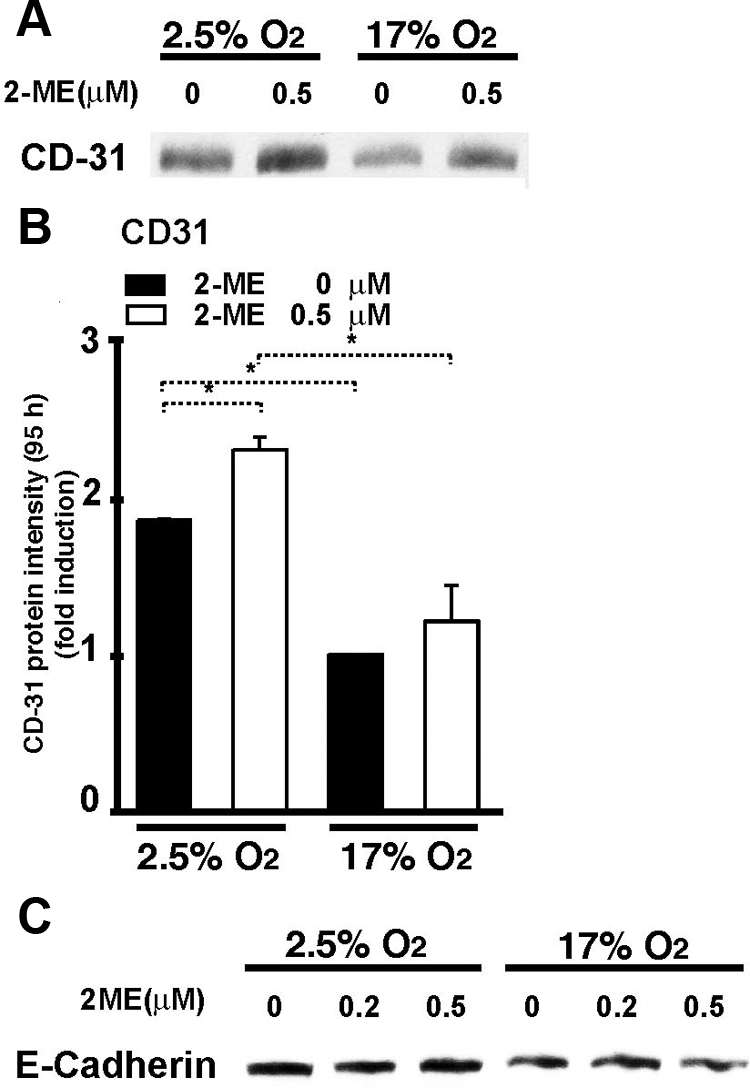
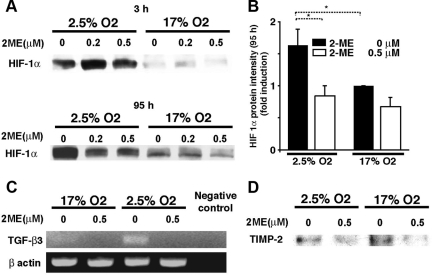
Endothelial cell-specific CD31 expression induced by 2-ME under hypoxic conditions. A: A representative western blot picture of CD31 (135 kDa) is shown. B: Densitometric analysis of a 135 kDa CD31 protein expression is shown. Results are shown as the relative expression against 17% O2 without 2-ME as in each set of western blots. Data are shown as the mean ± SE in the graph (n = 3). *P < 0.05. C: No alteration in the expression of the epithelial cell marker E-cadherin by the presence of 2-ME. Representative results from three independent experiments are shown.
Suppression of HIF-1α by 2-ME
HIF-1α was induced in trophoblasts that were cultured under 2.5% O2 conditions for 3 hours or 95 hours without 2-ME when compared with the cells in 17% O2 (Figure 4, A and B). In samples cultured in low-oxygen levels for 95 hours with 2-ME, the expression of HIF-1α was suppressed (Figure 4, A and B); however, the expression of HIF-1α at 3 hours remained unchanged even in the presence of 2-ME (Figure 4A).
Figure 4.
