Abstract
Dysfunction in macrophage-mediated phagocytosis of aberrant cells that undergo retrograde transport to the peritoneal cavity is considered an important factor in the development of endometriosis. However, the mechanisms responsible for the loss of function of macrophages remain largely unknown. Herein, we report that prostaglandin (PG) E2, via the EP2 receptor-dependent signaling pathway, inhibits the expression of CD36 in peritoneal macrophages, resulting in reduced phagocytic ability. PGE2-mediated inhibition of macrophage phagocytic capability was restored by ectopic expression of CD36. Treatment with PGE2 inhibited CD36-dependent phagocytosis of peritoneal macrophages and increased the number and size of endometriotic lesions in mice. In contrast, blockade of PGE2 production by cyclooxygenase inhibitors enhanced the phagocytic ability of peritoneal macrophages and reduced endometriotic lesion formation. Taken together, our findings reveal a potential mechanism of immune dysfunction during endometriosis development and may contribute to the design of an effective prevention/treatment regimen.
Endometriosis is a highly prevalent gynecological disease with complex etiology. The cost of treating endometriosis patients has been estimated to be $22 billion annually in United States.1 A growing body of evidence suggests that retrograde menstruation is a major contributing factor in the development of endometriosis.2,3,4 During menstruation, shed endometrial tissues may move retrogradely through the fallopian tube to the peritoneal cavity. In most cases, the retrograded tissues will be cleared by immune cells, especially macrophages, recruited to the peritoneum.5,6 The recruited peritoneal macrophages either activate other immune cells (such as nature killer cells and lymphocytes) to launch anti-proliferation responses or directly phagocytose any incongruous cells.7 Ideally, immune system activation would impair ectopic cell growth and even eliminate such cells. Owing to mechanisms unknown, however, infiltrated macrophages fail to mount an efficient phagocytic response, thus allowing the implantation and propagation of endometrial tissues ectopically.
Macrophage phagocytic function is mediated by scavenger receptors present on the macrophage plasma membrane.8,9 Scavenger receptors are a family of structurally diverse receptors having broad ligand specificity that includes low-density lipoproteins, phosphatidylserine, polyanions, and apoptotic cells.10,11,12 The known scavenger receptors that participate in phagocytosis of apoptotic cells include class A and B scavenger receptors (SR-AI, SR-AII, SR-AIII, SR-BI, SR-BII, and SR-BIII).11,13,14 Reduced expression of any one of these scavenger receptors may result in loss of phagocytic ability. We previously demonstrated that peritoneal macrophages isolated from endometriosis patients had lower SR-BIII (better known as CD36) expression and displayed reduced phagocytic ability.15 However, the underlying mechanism responsible for the reduced expression of CD36 was unclear.
The CD36 cDNA predicts a polypeptide of 55 kDa with 10 potential N-linked glycosylation sites.16 Depending on the cell type, CD36 displays different molecular masses (77, 88, or 94 to 120 kDa) corresponding to different glycoforms.17,18 The expression of a mature plasma surface CD36 during monocyte/macrophage differentiation includes the synthesis of a 72- to 74-kDa intracellular precursor as a resident protein of the endoplasmic reticulum; subsequent cleavage and differential glycosylation of this precursor yields a mature 88- to 120-kDa receptor. Increased expression of CD36 in macrophages is associated with the development of atherosclerosis and Alzheimer’s disease.8,19,20,21 In addition, CD36 has been shown to transduce signals regulating apoptotic and inflammatory responses in endothelial cells and macrophages.22,23,24 These data suggest that CD36 is a critical scavenger receptor controlling the phagocytic potential of macrophages and is associated with important human diseases.
Prostaglandin E2 (PGE2), the product of cyclooxygenase (COX), is a multifunctional eicosanoid that regulates many important physiological and pathological processes.25 PGE2 function is mediated through its binding to cognate receptors in the plasma membrane. There are four PGE2 receptors in mammals, namely EP1, EP2, EP3, and EP4. Because each EP receptor utilizes a different signal transduction pathway, the downstream consequence(s) of PGE2 action is distinctively different depending on which receptor it binds to.25 A growing body of evidence indicates that PGE2 contributes to the pathophysiology of endometriosis.3,26 The concentrations of PGE2 in peritoneal fluid are higher in women with endometriosis compared with disease-free women,27 and this PGE2 is considered to play multiple roles in the etiology of endometriosis.3,28 Because CD36 expression is down-regulated in peritoneal macrophages but not peripheral blood-derived mononuclear cells of women with endometriosis,15 we hypothesized that reduced expression of CD36 may be mediated by local factors, such as PGE2, present in the peritoneal fluid of endometriosis patients. This study was designed to characterize the mechanisms underlying the reduced expression of CD36, and thus the phagocytic ability of macrophages, in patients with endometriosis.
