Abstract
Each extracellular matrix compartment in the kidney has a unique composition, with regional specificity in the expression of various laminin isoforms. Although null mutations in the majority of laminin chains lead to specific developmental abnormalities in the kidney, Lama4−/− mice have progressive glomerular and tubulointerstitial fibrosis. These mice have a significant increase in expression of platelet-derived growth factor (PDGF)-BB, PDGF-DD, and PDGF receptor β in association with immature glomerular and peritubular capillaries. In addition, mesangial cell exposure to α4-containing laminins, but not other isoforms, results in down-regulation of PDGF receptor mRNA and protein, suggesting a direct effect of LN411/LN421 on vessel maturation. Given the known role of overexpression of PDGF-BB and PDGF-DD on glomerular and tubulointerstitial fibrosis, these data suggest that failure of laminin α4-mediated down-regulation of PDGF activity contributes to the progressive renal lesions in this animal model. Given the recent demonstration that individuals with laminin α4 mutations develop cardiomyopathy, these findings may be relevant to kidney disease in humans.
Laminin (LN) is a large, heterotrimeric, cruciform molecule composed of α, β, and γ subunits.1 Five distinct α (LAMA1-5), 3 β (LAMB1-3), and 3 γ (LAMC1-3) chains1,2 variably assemble to create distinct isoforms3 that are temporally and spatially regulated, and each conveys a variety of biological functions.4,5,6,7,8,9,10,11 The LNα4-containing isoforms, LN411 (α4β1γ1) and LN421 (α4β2γ1), are abundant in microvessels. Studies of LNα4-deficient mutant mice (lama4−/−) reveal that although α4-LNs are not required for blood vessel formation, they play important roles in blood vessel maturation, and in stabilization of vessels that form with injury, inflammation and tumor growth.7,12,13 In vitro studies indicate that α4LNs directly regulate endothelial cell proliferation and inhibit apoptosis.14 α4-LNs are produced by endothelial cells in most microvessels; however, endothelial cells in the renal glomerulus do not express LNα4-containing isoforms.15 Instead, LN411 and LN421 at the endothelial-mesangial interface are produced by the mesangial cells (MCs).15 Platelet-derived growth factor (PDGF) is the primary growth factor responsible for MC proliferation and migration during glomerulogenesis,16 and we have shown that PDGF-induced MC migration requires LNα4.15 This function could not be replaced by LN111 or LN511/521.15 Together these observations suggested the possibility that deficiency of LNα4 might impair the ability of the kidney microvasculature to mature or be repaired in lama4−/− adult mice, resulting in kidney disease despite normal initial development.
Previous reports have documented a spectrum of developmental defects and tissue maintenance defects in lama4−/− mice. Early postal-natal hemorrhage from birth-related trauma to fragile blood vessels occurs in lama4−/− mice; yet, by three-weeks of age, accumulation of LNα5 stabilizes vessels, although they remain dilated.12 Vessel fragility recurs when new vessels form in response to injury.12 The heart forms normally, but lama4−/− mice develop cardiomyopathy with time.17 Neurological dysfunction occurs in lama4−/− mice, through independent defects in organizing presynaptic specializations at neuromuscular synapses,18 and in the ability of developing Schwann cells to properly sort and myelinate.19,20 This report details the characteristics of kidney abnormalities, including the development of glomerulosclerosis and tubulointerstitial fibrosis over time in lama4−/− mice.
Materials and Methods
Antibodies
Polyclonal rabbit antibodies to fibronectin, thrombospondin, EHS LN, LNα2, LNα3, and LNα4 were developed and characterized as described previously.15,21,22 Other antibodies used are listed in Table 1. Alexa Fluor secondary antibodies to mouse and rabbit IgG were purchased from Molecular Probes, Eugene OR. HRP-conjugated secondary antibodies to rabbit IgG were purchased from Pierce, Rockford, IL.
Table 1.
Antibodies
| Antibody | Type | Antigen | Source |
|---|---|---|---|
| LN α1 | mRat | LN111 | Dr. D. Abrahamson, University of Kansas |
| LN α2 | pRabbit | LAMA2 peptide | Dr. C. Abrass, University of Washington |
| LN α3 (5C5) | mMouse | Rat 804G cells | Dr. J. Jones, Northwestern University |
| LN α4 | pGoat | Mouse LAMA4, AA826-1816 | R & D Systems Inc. (Minneapolis, MN) |
| LN α5 (H160) | pRabbit | LAMA5 peptide | Santa Cruz Biotechnology (Santa Cruz, CA) |
| LN β1 (5A2) | mRat | LN111 | Dr. D. Abrahamson, University of Kansas |
| LN β2 (H300) | pRabbit | LAMB2 peptide | Santa Cruz Biotechnology |
| LN111 | pRabbit | EHS LN111 | Dr. C. Abrass, University of Washington |
| Collagen IV (α3) | mMouse | Human | SciMedix (Denville, NY) |
| WT-1 | mMouse | Human | Santa Cruz Biotechnology |
| vWF | pRabbit | Human | Sigma-Aldrich (St. Louis, MO) |
| αSMA | mMouse | Human | Sigma-Aldrich |
| NG2 | mMouse | Rat | Sigma-Aldrich |
| PDGF-BB | pRabbit | Human AA101-116 | Calbiochem (La Jolla, CA) |
| PDGF-Rβ | pRabbit | Human | Upstate, Biotechnology (Lake Placid, NY) |
| PDGF-C | pRabbit | Human | Zymogenetics 3640 (Seattle, WA) |
| PDGF-D | pRabbit | Human | Zymogenetics 3812 |
| VEGF | pRabbit | Human | Santa Cruz Biotechnology |
| Angiopoietin 2 | pGoat | Human | Sigma-Aldrich |
| Activated caspase-3 | pRabbit | Human | Cell Signaling (Danvers, MA) |
| GAPDH | pRabbit | Human | Abcam (Cambridge, MA) |
Lama4−/− Mouse
Animal use was per the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of both the Oregon Health and Sciences University and the VA Puget Sound Health Care System. C57BL/6J mice were purchased from Jackson Laboratories. Lama4 null mice originally obtained from Dr. Karl Tryggvason12 were back-crossed for five generations to C57BL/6J mice.18,19 Genotypes were confirmed by polymerase chain reaction (PCR) of tail-snip DNA, using sense and anti-sense primers.19 Animals examined included male and female mice, aged 7 weeks to 23 months (wild type, n = 8; lama4−/−, n = 12).
Renal Function
Renal function studies were performed on serum and urine samples from 10 wild-type and 10 lama4−/− mice ages 2–11 months. Urine albumin was measured by enzyme-linked immunosorbent assay using the Albuwell M kit (Exocell Inc., Philadelphia, PA) per the manufacturer’s instructions. Serum and urine creatinines were determined using the QuantiChrom creatinine assay kit (DKT-500, BioAssay Systems, Hayward, CA). Albumin excretion was expressed as μg albumin/mg creatinine. No differences were observed with either group by age; thus, animals were grouped for statistical comparison.