Suppression of HIF-1α, TGF-β3, and TIMP-2 by 2-ME under hypoxic condition. A: Trophoblasts cultured for 3 hours or 95 hours in 17% or 2.5% O2 were harvested, and HIF-1α levels were analyzed by using Western blot analysis. The representative results are shown from at least three independent experiments. B: Densitometric analysis of a HIF-1α protein expression at 95 hours is shown. Results are shown as the relative expression against 17% O2 without 2-ME as in each set of western blots. Data are shown as the mean ± SE in the graph (n = 3). *P < 0.05. C: Total RNA was extracted from HTR-8 cultures after 95 hours of incubation with or without 2-ME under 17% or 2.5% O2. Complementary cDNA was synthesized by using 0.5 μg of total RNA, and TGF-β3 expression levels were analyzed by PCR. The experiment was performed twice and showed the same results. D: Western blot analysis for TIMP-2 by using cultured trophoblast after 95 hours of incubation with or without 2-ME under 17% or 2.5% O2is shown. The representative results are shown in three independent experiments.
Suppression of TGF-β3 and TIMP-2 by 2-ME under Hypoxic Condition
HIF-1α-induced expression of TGF-β3 is reported to mediate the inhibition of EVT differentiation.16,18 To define whether 2-ME had an effect on the expression of TGF-β3, we evaluated the mRNA expression of TGF-β3 by using RT-PCR. Consistent with previous reports,18 TGF-β3 was induced in trophoblasts cultured under low-oxygen levels (Figure 4C), and 2-ME down-regulated mRNA expression of TGF-β3 (Figure 4C). Such suppression of TGF-β3 by 2-ME under hypoxic condition is associated with TIMP-2 suppression (Figure 4D), even though TIMP-2 is not increased under low-oxygen conditions (Figure 4D).
Down-Regulation of TGF-β3 and TIMP-2 by 2-ME in Placentas of COMT−/− Mice
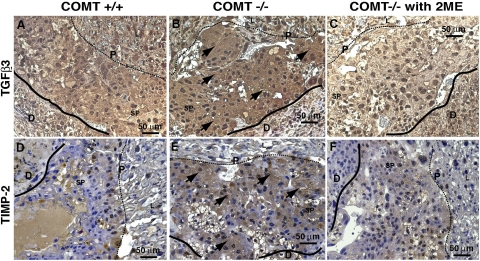
To correlate the in vitro data collected thus far with in vivo processes, the role of 2-ME was analyzed in the placentas of COMT−/− mice. In immunohistochemical analysis of COMT−/− placentas, expression levels of TGF-β3 significantly increased in the spongiotrophoblast layer (Figure 5,A and B), a region associated with decreasing levels of oxygen and HIF-1α accumulation.23 The TGF-β3 expression significantly diminished in COMT−/− mice receiving 2-ME (Figure 5C). An imbalance in the synchronized expression patterns of matrix metalloproteinases and TIMPs could lead to excessive cytotrophoblast invasion, such as that found in choriocarcinoma,45 or could lead to restricted invasion, such as that found in preeclampsia and fetal growth restriction.8 TIMPs have been reported to inhibit cytotrophoblast invasion.46 Immunohistochemical staining revealed an increased expression of TIMP-2 that was localized to the spongiotrophoblast layer of COMT−/− mice placentas (Figure 5, D and E). In 2-ME-treated COMT−/− mice, immunoreactivity and expression levels were restored to wild-type levels (Figure 5F).
Figure 5.
Down-regulation of TGF-β3 and TIMP-2 by 2-ME in placentas of COMT−/− mice. A–C: The immunohistochemical labeling of TGF-β3 in placenta/decidua from COMT+/+ (A), COMT−/− (B), and 2-ME-administered COMT−/− mice (C) is shown. D–F: The immunohistochemical labeling of TIMP-2 in placenta/decidua from COMT+/+ (D), COMT−/− (E), and 2-ME-administered COMT−/− mice (F) is shown. Formalin-fixed paraffin embedded sections were used in the analysis. Brownish staining represents positive immunostaining (arrows in B and E). The solid lines indicate the border between placenta and decidua. The dotted lines indicate the border between the spongiotrophoblast layer and the labyrinth layer of placenta (P, placenta; D, decidua; SP, spongiotrophoblast layer; L, labyrinth layer). Wild-type mice are designated as COMT+/+ mice in the figure. The representative results are shown from three independent experiments.