Materials and Methods
Chemicals and Antibodies
The mouse monoclonal antibody against human CD36, rabbit polyclonal anti-human CD36, PGE2, and AH6809 (EP2 antagonist) were purchased from Cayman Chemical (Ann Arbor, MI). Fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-human CD36 was from Abcam (Cambridge, UK). FITC-conjugated anti-mouse IgA was from Molecular Probes (Carlsbad, CA). Anti-β-actin was from Oncogene Research Products (Cambridge, MA). The Complete cocktail of protease inhibitors was purchased from Roche (Mannheim, Germany). Indomethacin, ketorolac, NS-398, 12-O-tetradecanoylphorbol 13-acetate (TPA), H89, and U0126 were purchased from Sigma-Aldrich (St. Louis, MO). ONO-D1-004 (EP1 agonist), ONO-AE1-259-01 (EP2 agonist), ONO-AE-248 (EP3 agonist), and ONO-AE1-329 (EP4 agonist) were a kind gift from Ono Pharmaceutical Co. Ltd. (Tokyo, Japan).
Patients
Endometriosis patients were diagnosed and classified according to the revised classification of the American Society of Reproductive Medicine.29 Criteria for recruiting human subjects were as described.27 In the control group, women undergoing laparoscopy for benign gynecological conditions (uterine myoma and tubal factor) were recruited. All patients were of reproductive age with normal menstrual cycles. The patients were not receiving any endocrine therapy, such as GnRH analog, danazol, or pseudopregnancy therapy. The following cases were excluded from the study: malignant neoplasms other than cervical carcinoma in situ, ovarian neoplasms, pelvic inflammation, and pregnancy. Peritoneal macrophage specimens were obtained from patients scheduled for laparotomy or laparoscopy at the Department of Obstetrics and Gynecology, National Cheng Kung University Hospital. The experimental procedure was approved by the Clinical Research Ethics Committee at the National Cheng Kung University Medical Center, and informed consent was obtained from each patient.
Peritoneal Macrophage Isolation and Treatment
Macrophages were isolated as described27 from the peritoneal fluid of each individual, which was collected in a sterile manner at the time of laparoscopy. The purity of peritoneal macrophages was greater than 95% as determined by flow cytometry.27 Purified peritoneal mononuclear cells were allowed to adhere to the surface of 30-mm Petri dishes for 30 minutes in the presence of 2 ml of Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum and cultured in a humidified atmosphere with 5% CO2 at 37°C. Peritoneal macrophages were treated with vehicle control, PGE2 (0.1, 1, or 10 μmol/L), EP1 agonist (ONO-D1-004, 10 μmol/L), EP2 agonist (ONO-AE1-259-01, 10 μmol/L), EP3 agonist (ONO-AE-248, 10 μmol/L), or EP4 agonist (ONO-AE1-329, 10 μmol/L) for 12 or 24 hours. In a separate experiment, macrophages were incubated with vehicle, PGE2 (1 μmol/L), or combined PGE2 with H89 (10 μmol/L), U0126 (1 μmol/L), or AH6809 (10 μmol/L) for various times as indicated in the figure legends, and then the cells were collected for CD36 mRNA and protein quantification or phagocytosis activity assay.
Macrophage Phagocytosis Assay
Macrophages (2 × 105 cells/ml) were mixed with 2 μl of FITC-labeled beads (109 beads/ml; fluoresbrite carboxylate 0.75-μm-diameter microspheres; Polysciences, Inc., Warrington, PA) for 1 hour with shaking at 37°C. The unbound beads were separated from cells using density gradient centrifugation in 2% bovine serum albumin. The binding of fluorescent beads to macrophages was determined by flow cytometry or by scanning confocal laser microscopy (Leica TCS SPII, Nussloch, Germany).