Histology
Kidneys were processed for light, electron and fluorescence microscopy by routine methods.23 For immunohistochemistry, anti-rabbit ImmPRESS reagent (Vector Laboratories, Burlingame, CA) was used followed by 3,3′-diaminobenzidine and counter staining with hematoxylin performed by the Seattle Mouse Metabolic Phenotyping Center. For fluorescence microscopy, slides were viewed using a Zeiss microscope equipped for epi-illumination. Slides were photographed using a digital RT-color Spot camera or a Zeiss AxioCam MRM black and white camera. Glomerular diameter was measured using the software contained in Zeiss Axiovision4.5.
Cell Culture
Cloned rat glomerular MCs (passages 8–12) were cultured without supplemental insulin by modification of routine methods.24,25,26 To assess the role of extracellular matrix composition on PDGF-Rβ expression, MCs were cultured on plates that had previously been coated with LN-111 (isolated from EHS-sarcoma cells, Sigma Chemical, St. Louis, MO) or LN411/421 prepared from MC cultures.15,27
RT-PCR
Total RNA was isolated using RNAqueous-4 PCR kit (Ambion, Austin, TX). The RNA was treated with RNase-free DNase, and the reverse transcription reaction was preformed with MuLV reverse transcriptase and oligo(dT) (Applied Biosystems Inc., Foster City, CA). Real-time quantitative PCR was performed using a ABI7900HT machine and SYBR Green SensiMix dT (Bioline, Taunton, MA).28 The following sequences were used for primers: rat PDGF-Rβ, 5′-ACACATCAAATACGCGGACA-3′ and 5′-GAGCACTGGTGAGTCGTTGA-3′; mouse PDGF-Rβ 5′-CCGGAACAAACACACCTTCT-3′ and 5′-TATCCATGTAGCCACCGTCA-3′, PDGF-BB, 5′-AAAGGCAAGCACCGAAAGT-3′ and 5′-GGGGCAATACAGCAAATACC-3′, PDGF-D, 5′-CCCAGGAGAAAACACGGATA-3′ and 5′-TTCCACAAAGTCATACCTACAAATG-3′ and 18s 5′-GACTCAACACGGGAAACCTC-3′ and 5′-AGACAAATCGCTCCACCAAC-3′. Primers to GAPDH were purchase from Qiagen, Valencia CA.
Western Blot
For whole cell lysates, cells were washed with phosphate-buffered saline and extracted in phosphate-buffered saline containing 0.1% sodium dodecyl sulfate, 0.5% Triton X-100, and protease inhibitors (Sigma-Aldrich). Western blots using were performed as described.22 Densitometry was performed using 1D software (v3.5, Kodak).
Statistical Analysis
Group means were compared by one-way analysis of variance with subgroup testing by contrasts. P < 0.05 was considered significant.
Results
Light Microscopy
Kidney samples were examined from wild-type and lama4−/− mice from 7 weeks to 23 months of age (Figure 1, A–F). No abnormalities were identified in wild-type mice over the ages examined. In lama4−/− mice aged 7–14 weeks, kidney development is generally normal; yet, glomerular capillary loops and peritubular capillaries are dilated. Consistent with dilated glomerular capillaries, glomeruli in lama4−/− mice were 21% larger than wild-type mice (glomerular diameter: wild-type: 89.9 ± 16.9 μm versus lama4−/− 109.2 ± 16.3 μm, P < 0.01). At ages older than 14 weeks, many glomeruli were hypercellular, and areas of perivascular inflammation were evident. With increasing age, sclerotic glomeruli were more common. Areas of tubulointerstitial fibrosis were also present in lama4−/− animals beyond 6 months of age. Fibrotic changes were accompanied by increased staining for collagen, thrombospondin, and fibronectin (not shown). The progressive sequence of these findings suggests that abnormalities in microvessel maturation or repair contribute to accelerated kidney fibrosis as these animals age. Studies were performed to determine whether proteinuria or functional abnormalities were present before the onset of fibrosis. Abnormalities in renal function were not demonstrated up to 11 months of age as serum creatinine values were not different from wild-type mice (wild-type: 0.19 ± 0.05 mg/dl, lama4−/−: 0.23 ± 0.07 mg/dl, P > 0.05). Urinary albumin excretion was also not elevated as compared with wild-type mice (wild-type: 0.35 ± 0.40 μg/mg creatinine; lama4−/−: 0.37 ± 0.23 μg/mg creatinine, P > 0.05); thus, functional abnormalities that develop later would reflect the increasing degrees of renal scarring.
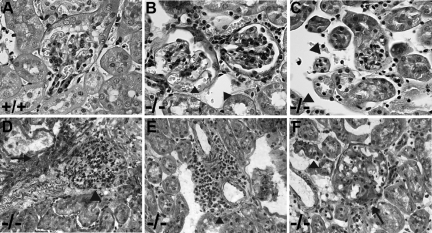
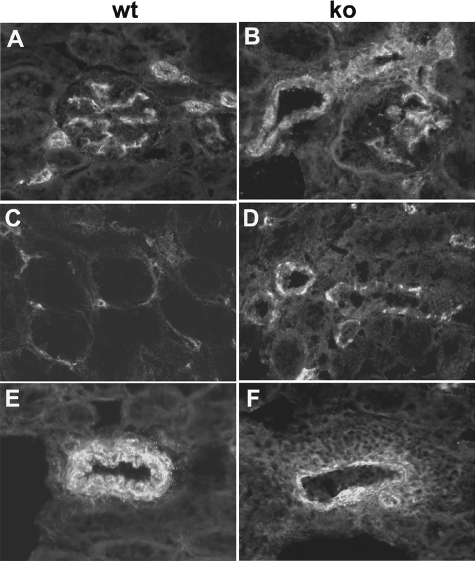
Figure 1.
Light microscopy. Paraffin sections were stained with periodic acid–Schiff. Samples from wild-type (wt) (representative glomerulus shown in A) and lama4−/− (ko) mice (B and C) at 7 weeks of age and from lama4−/− animals at 6 (D), 9 (E), and 18 (F) months of age are shown. Dilated glomerular capillaries (B) and peritubular capillaries (B and C) are shown (arrowheads). Note the proliferative changes in the glomerulus in D (arrowhead), as well as the area of tubulointerstitial fibrosis (arrow). A perivascular inflammatory infiltrate is shown in E (arrowhead). Glomerulosclerosis (arrow) and tubular atrophy (arrowhead) are apparent in F. No glomerular or tubulointerstitial fibrosis was seen in wild-type mice up to 23 months in age.