Down-Regulation of COMT and Up-Regulation of HIF-1α, TGF-β3, and TIMP-2 in Placentas of Preeclampsia Patients
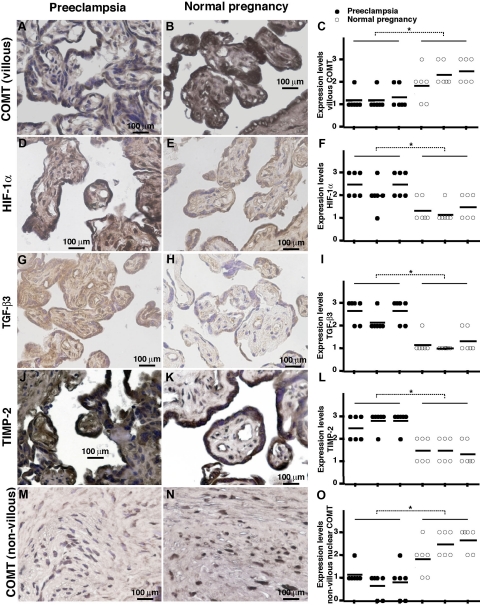
We also examined the immunoreactivity and spatial localization for COMT, HIF-1α, TGF-β3, and TIMP-2 in human placental samples (Figure 6). High levels of COMT activity were observed in placental and decidua vera samples obtained from pregnant women at term.47,48,49 Placentas from normal pregnancies showed strong COMT immunoreactivity in the villous syncytiotrophoblast layer and also in mesencymal cells, but relatively low immunoreactivity was present in placentas from preeclampsia patients (Figure 6, A–C). Consistent with in vitro data and mice experiments, positive immunoreactivity for HIF-1α, TGF-β3, and TIMP-2 was observed in the placentas from preeclampsia patients, in comparison with the much lower levels of expression in the placentas of women with normal pregnancy (Figure 6, D–L). With regards to spatial localization, HIF-1α immunostaining was localized to the syncytiotrophoblast, to the vascular endothelium, and, to a lesser extent, to the mesenchymal cells (Figure 6, D and E). TGF-β3 (Figure 6, G and H) and TIMP-2 (Figure 6, J and K) were mainly localized to syncytiotrophoblast and to a lesser degree to the mesenchymal cells. In non-villous placenta, COMT also exhibited strong expression and intranuclear accumulation (Figure 6, M and N). Such intranuclear accumulation is suppressed in preeclamptic placenta (Figure 6, M–O).
Figure 6.
Down-regulation of COMT is associated with up-regulation of HIF-1α, TGF-β3, and TIMP-2 in placentas of preeclampsia patients. Immunohistochemical analysis of villous COMT (A and B), HIF −1α (D and E), TGF-β3 (G and H), TIMP-2 (J and K), and nonvillous COMT (M and N) in placentas from patients with preeclampsia and normal pregnancy is shown. Brownish staining represents positive immunoreactivity. Each sample was analyzed by immunostaining intensity in six different areas (indicated as dot), scores were averaged, and statistical analyses were performed (C, F, I, L and O). Three normotensive control placentas and three severe cases of preeclamptic placentas were analyzed. Formalin-fixed paraffin-embedded sections are used in the analysis. *P < 0.05.
Discussion
In this report, we demonstrated that 2-ME enables cytotrophoblasts to differentiate into an invasive endovascular phenotype under low-oxygen conditions. At 8 to 10 weeks of gestation, the placenta is hypoxic (∼3% O2, ∼15 to 20 mmHg); the low level of oxygen results from the endovascular trophoblast plugs, which obstruct blood flow from the spiral arteries and prevent the influx of oxygenated maternal blood.50 The role of low-oxygen levels in trophoblast trans-differentiation and invasion is still poorly understood. We demonstrated that the combination of low-oxygen levels and 2-ME are vital initiators of cytotrophoblast invasion and trans-differentiation.