Detecting CD36 by Flow Cytometry and Immunofluorescence Imaging
Human macrophages were incubated first with a mouse monoclonal anti-human CD36 for 60 minutes on ice and second with an FITC-conjugated anti-mouse IgA for 60 minutes on ice in the dark. Mouse IgA was used as isotype control. Flow cytometry was performed in a FACScan machine (Becton Dickinson, Mountain View, CA), and at least 10,000 cells were analyzed using Cell Quest software (Becton Dickinson). For immunoimaging, macrophages were rinsed twice with PBS, fixed with 3.7% buffered paraformaldehyde, permeabilized with 0.5% (w/v) Triton X-100 for 15 minutes, and then stained with Hoechst 33258 and FITC-conjugated mouse monoclonal anti-CD36 for 15 minutes on ice in the dark. The cells were then detected by scanning confocal laser microscopy.
Real-Time Reverse Transcription-PCR
Procedures for RNA isolation, concentrations and quality determination, and real-time reverse transcription (RT)-PCR were as described.30,31,32 A negative control that omitted reverse transcriptase was included in all experiments during the reverse transcription step to ensure that RNA samples were free of genomic DNA contamination. All primers used in this study are listed in Table 1.
Table 1.
Sequences of Primers Used in this Study
| Gene Symbol | NCBI ref # | Sense | Antisense | Amplicon length (bp) |
|---|---|---|---|---|
| Human | ||||
| CD36 | NM_000072 | 5′-AGATGCAGCCTCATTTCCAC-3′ | 5′-CGTCGGATTCAAATACAGCA-3′ | 101 |
| SCARB1 (SR-BI) | NM_001082959 | 5′-AGATCCTGAAGGGCGAGAAG-3′ | 5′-CGGTGTCGTTGTTGTTGAAG-3′ | 102 |
| SCARB2 (SR-BII) | NM_005506 | 5′-CCAAATCAGGAAGACCATGAG-3′ | 5′-TCCCGTTTCAACAAAGTCATC-3′ | 126 |
| MSR1 (SR-AI) | NM_138715 | 5′-CTGGTCCAATATGGCTGAATG-3′ | 5′-CAGAATGTGAACAGGCTCTTG-3′ | 102 |
| MARCO (SR-AII) | NM_006770 | 5′-GAGTGGAAACACATTAAGACCA-3′ | 5′-ATGACTGTCCAGAGGTGAAG-3′ | 133 |
| SCARA3 (SR-AIII) | NM_182826 | 5′-TTTCCTGGAAGTCGAGGAC-3′ | 5′-GCCATATTGGACCAGTACTTA-3′ | 115 |
| RN1BS1 (IBS) | NR_003286 | 5′-GTGTGCCTACCCTACG-3′ | 5′-TGACCCGCACTTACTC-3′ | 114 |
| OAS1 | NM_016816 | 5′-TGGTTGTCTTCCTCAGTCCTC-3′ | 5′-CTCTCTTTGACAGGCTTCACG-3′ | 104 |
| PTGER1 (EP1)* | NM_000955 | 5′-CTGCCCATCTTCTCCATGAC-3′ | 5′-CCGGGTACTGCAGCTCATAG-3′ | 442 |
| PTGER2 (EP2)* | NM_000956 | 5′-TGCTGCTTCTCATTGTCTCG-3′ | 5′-CGACAACAGAGGACTGAACG-3′ | 388 |
| PTGER3 (EP3)* | NM_000957 | 5′-ATGCTGCTCACTGGTTTCG-3′ | 5′-TCTTCATGTGGCTCGCATAC-3′ | 340 |
| PTGER4 (EP4)* | NM_000958 | 5′-TGTGAACCCCATCCTAGACC-3′ | 5′-CCAAGGCCATTTTCACTGAG-3′ | 273 |
| Mouse | ||||
| Cd36 | NM_007643 | 5′-CTTGAAGAAGGAACCACTGC-3′ | 5′-GTTCTTTGCCACGTCATCTG-3′ | 108 |
| Scarb1 (SR-BI) | NM_016741 | 5′-TTTATGAACCGCACAGTTGG-3′ | 5′-TTTATGGGAAGCATGTCTGG-3′ | 83 |
| Scarb2 (SR-BII) | NM_007644 | 5′-TTATCGAAGCCATGCTGAAAG-3′ | 5′-AAATATGGACGAGGGACAAGA-3′ | 108 |
| Msr1 (SR-AI) | NM_031195 | 5′-GGAAGAAGAAGAGGGTCTGTCA-3′ | 5′-CTGGCTCAAGCTGTTGTCAT-3′ | 85 |
| Gapdh | NM_008084 | 5′-GGAGATTGTTGCCATCAACG-3′ | 5′-GAATTTGCCGTGAGTGGAGT-3′ | 85 |
All primers listed were used for both conventional and real time PCR except those labeled with asterisks.