Electron Microscopy
Normal glomerular structure in wild-type mice included endothelial cells with fenestrae and podocytes with foot processes. Small arterioles and peritubular capillaries with pericytes were also normal in wild-type mice (Figure 2, A–C). In contrast, lama4−/− mice (Figure 2, D–I) had enlarged glomeruli with dilated capillary loops. Mesangial cells (MCs) were more prominent at the glomerular hilus, with fewer MCs supporting peripheral capillary loops than normally occurs, and their irregular distribution coincided with irregular and dilated capillary loops. The glomerular capillary wall is generally normal, including podocyte foot processes, endothelial fenestrae and glomerular basement membrane (GBM); however, there were also frequent focal areas with outpockets of GBM. Apoptotic glomerular endothelial cells were identified in glomeruli examined by electron microscopy, and the GBM was split with mesangial interposition at some sites. Peritubular capillaries were dilated in lama4−/− mice, and pericytes were distant from the capillary edge. Small arterioles had increased extracellular matrix (ECM) and multiple pericytes, yet some were not tightly invested. Dilated glomerular capillary loops, GBM outpockets, mesangial interposition and apoptotic endothelial cells are likely harbingers of the sclerosis observed by light microscopy in lama4−/− animals at later ages.
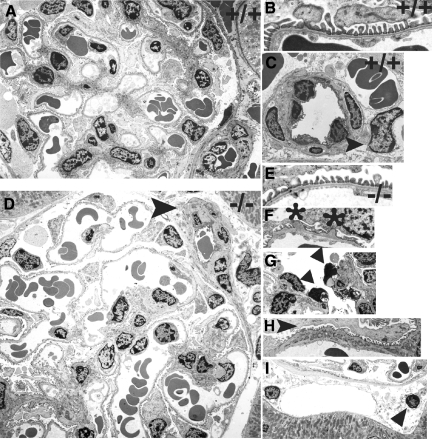
Figure 2.
Electron microscopy. Representative electron micrographs from wt (A–C) and (ko) (D–I) mice at 7–14 weeks of age are shown. Normal glomerular structure (A) including podocyte foot processes, GBM, and endothelial fenestrae (B) are shown in wild-type mice. The circumferential pattern of pericyte investment around an arteriole and a pericyte (arrowhead) adjacent to an erythrocyte-filled peritubular capillary is shown in (C). In lama4−/− mice dilated glomerular capillaries are present (D). Also shown in D (arrowhead) is a small arteriole with pericytes and abundant extracellular matrix. Lama4 null mice had normal regions of glomerular capillary wall with normal appearing GBM, podocyte foot processes and endothelial fenestrae similar to wild-type mice (E). Abnormal areas of GBM were also detected where out-pockets of GBM were associated with foot process effacement (F, asterisks). Although glomerular endothelial cells were abundant, apoptotic endothelial cells were also noted (G, arrowheads). Areas of mesangial interposition with split basement membranes were also seen in lama4−/− mice (H, arrowhead), as well as dilated peritubular capillaries with nearby and distant pericytes (I, arrowhead).
Distribution of Cell Types
Renal tissue was stained with antibodies to identify podocytes (WT-1), endothelial cells (PECAM CD31, vWF, isolectin B4), and MCs, vascular smooth muscle cells, and pericytes (α-SMA, NG2) (Figures 3 and 4, A–D). Staining showed similar numbers of podocytes and endothelial cells in wild-type and lama4−/− mice. The intensity of vWF staining was increased in lama4−/− mice suggesting that endothelial cells are activated.29 Using similar methods, Wang et al17 found early loss of microvascular endothelial cells in the heart along with the development of ischemia and cardiofibrosis. Significant differences in endothelial cell numbers were not observed in kidneys in animals 7–14 weeks of age, nor were apoptotic cells detected by staining for activated caspase 3 (less than 1% of total cells stained positively in either wild-type or lama4−/− mice, data not shown). Although a general increase in the number of apoptotic cells was not demonstrated, the presence of apoptotic glomerular endothelial cells in electron micrographs suggests that processes similar to those demonstrated in the heart may be occurring in the kidney, albeit at a slower rate. In older mice, reduced numbers of endothelial cells were observed in areas of tubulointerstitial fibrosis; thus, in the late stages of interstitial fibrosis, ischemia may contribute to fibrosis as occurs in the heart.17
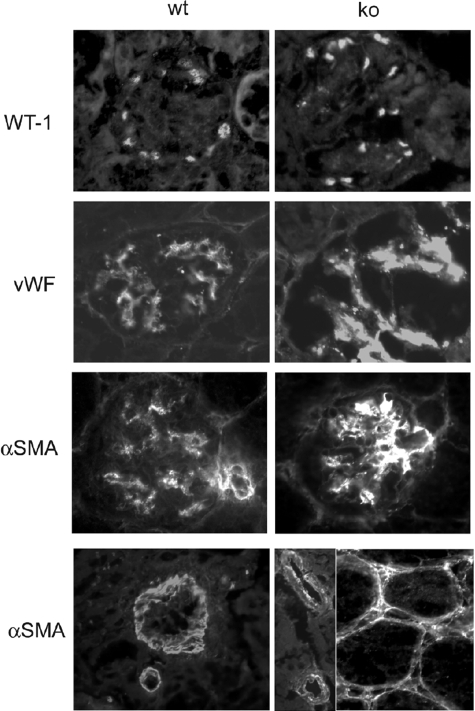
Figure 3.
Immunofluorescence microscopy: identification of cells. Samples from wt and ko tissues were stained as indicated. Podocytes were identified by staining for WT-1. Endothelial cells were identified by staining for von Willebrand factor (vWF). Note increased staining with vWF in lama4−/− mice indicating endothelial cell activation. Vascular smooth muscle cells and MCs were identified by staining for α-smooth muscle actin (αSMA). Note increased αSMA expression in the glomerular hilum and discontinuous staining of arteriole walls of the lama4−/− mice.
Figure 4.
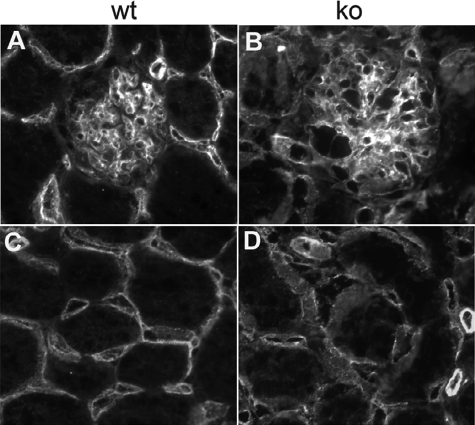
Endothelial cell staining. Endothelial cells were identified by staining with fluorescein-labeled isolectin B4. A and C: Wild-type mice. B and D: Lama4−/− mice.
MCs, vascular smooth muscle cells, and capillary pericytes were identified by staining for α-SMA and NG230 (Figure 3). Glomerular staining indicated the presence of MCs and their distribution coincided with what was seen by electron microscopy. During development, α-SMA is prominent in MCs, as well as afferent and efferent arterioles. Mesangial expression declines as glomerulogenesis is completed.31 In contrast to the normal pattern confirmed in wild-type mice, lama4−/− mice had increased α-SMA staining in the glomerular hilum, and discontinuous staining in the walls of arterioles. In some lama4−/− mice, increased α-SMA was noted in cells associated with peritubular capillaries. α-SMA staining is normally absent in the interstitium, but has been observed in lesions associated with progressive tubulointerstitial fibrosis.32
To further explore the degree of pericyte investment, particularly given the discontinuous staining for α-SMA, NG2 (Figure 5, A–F) staining was performed. NG2 was identified throughout the mesangium in wild-type animals, and it was clustered at the hilus in lama4−/− mice, similar to α-SMA. In arterioles and peritubular capillaries of wild-type mice, NG2-positive cells formed a tight ring around endothelial cells. Although abundant NG2-positive pericytes surrounded blood vessels in lama4−/− mice, they did not tightly encase the vessels. The relationship of these cells to endothelial cells was confirmed by dual staining for CD31 and isolectin B4, which showed a discrete ring of CD31/isolectin B4 positive cells forming the lumen in all animals (not shown). These findings suggested an increase in number of pericytes and migration toward the vessel wall; yet, inadequate investment of endothelial cells.