The process of EVT invasion of the maternal uterus is critical for the establishment of utero-placental circulation. Uterine invasion by EVTs occurs via two pathways: (1) an endovascular pathway, where EVTs migrate to the lumen of the uterine spiral arteries, and (2) an interstitial pathway, which requires the attachment of cytotrophoblasts to the maternal decidua, the proteolytic degradation of extracellular matrix, and finally the migration of EVTs between decidual and myometrial cells.5,6 EVTs that have invaded the maternal decidua express an endothelial cell surface marker (CD31) and lose epithelial markers such as E-cadherin.44,51 In our analysis, despite the CD31 expression, the loss of E-cadherin was not observed in the invaded trophoblasts under low-oxygen levels with 2-ME. It is possible that our results reveal a “transitional phase” of cytotrophoblast conversion into an endovascular phenotype, still preserving the epithelial marker, E-cadherin, at this stage. In this regard, Floridon et al52 have described that functional E-cadherin expression is restored in endovascular- and perivascular-invaded cytotrophoblasts, supporting our observation of E-cadherin expression in the trophoblasts with an invasive phenotype.
The transcription factor HIF-1α is responsible for the induction of genes that facilitate the adaptation and survival of cells during low-oxygen levels.28,53 It has been reported that in the first-trimester cytotrophoblasts, the expression of HIF-1α parallels that of TGF-β3, which is an inhibitor of early cytotrophoblast differentiation16,18 and likely impairs the ability of cytotrophoblasts to adopt an invasive phenotype. Also, it has been found that antisense inhibition of HIF-1α down-regulates TGF-β3 mRNA expression in villous explants.18 Consistent with these findings, we observe that 2-ME, an inhibitor of HIF-1α, down-regulates TGF-β3 mRNA expression in cytotrophoblasts cultured under low-oxygen levels. TGF-β3 expression is also associated with TIMP-2 expression in mice.54 TGF-β3 and TIMP-2 are induced in the spongiotrophoblast layer of placentas in the COMT−/− mice. Spogiotrophoblast-associated glycogen cells, which can invade the decidua, exhibit similar features as the human cytotrophoblasts55 and have been proposed to contribute to the establishment of maternal-placental circulation. Although the role of these spongiotrophoblast-associated glycogen cells are not clear, overproduction of TGF-β3 and TIMP-2 in the placenta of COMT−/− mice may result in poor establishment of utero-placental circulation, resulting in decreasing levels of oxygen in the placenta and emergence of preeclampsia-like phenotype in these mice.23
Invasion of the placenta by EVTs is believed to occur primarily in the first trimester.4 However, throughout pregnancy the placenta maintains a population of undifferentiated mononuclear cytotrophoblasts within villous tissue.56 Even at term, these villous cytotrophoblasts can be isolated as a relatively pure population of undifferentiated cells.56 Moreover, EVTs retain the capacity for sustained invasion of the uterus even in the third trimester of pregnancy.57 We have shown that 2-ME administration in COMT−/− mice in the latter half of pregnancy suppresses a preeclampsia-like phenotype.23 Collectively, these results suggest that low-oxygen levels/2-ME maintain placental homeostasis throughout the gestational period via appropriate regulation of cytotrophoblast differentiation and function.
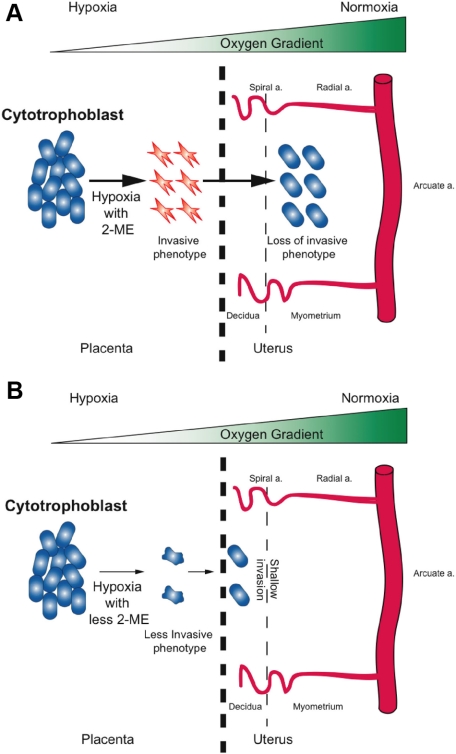
EVT invasion is strictly regulated and does not go past the proximal third of uterine wall4; however, the underlying mechanism for this organized and tempered migration is not well understood. Our results reveal that neither low-oxygen levels nor 2-ME alone stimulated invasion of HTR-8 cytotrophoblast cells in our model system. Our experiments also demonstrated that the invasive phenotype of cytotrophoblasts (induced by 2.5% O2 and 2-ME), were reversible when the chamber conditions were returned to high-oxygen concentrations (∼17% O2). We hypothesize that under hypoxia and adequate concentrations of 2-ME, cytotrophoblasts invade the maternal decidua and uterus (Figure 7A). Further invasion into the myometrium (proximal third of the uterine wall) is then regulated by the gradient of oxygen concentration between the placenta, deciduas, and uterine wall.1,6,17 As cytotrophoblasts invade the uterine wall and encounter higher levels of oxygen, the cells lose their invasive phenotype (Figure 7A) and their invasion is restricted. In cases where there is both an inadequate concentration of 2-ME and placental hypoxia, as speculated in preeclampsia, cytotrophoblasts still retain a minimal capacity to invade; however, this compromised capacity results in shallow invasion by trophoblasts—a phenotype observed in preeclamptic placentas (Figure 7B).