Primers for conventional PCR.
Forced Expression of CD36
Human CD36 cDNA was purchased from Becton Dickinson and subcloned into pTagRFP-N vector. The pTagRFP-N/CD36 fusion plasmid was then transiently transfected into macrophages using MicroPorator MP-100 (Digital Bio Technology, Seoul, Korea). Transfection efficiency was evaluated by direct examination via epifluorescence microscopy.
Animals
Mature (8 to 10 weeks old) female C57BL/6NCrj mice were purchased from the Animal Center at the College of Medicine, National Cheng Kung University. The mice were maintained on standard chow and water, which were available ad libitum, in animal facilities illuminated between 0600 and 1800 hours. All procedures were performed in accordance with the Guidelines of the National Cheng Kung University Animal Center for the handling of laboratory animals.
Surgical Induction of Endometriosis
Endometriosis was surgically induced in female mice (C57BL/6NCrj) using a technique modified from a previous report.33 Briefly, to increase the amount of endometrium harvested, the donor mice were implanted subcutaneously with capsules containing 17β-estradiol (25 μg/ml dissolved in sesame oil) for 2 days. Mice were anesthetized with sodium pentobarbital, and their uterine horns were removed and endometrial fragments were obtained by peeling off the serosa and myometrium gently. After rinsing with sterile culture medium, endometria were cut to 5 mm × 5 mm pieces using a razor blade. Eight pieces of minced endometrial fragments suspended in 0.5 ml PBS were injected into recipients with an 18-gauge needle through the abdominal wall just below the umbilicus into the peritoneal cavity. Mice were randomly assigned to experimental groups of 10 animals each and treated with PGE2 (3 mg or 15 mg per kg body weight), ketorolac (10 mg/kg body weight), NS398 (10 mg/kg body weight), indomethacin (10 mg/kg body weight), or vehicle subcutaneously in a double-blind setting. Treatment was initiated on postoperative day 1 and continued to 1 day before sacrifice to determine and compare the effects of PGE2 or COX inhibitors on disease establishment and progression. All treatment periods were 4 weeks in duration, and the mice were monitored daily. No evidence of toxicity was noted at the doses administered based on body weight, food consumption, grooming behavior, or activity levels compared with controls. All of the inhibitors were suspended in PBS in sterile water by vigorous agitation for 15 minutes just before administration. Control mice similarly received daily injection of sterile PBS. All of the reagents used were labeled with only a code to ensure that all personnel involved in the experiment remained blind to the treatments.
Isolation of Peritoneal Macrophages from Mice
Mice were sacrificed by CO2 asphyxiation, and peritoneal fluid was collected by lavaging the peritoneum twice with 5 ml ice-cold growth media (glucose-free RPMI 1640 media supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin sulfate, and 0.625 μg/ml fungizone, 10 mmol/L HEPES pH 7.4), followed immediately by analysis. Cells were counted with the use of a hemocytometer. After 30 minutes, plates were washed twice to remove nonadherent cells, resulting in approximately 95% pure monocytes as assessed by CD14 staining.27
Measurement of PGE2 in the Peritoneal Fluid of Mice
The PGE2 concentration in peritoneal fluid flushed from each mouse was measured with the Monoclonal PGE2 EIA kit (Cayman, Ann Arbor, MI) as described.27 Because the peritoneal fluid collected was flushed with 10 ml low-glucose growth media, the absolute concentration of PGE2 in peritoneal fluid could not be calculated. Therefore, the PGE2 concentrations measured by enzyme immunoassay were normalized against PGE2 concentrations of sham control (disease-free) mice.