Figure 5.
Pericyte staining. Samples from wt (A, C, E) and ko (B, D, F) mice were stained with antibody to NG2. Note the increased staining in the extra-glomerular mesangium and around afferent and efferent arterioles in B. Note the enlarged vessels with discontinuous pericytes in D. Note the large number of pericytes that surround, but are distant from the vessel wall in F.
Laminin Subunit Expression in lama4−/− Mice
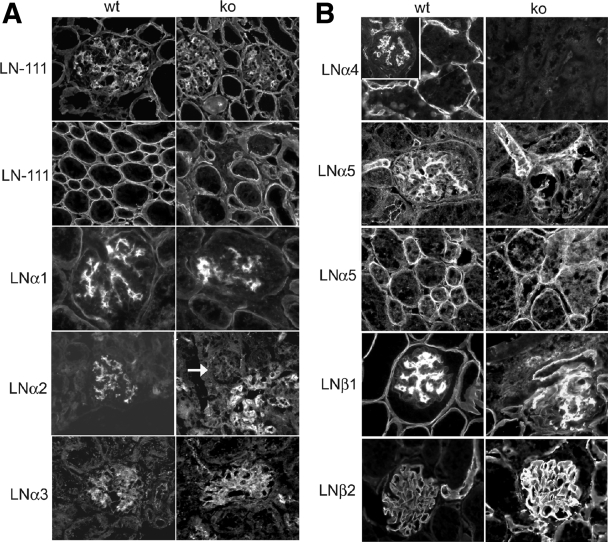
Key transitions in LN isoforms occur during normal glomerulogenesis resulting in unique composition in each ECM compartment. In some renal diseases, the normal transitions and final composition are altered.33,34,35,36 Although developmental transitions in expression of LN chains occurred in lama4−/− mice, notable alterations in some subunits occurred (Figure 6, A and B). Staining with antibody to LN111 shows irregular organization of mesangial matrix in lama4−/− mice. Wide separation between adjacent tubular basement membranes (TBM) representing the space occupied by dilated peritubular capillaries is apparent. Although the reported distribution of LNα4 varies,37,38,39 the α4 subunit is usually detected in mesangial matrix and surrounding peritubular capillaries as seen in wild-type mice. As expected, LNα4 was not detected in lama4−/− mice.
Figure 6.
Immunofluorescence microscopy: patterns and transitions in laminin isoforms. Kidneys from wt and ko mice were stained with antibodies to the following proteins: A: LN111, LNα1, LNα2, LNα3. Anti-LN111 stains all kidney basement membranes showing overall structure. Typically this antibody stains mesangial matrix more brightly than GBM. This was true in wild-type and lama4−/− mice. Note the irregular organization of mesangial matrix and wide separation between adjacent tubular basement membranes, which represents the space occupied by dilated peritubular capillaries. In the mouse, staining for LNα1 is normally detected in GBM at early stages of glomerulogenesis, and disappears from this site as glomerulogenesis is completed, with detectable LNα1 remaining in the mesangium and TBM of proximal tubules. These transitions in LNα1 were identical in 7-to 14-week-old wild-type and lama4−/− mice. Note the absence of glomerular staining for LNα2 in lama4−/− mice (arrow) and the increase in staining around afferent and efferent arterioles at the glomerular hilum. LNα3 has a limited distribution on the endothelial side of GBM. No significant change in LNα3 was identified in lama4−/− mice. B: LNα4, LNα5, LNβ1, and LNβ2. LNα4 is detected in the mesangial matrix and surrounding peritubular capillaries in wild-type mice and is not detected in lama4−/− mice. During normal glomerulogenesis, LNα5 replaces LNα1 in the GBM and it facilitates maturation and condensation of the mesangium. LNα5 is a normal component of TBM and the peritubular capillary basement membrane. In lama4−/− mice, LNα5 staining of GBM, mesangial matrix, TBM and peritubular capillaries was not different from wild-type mice; however, structural differences in the mesangium were apparent with staining for this LN chain. The brightly stained, condensed mature mesangium was not uniformly present in lama4−/− mice. As glomeruli become fully mature, LNβ1 in GBM is replaced by LNβ2. This transition occurred normally in lama4−/− mice. In wild-type and lama4−/− mice, LNβ1 persists in other ECM compartments including the mesangium and TBM. Adjacent to TBM, a fibrillar matrix that supports peritubular capillaries normally contains LN411 and LN511. Of note, LNβ1 was not detected in this location, but LNβ2 was abundant, which indicates that peritubular capillary matrix is altered and contains only LN521 in lama4−/− mice.
As GBM matures, LNα1 and LNβ1 are replaced by LNα5 and LNβ2.40,41 LNα3 is detected only on the subendothelial side of GBM.23 TBM is usually composed of LNα1, LNα5, and LNβ1. These normal patterns of LN subunit staining in GBM and TBM are unaffected in lama4−/− mice.
As the mesangium matures in wild-type mice, LNα1 persists and LNα2, LNα4 and LNα5 appear. As expected, LNα4 was not detected in lama4−/− mice. LNα1 and LNα5 were present and irregularly distributed in keeping with structural changes described above. LNα2, which is normally expressed in mature mouse mesangium, was not identified in glomeruli of lama4−/− mice, but instead was abundant in cells adjacent to afferent and efferent arterioles. In mice, LNα2 is a product of MCs; thus, this observation suggests that MCs are abundant in the hilar region and adjacent to the afferent and efferent arterioles (extraglomerular mesangium), but less abundant in peripheral loops of the glomerulus. This coincides with the distribution of MCs described above.
Peritubular capillaries normally stain with antibodies to LNα4, LNβ1, and lower amounts of LNα5 (see wild-type mice in Figure 6). The appearance of LNα5 at 1–3 weeks after birth is reported to stabilize microvessels in other organs in the lama4−/− model (12); similar to those reports, peritubular capillaries remain dilated. New expression of LNβ2 was seen in the lama4−/− mice in the fibrillar matrix that supports peritubular capillaries. LN β1 that is normally expressed in this location was not detected. We previously reported a similar change in focal capillaries around proximal tubules in individuals with transplant rejection.42 The functional significance of new expression of LNβ2 in this location is unknown, although similar transitions have been reported in other capillaries during active angiogenesis and microvessel maturation.7,43 The expression of LNα2 and LNα5 in the face of altered peripheral migration of MCs and poor pericyte investment of other microvessels argues that these LN subunits cannot substitute for LNα4 in mediating these functions.