Figure 7.
Schematic illustration of extravillous cytotrophoblast invasion into the uterus. A: With sufficient 2-ME, cytotrophoblast with an invasive phenotype can invade up to the proximal third of the myometrium. B: With less 2-ME, the cytotrophoblasts are less invasive; therefore, shallow invasion of cytotrophoblasts develops.
In conclusion, we demonstrated unique synergistic roles of hypoxia and 2-ME in the regulation of appropriate and organized cytotrophoblast invasion. Our findings shed further light on the complex interplay between oxygen physiology and the endothelium-like function of trophoblast in placental homeostasis.
Footnotes
Address reprint requests to Raghu Kalluri, M.D., Ph.D., Division of Matrix Biology, CLS 11086, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Ave, Boston, MA 02215. E-mail: rkalluri@bidmc.harvard.edu.
Supported by Cynthus Biosciences. Supported in part by NIH grant DK 55001 and research funds to the Division of Matrix Biology from the Department of Medicine at the Beth Israel Deaconess Medical Center. S.B.L. is funded by Pusan National University and Pusan National University Hospital, Busan, Korea.
S.B.L., A.P.W., and K.K. contributed equally to this work.
References
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Strickland S, Richards WG. Invasion of the trophoblasts. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Gauster M, Orendi K, Konig J, Moser G. Oxygen as modulator of trophoblast invasion. J Anat. 2009;215:14–20. doi: 10.1111/j.1469-7580.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof P, Irminger-Finger I. The human cytotrophoblastic cell, a mononuclear chameleon. Int J Biochem Cell Biol. 2005;37:1–16. doi: 10.1016/j.biocel.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;5:6. doi: 10.1186/1477-7827-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46:266–273. doi: 10.1111/j.1479-828X.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64:126–130. doi: 10.1159/000101734. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chiu K, Brescia RJ, Combs CA, Katz MA, Kitzmiller JL, Heilbron DC, Fisher SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- Graham CH, Postovit LM, Park H, Canning MT, Fitzpatrick TE. Adriana and Luisa Castellucci award lecture 1999: role of oxygen in the regulation of trophoblast gene expression and invasion. Placenta. 2000;21:443–450. doi: 10.1053/plac.2000.0543. [DOI] [PubMed] [Google Scholar]
- Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Tahara M, Yamamoto T, Minekawa R, Isobe A, Tasaka K, Murata Y. Hypoxia up-regulates hypoxia-inducible factor-1alpha expression through RhoA activation in trophoblast cells. J Clin Endocrinol Metab. 2005;90:1712–1719. doi: 10.1210/jc.2004-1547. [DOI] [PubMed] [Google Scholar]
- Robins JC, Heizer A, Hardiman A, Hubert M, Handwerger S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta. 2007;28:1141–1146. doi: 10.1016/j.placenta.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Crocker IP, Wareing M, Ferris GR, Jones CJ, Cartwright JE, Baker PN, Aplin JD. The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J Pathol. 2005;206:476–485. doi: 10.1002/path.1801. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester villous explant model. Hum Reprod. 2006;21:2699–2705. doi: 10.1093/humrep/del212. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Low oxygen concentrations inhibit trophoblast cell invasion from early gestation placental explants via alterations in levels of the urokinase plasminogen activator system. Biol Reprod. 2006;74:403–409. doi: 10.1095/biolreprod.105.047332. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh) 1983;103:282–288. doi: 10.1530/acta.0.1030282. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–8673. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- Becker CM, Rohwer N, Funakoshi T, Cramer T, Bernhardt W, Birsner A, Folkman J, D'Amato RJ. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172:534–544. doi: 10.2353/ajpath.2008.061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Jorge PAS, Caldas P, Da Silva J, Rosa CC, Oliva AG, Santos JL, Farahi F. Luminescence-based optical fiber chemical sensors. Fiber and Integrated Optics. 2005;24:201–225. [Google Scholar]