Statistical Analysis
The data are expressed as the mean ± SD and were analyzed by one-way analysis of variance using GraphPad Prism 4.02 (GraphPad Software, San Diego, CA). Tukey’s procedures were used to test differences between individual treatment groups, whereas Dunnett’s test was applied to compare treatment groups verses control groups once significance was established by the F-test. The Student’s t-test was used for two-sample comparisons.
Results
PGE2-Induced Suppression of Macrophage CD36 Expression and Phagocytic Activity
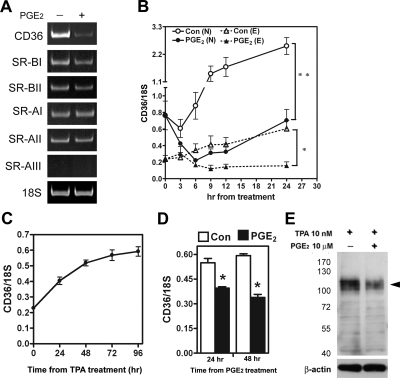
Toward our goal of addressing mechanistic issues, we first aimed to identify local-acting factors that could contribute to the observed reduced expression of CD36 in endometriotic peritoneal macrophages. We screened several previously identified candidate factors (including PGE2) that are elevated in peritoneal fluid of women with endometriosis34 for their potential effects on the mRNA levels of scavenger receptors, and we found that PGE2 potently down-regulated CD36 mRNA level in macrophages (Figure 1A). In contrast, other class A and class B scavenger receptors (SR-AI, SR-AII, SR-BI, and SR-BII) were not affected by PGE2 treatment. Transcripts of SR-AIII were not detected in macrophages (Figure 1A).
Figure 1.
PGE2 inhibits CD36 expression in normal and endometriotic macrophages. A: Macrophages from normal women were treated with 10 μM PGE2 or vehicle for 24 hr, and expression of scavenger receptors was determined by RT-PCR. This experiment was done four times using different batches of macrophages, and the results were similar. B: Normal (N) and endometriotic (E) macrophages were cultured for different periods as indicated in the absence (control) or presence of PGE2, and levels of CD36 were quantified by real-time quantitative RT-PCR. Data represent the mean and standard deviation (SD) of four independent experiments using different batches of cells and were analyzed by ANOVA with repeated measurement. C: U937 monocytic cells were treated with 10 nM TPA for various periods as indicated, and CD36 mRNA expression was quantified by real-time quantitative RT-PCR. Data represent the mean and SD of three independent experiments. D, E: U937 cells were incubated with TPA for 48 hr and then treated with PGE2 (10 μM) for 24 hr or 48 hr. Expression of CD36 mRNA (D) and protein (E) was determined (β-actin served as a loading control). The arrowhead indicates the glycosylated membrane form of CD36. Molecular size markers (kDa) are shown to the left. *P < 0.05, **P < 0.01.
Next, we used quantitative RT-PCR to test whether PGE2 can inhibit CD36 mRNA expression in both normal and endometriotic peritoneal macrophages. In normal macrophages, the level of CD36 mRNA increased over time in the absence of PGE2, indicating auto-activation of macrophages when cultured on a solid surface (Figure 1B). Treatment with PGE2 acutely and profoundly inhibited CD36 expression in macrophages derived from normal women, as well as endometriosis patients (Figure 1B). Notably, the basal level of CD36 mRNA in endometriotic macrophages was significantly lower than that in normal macrophages, and the level in PGE2-treated normal macrophages was similar to that in endometriotic macrophages without PGE2 treatment after 24 hours (Figure 1B). These results implied that reduced expression of CD36 in endometriotic macrophages is likely due to exposure to PGE2 in the peritoneal fluid of women with endometriosis.
To test whether the pattern of CD36 expression we observed is a unique feature of peritoneal macrophages, human U937 monocytic cells were induced by TPA (10 nmol/L) to differentiate into macrophages, and levels of CD36 were determined after PGE2 treatment. Indeed, CD36 expression was observed in TPA-induced U937 cells. Similar to what was observed in human peritoneal macrophages, the levels of CD36 mRNA in TPA-induced U937 cells increased with time in culture (Figure 1C), and treatment with PGE2 significantly decreased the levels of CD36 mRNA and protein (Figure 1, D and E). These data demonstrated that regulation of CD36 by PGE2 is a common phenomenon of the monocyte/macrophage lineage.