Growth Factor Expression Over Time in lama4−/− Mice
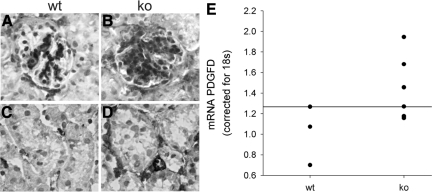
Although microvessels form in lama4−/− mice, each of the changes described above indicate that there is poor pericyte investment and failure of vessel maturation. Normally, these processes are carefully orchestrated by sequential expression and down-regulation of a series of growth factors. During development, VEGF-A drives endothelial cell proliferation and migration. Then, endothelial cells secrete PDGF-BB, which recruits PDGF-Rβ-bearing pericytes to blood vessels and MCs to the glomerulus.31,44 As pericytes coat the expanding endothelial tubes, they initiate a series of reciprocal changes between the two cells that slow vessel growth and enhance cell differentiation. In keeping with this, VEGF and PDGF-BB are normally present in developing glomeruli, but, their expression is significantly reduced when maturation is complete.31 In lama4−/− mice 7–14 weeks of age, expression of VEGF, VEGFR2, VEGFR1, angiopoietin 2 (Ang2) and Tie2 were not different from wild-type mice (not shown); yet, PDGF-BB (mRNA and protein) and PDGF-Rβ expression were significantly increased in glomerular and peritubular areas (Figure 7, A–J). These data indicate that reductions in pericyte coverage do not result from absence of PDGF/PDGF-Rβ; however, in the absence of LNα4, appropriate down-regulation of the PDGF/PDGF-Rβ system that is normally associated with vessel maturation did not occur.
Figure 7.
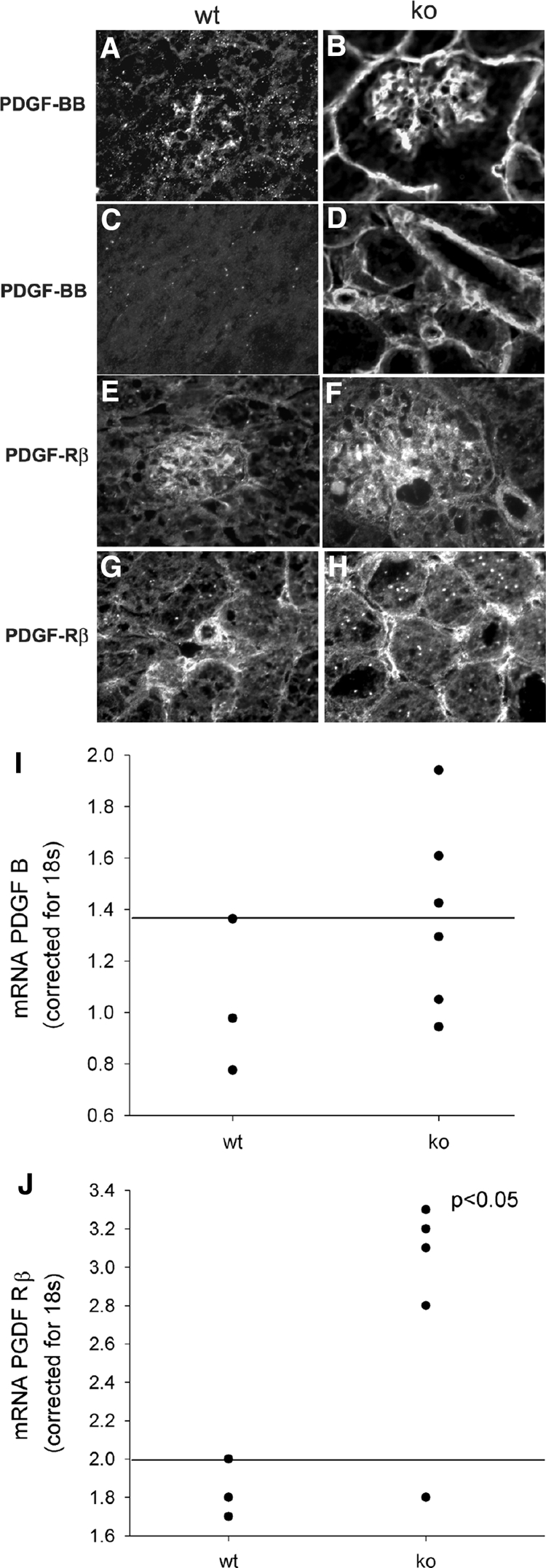
PDGF and PDGF-Rβ. Staining for PDGF-BB (A–D) and PDGF-Rβ (E–H) are shown in wt (A, C, E, G) and ko (B, D, F, H) mice. Note increased staining for both PDGF and PDGF-Rβ in lama4 mice. qPCR for PDGF-B mRNA (I) and PDGF-Rβ mRNA (J). mRNA in whole kidney cortex was determined by qPCR and corrected for 18S RNA. The line represents the upper value detected in normal mice. Note that mRNA for PDGF-Rβ was significantly increased (P < 0.05) in ko mice.
PDGF-BB is known to lead to glomerulosclerosis through stimulation of mesangial proliferation and an increase in matrix synthesis. PDGF-BB also contributes to interstitial fibrosis in a variety of kidney diseases.45,46 PDGF-BB may indirectly promote renal fibrosis as prolonged stimulation with PDGF-BB leads to transforming growth factor-β and chemokine synthesis.45 Based on these known effects of PDGF-BB, and because the increase in PDGF-BB and PDGF-Rβ observed in the first three months of life persisted as animals aged, we postulate that PDGF-BB contributes to the development of glomerulosclerosis and tubulointerstitial fibrosis in lama4−/− mice. There has been recent interest in the potential role of PDGF-CC and PDGF-DD in microvessel development and renal disease progression.45 Although we did not detect significant amounts of PDGF-CC in wild-type or lama4−/− mice (data not shown), distinct increases in PDGF-DD (mRNA or protein) were observed (Figure 8, A–E). In the context of the present studies, expression of both PDGF-BB and PDGF-DD could stimulate angiogenesis of peritubular capillaries, retard their maturation, and drive fibrosis in both the glomerulus and interstitium. As glomerulosclerosis and tubulointerstitial fibrosis develop, ischemia may stimulate the production of other cytokines that further aggravate fibrosis. The increases in VEGF-A and Ang2 that were observed in fibrotic kidneys in older lama4−/− mice (Figures 9, A–D, and 10, A–D) are likely a consequence of the earlier changes, but they may contribute to progressive fibrosis as mice age.
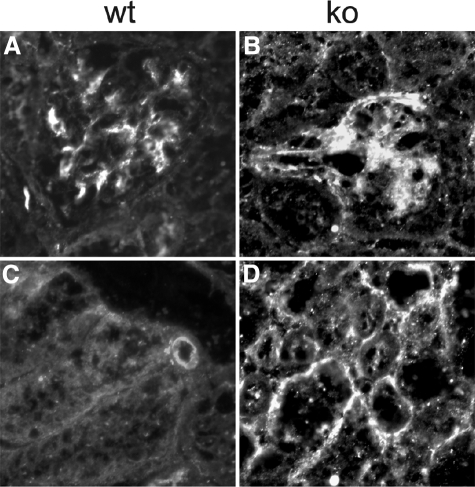
Figure 8.