- Jorge PAS, Caldas P, Rosa CC, Oliva AG, Santos JL. Optical fiber probes for fluorescence based oxygen sensing. Sensors and Actuators B-Chemical. 2004;103:290–299. [Google Scholar]
- Choi MMF, Xiao D. Single standard calibration for an optical oxygen sensor based on luminescence quenching of a ruthenium complex. Analytica Chimica Acta. 2000;403:57–65. [Google Scholar]
- Li XM, Ruan FC, Wong KY. Optical characteristics of a ruthenium(Ii) complex immobilized in a silicone-rubber film for oxygen measurement. Analyst. 1993;118:289–292. [Google Scholar]
- Kaneko M, Iwahata S, Asakura T. Quenching of photoexcited 4,4′-dicarboxy-2,2′-bipyridinebis(2,2′-bipyridine) ruthenium(Ii) by oxygen in aqueous-solution and in silk fibroin membrane. Photochemistry and Photobiology. 1992;55:505–509. [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Blott SJ, Pye K. Particle shape: a review and new methods of characterization and classification. Sedimentology. 2008;55:31–63. [Google Scholar]
- Wadell H. Volume, shape and roundness of quartz particles. Journal of Geology. 1935;43:250–280. [Google Scholar]
- Soll DR, Voss E, Varnumfinney B, Wessels D. Dynamic morphology system - a method for quantitating changes in shape, pseudopod formation, and motion in normal and mutant amebas of Dictyostelium-Discoideum. Journal of Cellular Biochemistry. 1988;37:177–192. doi: 10.1002/jcb.240370205. [DOI] [PubMed] [Google Scholar]
- Wadell H. Volume, shape, and roundness of rock particles. J Geol. 1932;40:443–451. [Google Scholar]
- Riley N. Projection sphericity. Journal of Sed Petrol. 1941;11:94–97. [Google Scholar]
- Zeisberg M, Maeshima Y, Mosterman B, Kalluri R. Renal fibrosis: extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. Am J Pathol. 2002;160:2001–2008. doi: 10.1016/S0002-9440(10)61150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics ACOG practice bulletin: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- Lyall F, Simpson H, Bulmer JN, Barber A, Robson SC. Transforming growth factor-beta expression in human placenta and placental bed in third trimester normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;159:1827–1838. doi: 10.1016/s0002-9440(10)63029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Niu R, Yamada S, Osawa M. Reduced expression of tissue inhibitor of metalloproteinase (TIMP)-2 in gestational trophoblastic diseases. Mol Hum Reprod. 2002;8:392–398. doi: 10.1093/molehr/8.4.392. [DOI] [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén O, Saarikoski S. The simultaneous function of catechol-O-methyltransferase and monoamine oxidase in human placenta. Acta Obstet Gynecol Scand. 1974;53:41–47. doi: 10.3109/00016347409156887. [DOI] [PubMed] [Google Scholar]
- Casey ML, MacDonald PC. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am J Obstet Gynecol. 1983;145:453–457. doi: 10.1016/0002-9378(83)90316-2. [DOI] [PubMed] [Google Scholar]
- Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-o-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5:121–127. doi: 10.1055/s-2007-999669. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta. 2006;27:468–474. doi: 10.1016/j.placenta.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Localization of E-cadherin in villous, extravillous and vascular trophoblasts during intrauterine, ectopic and molar pregnancy. Mol Hum Reprod. 2000;6:943–950. doi: 10.1093/molehr/6.10.943. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, Kaartinen V. TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell. 2001;12:1457–1466. doi: 10.1091/mbc.12.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Kliman H, Nestler J, Sermasi E, Sanger J, Strauss Jr. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am J Obstet Gynecol. 1994;171:832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]