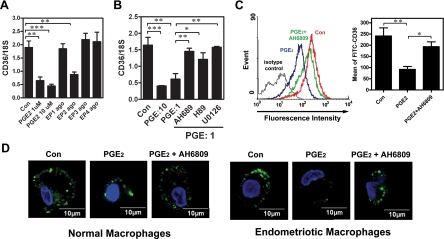
PGE2-Induced Suppression of CD36 Expression Is Mediated by the EP2 Receptor
We next addressed the mechanism of PGE2-induced suppression of CD36 expression. Results from RT-PCR revealed that all four EP receptors were expressed by macrophages, with no substantial difference between normal and endometriotic cells (Supplemental Figure S1 at http://ajp.amjpathol.org). Treatment of macrophages with PGE2 (1 or 10 μmol/L) and various EP receptor agonists revealed that PGE2-induced inhibition of CD36 expression was mediated by the EP2 receptor (Figure 2A). These data were supported by the result that treatment with the EP2 receptor antagonist (AH6809) or inhibitors of signaling kinases (protein kinase A and mitogen-activated protein kinase kinase) acting downstream of the EP2 receptor significantly blocked PGE2-induced CD36 down-regulation (Figure 2B). Concordant with the mRNA data, the membrane form of CD36 was markedly decreased in PGE2-treated macrophages, whereas administration of the EP2 receptor antagonist abolished that down-regulation (Figure 2C). These results were confirmed by confocal microscopy, which clearly showed a reduction in CD36-specific fluorescence intensity in PGE2-treated macrophages but not in macrophages pre-treated with AH6809 (Figure 2D).
Figure 2.
Inhibition of CD36 by PGE2 is mediated by the EP2 receptor signaling pathway. A: Macrophages isolated from normal women were treated with vehicle, PGE2 (1 μM), or selective agonists for EP1 (ONO-D1-004, 10 μM), EP2 (ONO-AE1-259-01, 10 μM), EP3 (ONO-AE-248, 10 μM), or EP4 (ONO-AE1-329, 10 μM) for 12 hr. CD36 mRNA expression was detected by real-time quantitative RT-PCR. B: Macrophages isolated from normal women were treated with PGE2 (1 or 10 μM) or PGE2 plus inhibitors as indicated for 12 hr, and CD36 mRNA expression was determined by real-time quantitative RT-PCR. C: Macrophages isolated from normal women were treated with vehicle (Con), PGE2, or PGE2+AH6809 (10 μM) for 96 hr, and CD36 expression was assessed by staining with anti-CD36 antibody or isotype-matched FITC-IgA (isotype control) followed by quantification via flow cytometry. Representative flow cytometric data are shown in the left panel. The right panel shows the mean and SD of four independent experiments using different batches of peritoneal macrophages. D: Representative confocal images show CD36 staining in macrophages isolated from normal women treated with vehicle (Con), PGE2, or PGE2+AH6809 as described in (C). This experiment was done four times using different batches of cells. *P < 0.05, **P < 0.01, ***P < 0.001.
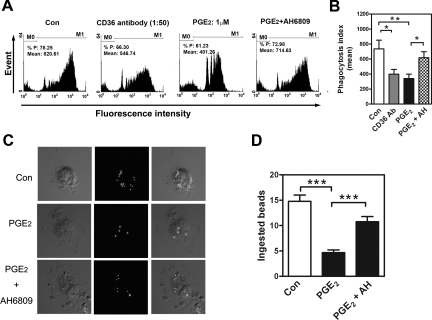
Down-Regulation of CD36 by PGE2 Inhibits Phagocytosis
To directly test whether PGE2 treatment of macrophages inhibited their phagocytic function, macrophages isolated from normal women (those with high CD36 levels and a high level of phagocytic ability) were treated with PGE2; indeed, PGE2 significantly decreased both the phagocytic capacity of each cell and the total number of phagocytic cells (Figure 3, A and B). Both effects were reversed by co-treatment with the EP2 receptor antagonist (Figure 3, A and B). These flow cytometry results were confirmed by confocal laser microscopy, which could distinguish phagocytosed beads from potentially adhered beads. Figure 3C shows representative images of macrophages with ingested beads, the mean values of which were 15.0 ± 1.5, 4.7 ± 1.1, and 10.3 ± 1.5 in the control, PGE2-, and PGE2+AH6809-treated groups, respectively (Figure 3D). These results demonstrated that PGE2 suppresses macrophage phagocytosis via an EP2 receptor-dependent pathway.