PDGF-DD. Staining for PDGF-DD shown in wt (A and C) and ko (B and D) mice. Note increased staining in lama4−/− mice. E: PDGF-D mRNA. mRNA in whole kidney cortex was determined by qPCR corrected for 18S RNA. The line represents the upper value detected in normal mice.
Figure 9.
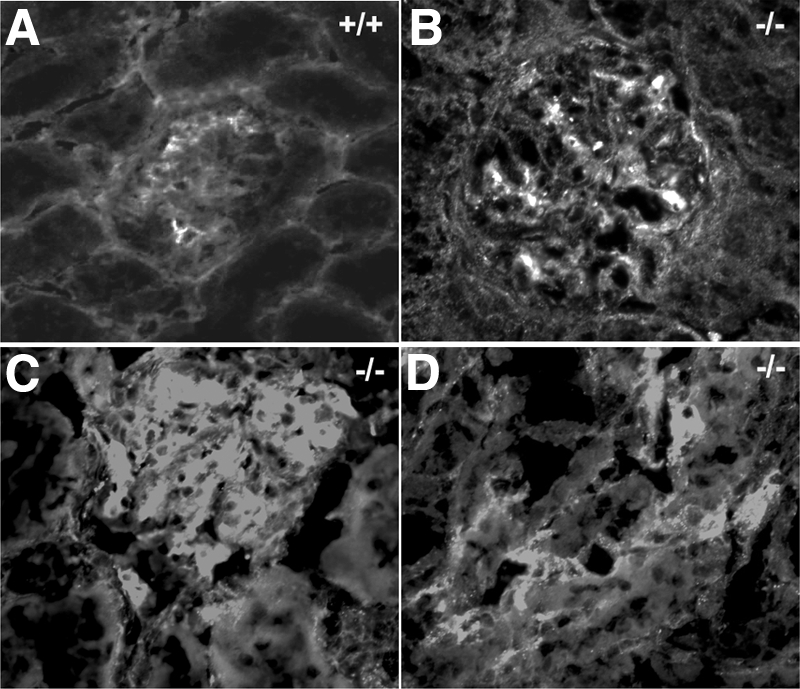
VEGF Staining. In lama4−/− mice (B, C, D) over 9 months of age, there was an increase in VEGF staining in both the glomerulus and peritubular capillaries as compared with wt mice (A).
Figure 10.
Angiopoietin 2 Staining. In animals over 9 months of age, there was a modest increase in Ang2 staining in both the glomerulus and peritubular capillaries in ko mice (B and D) as compared with wt mice (A and C).
In Vitro Studies
There is growing evidence that expression of LN411/421 is important for blood vessel growth and repair after development,7,13 although the mechanisms responsible for these effects are unknown. To investigate this process, we asked if LNα4 expression played a direct role in controlling growth factor and/or growth factor receptor expression. MCs were cultured on plates coated with LN111 or LN411/421. mRNA was extracted and analyzed by qPCR, and cell lysates were analyzed by Western blot. MCs plated on LN411/421 exhibited marked down-regulation of PDGF-Rβ mRNA and protein in comparison with cells plated on LN-111 (Figure 11). PDGF-BB mRNA was not detected in MCs plated on either substrate. Our previous findings showing that LNα4 is required for PDGF-induced migration,15 indicate that pericyte investment might be reduced when LNα4 expression is reduced even when PDGF and PDGF-Rβ are abundant. These findings complement those made in vivo and suggest that LNα4 plays a role in pericyte recruitment and the cooperative interaction between these cells that promotes glomerular and blood vessel maturation.
Figure 11.
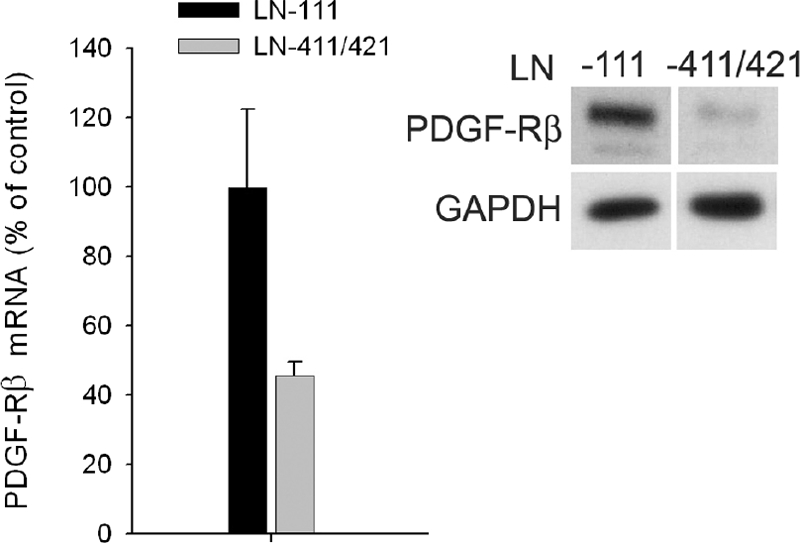
In vitro studies. Plating of MCs on LN411/421 as compared with LN111 was associated with a decrease in PDGF-Rβ mRNA (bar graph, left) and protein (Western blot, right).
Discussion
Evaluation of kidneys in lama4−/− mice shows reduced MC/pericyte investment of glomeruli and peritubular capillaries. With reduced pericyte investment, capillaries remain dilated, and PDGF/PDGF-Rβ, which can drive blood vessel growth and fibrosis are not appropriately down-regulated. With time, glomerulosclerosis and tubulointerstitial fibrosis develop. In vitro studies support a role for LN411/421 in microvessel growth and maturation,7,13 including PDGF-BB-mediated MC migration15 and down-regulation of PDGF-Rβ expression. LNα4 protects endothelial cells from apoptosis14; thus, without this subunit, endothelial loss ultimately contributes to ischemia driven fibrosis, particularly in the interstitium. This occurs at an earlier age in the heart than in the kidney.17 Recent evidence showing that mutations affecting LNα4 expression contribute to the development of cardiomyopathy in humans suggests that these individuals may also be prone to kidney fibrosis. The rate of kidney fibrosis may be influenced by the frequency of minor injury to the kidney where microvessel repair is altered.
The role of angiogenic growth factors and their receptors in blood vessel and glomerular development has been extensively reviewed.47,48,49 Elegant studies from Quaggin and colleagues and others47,48 have established the importance of VEGF-A in endothelial proliferation and migration into the vascular cleft of the developing glomerulus and other blood vessels.50 Podocyte-derived angiopoietin-1 (Ang1) synergizes with VEGF to enhance proliferation and migration and prevent endothelial apoptosis via Tie2-mediated activation of PI3K/Akt.51 Regulated expression of various receptors also influences the sequence of vessel maturation.51 For example, VEGFR-2 initiates vasculogenic formation of glomerular endothelial cells (GEnC), whereas VEGFR-1 modulates development into blood vessels.51 Podocyte-GEnC interactions are critical in the early stages of glomerulogenesis and in maintenance of the slit diaphragm.52 These relationships appear normal in lama4−/− mice as the capillary wall formed normally, with appropriate transitions in ECM composition and no detectable changes in VEGF mRNA or protein.