Figure 3.
PGE2 inhibits phagocytosis via the EP2 receptor–dependent signaling pathway. A: Macrophages isolated from normal women were treated with vehicle (Con), anti-CD36, PGE2 (1 μM), or PGE2 plus EP2 antagonist (AH6809, 10 μM) for 24 hr, and fluorescence intensity was determined by flow cytometry. M0: no bead was ingested; M1: with at least one bead ingested. B: Mean and SD of M1 obtained from four experiments using different batches of cells. C: Representative confocal microscopic images of phagocytic macrophages isolated from normal patients. Macrophages were treated with vehicle (Con), PGE2, or PGE2+AH6809 and then incubated with FITC-conjugated beads for 1 h. D: Mean and SD of the number of beads phagocytosed by macrophages isolated from normal women after treatment with vehicle (Con), PGE2, or PGE2+AH6809. Four independent experiments using different batches of macrophages were conducted, and at least 20 cells per experiment were counted. *P < 0.05, **P < 0.01, ***P < 0.001.
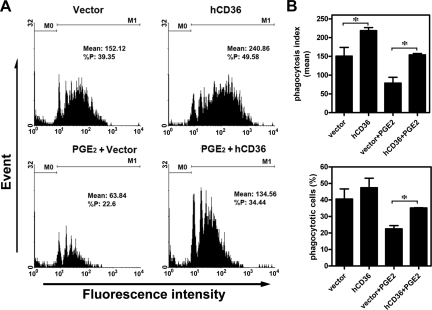
Ectopic Expression of CD36 Rescues PGE2-Inhibited Phagocytosis
We next used a CD36 overexpression approach to demonstrate that CD36 is indispensable for PGE2-mediated suppression of the phagocytic ability of peritoneal macrophages. Human CD36 cDNA was ectopically expressed in peritoneal macrophages isolated from women with endometriosis (those with low CD36 levels) to restore phagocytic ability. After trying all currently available methodologies, electroporation yielded the best result with roughly 25% of macrophages being successfully transfected with full-length human CD36 cDNA (data not shown). Ectopic expression of CD36 in endometriotic macrophages enhanced phagocytic ability (Figure 4, A and B). More significantly, PGE2-inhibited phagocytic ability was also restored by ectopic expression of CD36 (Figure 4, A and B). The mean number of beads ingested by macrophages was higher in groups with ectopic expression of CD36, whereas the total number of macrophages with phagocytic ability was not significantly increased owing to the small percentage of macrophages that were successfully transfected. Nevertheless, these data clearly demonstrated that ectopic expression of CD36 is sufficient to rescue the loss of phagocytic ability in low-CD36 endometriotic macrophages and in PGE2-treated macrophages.
Figure 4.
Ectopic expression of CD36 rescues PGE2-inhibited phagocytosis. A: Macrophages isolated from women with endometriosis were transfected with vector (as a control) or vector containing human CD36 cDNA (hCD36) for 24 hr. Cells were then treated without or with PGE2, and phagocytic ability was determined by flow cytometry. M0: no bead was ingested; M1: with at least one bead ingested. B: Mean and SD of phagocytic ability (upper panel) and percent of phagocytic cells (lower panel) from four independent experiments using different batches of macrophages. *P < 0.05.
PGE2 Promotes the Formation of Endometriosis in Vivo
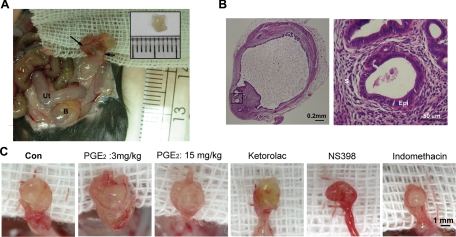
The in vitro experiments demonstrated that PGE2 inhibits the phagocytic capability of macrophages, primarily via EP2 receptor-mediated down-regulation of CD36. Thus, we next asked whether this indeed contributes to the development of endometriosis in vivo. To address this possibility, we established a mouse model of endometriosis. Induction of endometriosis in mice resulted in numerous intestinal and mesenteric adhesions (Figure 5A). The endometriotic lesions were large, cystic, and vascularized, grossly resembling the human disease (Figure 5, A and B). Four weeks after induction of endometriosis, the PGE2-treated groups displayed significant gross vascularity and hyperemia within the lesions, as compared with controls (Figure 5C). Microscopic examination revealed that the cystic tissues contained endometrial stroma and epithelium, again resembling human endometriotic lesions (Figure 5B).