GEnC-MC and podocyte-MC interactions are important for completion of glomerulogenesis and for repair in glomerular disease.53,54,55 Recruitment of mesangial progenitors into the developing glomerulus is dependent on GEnC secretion of PDGF-BB and MC expression of PDGFRβ.16,56 Once pericytes or MC contact endothelial cells, they secrete Ang2, which antagonizes Ang1-mediated activation of Tie2 on endothelial cells, thereby suppressing endothelial cell proliferation and migration.57,58 PDGF plays a similar role in pericyte recruitment to vessels in other organs.44 VEGF remains cell-associated, presumably bound to ECM,59 where it increases the degree of pericyte coverage of capillaries.60 In turn, the density of pericyte investment influences the stability of endothelial cells and protects them from apoptosis. Normally, PDGF-BB secretion by endothelial cells falls following contact with pericytes, which in turn controls the density of pericyte investment.57 Contact with endothelial cells reduces pericyte expression of αSMA and enhances expression of differentiation markers including desmin, smooth muscle myosin and NG2.61 These data show that reciprocal interactions between endothelial cells and pericytes determine the degree of pericyte investment of blood vessels and the maturation of both cell types. Disruption of the final stages of glomerular and blood vessel maturation with persistent overexpression of PDGF-BB and PDGF-Rβ in lama4−/− mice suggest that LN411/LN421 are critical to this process.
Matrix attachment of PDGF and VEGF are required for, or enhance, the proliferative and migratory responses of cells to these growth factors.62,63 Although it is not known if LNα4-containing isoforms bind, retain, and present growth factors to the cell, it is possible that lack of LNα4 reduces or abolishes the effect of PDGF-BB on MC/pericyte proliferation, migration, or differentiation. We have shown that PDGF-BB-induced MC migration requires interaction with the LNα4 chain of LN411.15 This may explain the inadequate migration of MCs and pericytes despite abundant PDGF-BB, which is manifested by a reduction in MCs in the periphery of the glomerulus and an irregular pericyte investment in other microvessels. Without reciprocal interaction with endothelial cells, PDGF-Rβ expression and that of other proangiogenic factors fail to be properly modulated to promote vessel maturation. Although much less is known about the biology of PDGF-DD, if it remains uncleaved following secretion, it can inhibit binding of PDGF-BB to the PDGF-Rβ.64 This might further prevent vessel maturation. Because PDGF-BB and PDGF-DD are potent growth factors that can induce progressive glomerulosclerosis and tubulointerstitial fibrosis when elevated expression persists, we postulate that continued overexpression of PDGF-BB and PDGF-DD contributes to the late stage of kidney fibrosis in the lama4−/− mice. The importance of LNα4 to this process was shown in vitro, in which we found that MC exposure to LN411/421 directly led to PDGF-Rβ down-regulation.
This report provides new insights into the cell and molecular mechanisms controlling the structure of the peritubular capillary. Normally these vessels are small, surrounded by fibrillar ECM composed of LN511 and LN411, with a limited number of pericytes. In lama4−/− mice, there was an increase in LN521 with a reduction or loss of LN511. Only on rare occasions is LNβ2 detected in cortical peritubular capillaries. The significance of the increase in LNβ2 in this region is not known. As we have found that PDGF-BB does not induce a change in LNβ2 transcription or protein (data not shown), it suggests that other factors contribute to this change in LNβ2. Although the lack of LNα4 does not lead to major developmental abnormalities, it influences maturation of microvessels and responses to injury that could contribute to the degree and speed with which kidney fibrosis occurs. Similarities between cardiomyopathy observed in lama4−/− mice and humans with mutations in this gene raise the possibility that these individuals will also develop abnormalities in their kidneys.
Acknowledgments
We appreciate the excellent technical assistance of Anne K. Berfield and thank the Seattle Mouse Metabolic Phenotyping Center and Dr. Charles Alpers for staining tissues for PDGF-DD.
Footnotes
Address reprint requests to Christine K. Abrass, M.D., Professor of Medicine, University of Washington School of Medicine, UW Medicine South Lake Union, 815 Mercer Street, Seattle, WA 98109. E-mail: cabrass@u.washington.edu.
Supported by National Institutes of Health grants (C.K.A., R01 DK49771; B.L.P. NS040759 and NS064397). This work was supported by resources and the use of facilities at the VA Puget Sound Health Care System, Seattle, WA.
References
- Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitamins Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Beck K, Kitagawa Y. Heparin binds to the laminin α4 chain LG4 domain at a site different from that found for other laminins. J Mol Biol. 2004;335:1145–1149. doi: 10.1016/j.jmb.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin α4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- Nomizu M, Song S-Y, Kuratomi Y, Tanaka M, Kim WH, Kleinman HK, Yamada Y. Active peptides from the carboxy-terminal globular domain of laminin α2 and Drosophila α chains. FEBS Lett. 1996;396:37–42. doi: 10.1016/0014-5793(96)01060-5. [DOI] [PubMed] [Google Scholar]
- Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin α5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–4816. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Collagen IV α3, α4, and α5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol. 1994;127:879–891. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M, Saladin K. Mouse heart laminin. J Biol Chem. 1989;264:18726–18732. [PubMed] [Google Scholar]
- Marinkovich MP, Lundstrum GP, Keene DR, Burgeson RE. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sanes JR. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990;1:731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Doi M, Wang J, Cao R, Liu B, Chan KM, Kortesmaa J, Sorokin L, Cao Y, Tryggvason K. Deletion of laminin-8 results in increased tumor neovascularization and metastasis in mice. Cancer Res. 2004;64:4059–4063. doi: 10.1158/0008-5472.CAN-04-0291. [DOI] [PubMed] [Google Scholar]
- DeHahn KC, Gonzales M, Gonzalez AM, Hopkinson SB, Chandel NS, Brunelle JK, Jones JC. The α4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004;294:281–289. doi: 10.1016/j.yexcr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hansen KM, Abrass CK. Laminin-8/9 is synthesized by rat glomerular mesangial cells and is required for PDGF-induced mesangial cell migration. Kidney Int. 2003;64:110–118. doi: 10.1046/j.1523-1755.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF β-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J, Jr, Tryggvason K, Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin α4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4:597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lännergren J, Domogatskaya A, Ögren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin α4. J Neuro Sci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrass CK, Spicer D, Berfield AK, St John PL, Abrahamson DR. Diabetes induces changes in glomerular development and laminin β2 (s-laminin) expression. Am J Pathol. 1997;151:1131–1140. [PMC free article] [PubMed] [Google Scholar]
- Hansen K, Berfield AK, Spicer D, Abrass CK. Rat mesangial cells express two unique isoforms of laminin which modulate mesangial cell phenotype. Matrix Biol. 1998;17:117–130. doi: 10.1016/s0945-053x(98)90025-7. [DOI] [PubMed] [Google Scholar]
- Abrass CK, Berfield AK, Ryan MC, Carter WG, Hansen KM. Abnormal development of glomerular endothelial and mesangial cells in mice with targeted disruption of the lama3 gene. Kidney Int. 2006;70:1062–1071. doi: 10.1038/sj.ki.5001706. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Karnovsky MJ. Glomerular cells in culture. Kidney Int. 1983;23:439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- Abrass CK, Spicer D, Raugi GJ. Induction of nodular sclerosis by insulin in rat mesangial cells in vitro: studies of collagen. Kidney Int. 1995;47:25–37. doi: 10.1038/ki.1995.3. [DOI] [PubMed] [Google Scholar]
- Abrass CK, Spicer D, Raugi GJ. Insulin induces a change in extracellular matrix glycoproteins synthesized by rat mesangial cells in culture. Kidney Int. 1994;46:613–620. doi: 10.1038/ki.1994.313. [DOI] [PubMed] [Google Scholar]
- Berfield AK, Hansen KM, Abrass CK. Rat glomerular mesangial cells require laminin-9 to migrate in response to insulin-like growth factor binding protein-5. Am J Physiol Cell Physiol. 2006;291:C589–C599. doi: 10.1152/ajpcell.00623.2005. [DOI] [PubMed] [Google Scholar]
- Arya M, Shergill IS, Williamson M, Gommersall L, Arya N, Patel HRH. Basic principles of real-time quantitative PCR. Exp Rev Mol Diagn. 2005;5:209–219. doi: 10.1586/14737159.5.2.209. [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade Bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, van Noorden CJF, Vrensen GFJM, Schlingemann RO. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-A. J Histochem Cytochem. 2004;52:39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
- Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF. Developmental patterns of PDGF B-chain. PDGF-receptor, and actin expression in human glomerulogenesis. Kidney Int. 1992;42:390–399. doi: 10.1038/ki.1992.300. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Rottoli D, Corna D, Tomasoni S, Remuzzi G. Proximal tubular cells promote fibrogenesis by TGF-β1-mediated induction of peritubular myofibroblasts. Kidney Int. 2002;61:2066–2077. doi: 10.1046/j.1523-1755.2002.00380.x. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, St John PL. Loss of laminin epitopes during glomerular basement membrane assembly in developing mouse kidneys. J Histochem Cytochem. 1992;40:1943–1953. doi: 10.1177/40.12.1280666. [DOI] [PubMed] [Google Scholar]
- Peutz-Kootstra CJ, Hansen K, de Heer E, Abrass CK, Bruijn JA. Differential expression of laminin chains and anti-laminin autoantibodies in experimental lupus nephritis. J Pathol. 2000;192:404–412. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH707>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, Prettyman AC, Robert B, St John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–834. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- St John PL, Wang R, Yin Y, Miner JH, Robert B, Abrahamson DR. Glomerular laminin isoform transitions: errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol. 2001;280:F695–F705. doi: 10.1152/ajprenal.2001.280.4.F695. [DOI] [PubMed] [Google Scholar]
- Petäjäniemi N, Korhonen M, Kortesmaa J, Tryggvason K, Sekiguchi K, Fujiwara H, Sorokin L, Thornell L-E, Wondimu Z, Assefa D, Patarroyo M, Virtanen I. Localization of laminin α4-chain in developing and adult human tissues. J Histochem Cytochem. 2002;50:1113–1130. doi: 10.1177/002215540205000813. [DOI] [PubMed] [Google Scholar]
- Frieser M, Nockel H, Pausch F, Roder C, Hahn A, Deutzmann R, Sorokin LM. Cloning of the mouse laminin α4 cDNA. Expression in a subset of endothelium. Eur J Biochem. 1997;246:727–735. doi: 10.1111/j.1432-1033.1997.t01-1-00727.x. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Thesleff I, Tryggvason K. Primary structure, developmental expression, and immunolocalization of the murine laminin α4 chain. J Biol Chem. 1997;272:27862–27868. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol. 2005;16:857–861. doi: 10.1681/ASN.2004121139. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, St John PL, Isom KS, Robert B, Miner JH. Partial rescue of glomerular laminin α5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 2007;18:2285–2293. doi: 10.1681/ASN.2007020207. [DOI] [PubMed] [Google Scholar]
- Abrass CK, Berfield AK, Stehman-Breen C, Alpers CE, Davis CL. Unique changes in interstitial extracellular matrix composition are associated with rejection and cyclosporine toxicity in human renal allograft biopsies. Am J Kidney Dis. 1999;33:11–20. doi: 10.1016/s0272-6386(99)70252-0. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- Eitner F, Bücher E, van Roeyen C, Kunter U, Rong S, Seikrit C, Villa L, Boor P, Fredriksson L, Bäckström G, Eriksson U, Östman A, Floege J, Ostendorf T. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J Am Soc Nephrol. 2008;19:281–289. doi: 10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Mathieson PW. Angiopoietins: microvascular modulators with potential roles in glomerular pathophysiology. J Nephrol. 2003;16:168–178. [PubMed] [Google Scholar]
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber H-P, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatsi-Joannou M, Li XZ, Suda K, Yuan HT, Woolf AS. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev Dyn. 2001;222:120–126. doi: 10.1002/dvdy.1170. [DOI] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Woolf AS, Yuan HT. Angiopoietin growth factors and Tie receptor tyrosine kinases in renal vascular development. Pediatr Nephrol. 2001;16:177–184. doi: 10.1007/s004670000509. [DOI] [PubMed] [Google Scholar]
- Liu A, Dardik A, Ballermann BJ. Neutralizing TGF-β1 antibody infusion in neonatal rat delays in vivo glomerular capillary formation. Kidney Int. 1999;56:1334. doi: 10.1046/j.1523-1755.1999.00661.x. 1348. [DOI] [PubMed] [Google Scholar]
- Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, Carmeliet P. Loss of the VEGF164 and VEGF188 isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol. 2002;13:1548–1560. doi: 10.1097/01.asn.0000013925.19218.7b. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-H, Joly AH, Oh S-W, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: i. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- Yuan HT, SURI CHIT, Landon DN, Yancopoulos GD, Woolf AS. Angiopoietin-2 Is a site-specific factor in differentiation of mouse renal vasculature. J Am Soc Nephrol. 2000;11:1055–1066. doi: 10.1681/ASN.V1161055. [DOI] [PubMed] [Google Scholar]
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Balke M, Schmidt A, Bloch W, Kurz H, Javerzat S, Rousseau B, Wilting J, Bikfalvi A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dyn. 2004;230:23–33. doi: 10.1002/dvdy.20020. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF. TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralem T, Steinberg R, Price D, Avraham H. VEGF165 requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene. 2001;20:5511–5524. doi: 10.1038/sj.onc.1204753. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]