Figure 5.
Macroscopic and microscopic images of endometriotic-like lesions in female C57BL/6NCrj recipients after syngeneic uterine tissue transfer. A: Four weeks after injection, a recipient displayed significant gross vascularity and hyperemia within the lesion and marked vascular recruitment along the periphery of the peritoneal attachment site (arrows). Ut: uterus; B: bladder. The inset shows the endometrial tissue peeled from a donor mouse. Eight pieces of such endometrial fragments were injected into the peritoneal cavity of each recipient. B: Representative images showing H&E–stained adhesive endometriotic tissues in a surgery-induced endometriotic mouse. The right panel is a high-magnification image from the square indicated in the left panel. S: stroma, Epi: epithelium. C: Macroscopic images of endometriotic-like lesions in mice treated with vehicle (Con), PGE2 (3 mg/kg-body weight), PGE2 (15 mg/kg-body weight), COX-1 inhibitor (ketorolac, 10 mg/kg body weight), COX-2 inhibitor (NS398, 10 mg/kg body weight), or nonselective COX-1/COX-2 inhibitor (indomethacin, 10 mg/kg body weight).
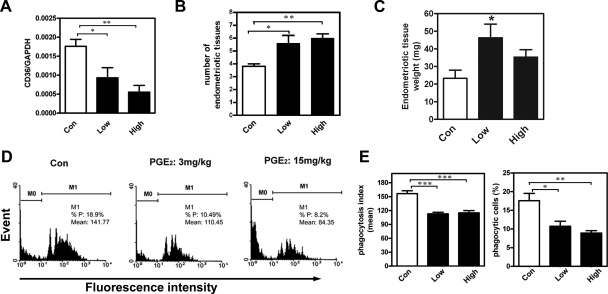
We then analyzed levels of CD36 mRNA in macrophages isolated from peritoneal fluid of mice treated with vehicle or a low dose (3 mg/kg body weight) or high dose (15 mg/kg body weight) of PGE2. Quantitative RT-PCR revealed a significant dose-dependent decrease in CD36 mRNA levels in peritoneal macrophages of PGE2-treated mice (Figure 6A), which was consistent with results for human macrophage experiments (Figure 2A). The mRNA level of other scavenger receptors such as SR-AI, SR-BI, and SR-BII in mouse peritoneal macrophages did not differ between vehicle and PGE2-treated groups (Supplemental Figure S2A at http://ajp.amjpathol.org). Interestingly, CD36 levels on macrophages correlated inversely with the number of adherent lesions in mice (Figure 6B). The total wet-weight of endometriotic-like tissues was greater in mice treated with 3 mg/kg body weight PGE2 compared with vehicle-treated mice (Figure 6C). Mice treated with 15 mg/kg body weight PGE2 developed diarrhea, and thus the cystic lesion weight was not statistically different (P = 0.084) from vehicle-treated mice (Figure 6C).
Figure 6.
PGE2 inhibits the phagocytic ability of peritoneal macrophages and enhances endometriosis formation in vivo (n = 10 animals per group). A: Mean and SD of levels of CD36 mRNA on peritoneal macrophages freshly isolated from mice treated with vehicle (Con), 3 mg/kg body weight PGE2 (Low), or 15 mg/kg body weight PGE2 (High). B: Mean and SD of the number of endometriotic-like lesions in mice treated without or with PGE2 for 4 weeks. C: Mean and SD of total wet-weight of endometriotic-like lesions in mice treated without or with PGE2 for 4 weeks. D: Representative flow cytometric results of the phagocytic ability of peritoneal macrophages freshly isolated from mice subjected to surgery-induced endometriosis. M0: no bead was ingested; M1: with at least one bead ingested. E: Percent of phagocytic macrophages and mean phagocytosis index of peritoneal macrophages freshly isolated from mice subjected to surgery-induced endometriosis. *P < 0.05, **P < 0.01, ***P < 0.001, as compared with control.
To characterize whether the observed increase in endometriotic adhesive lesions in PGE2-treated mice was due to decreased phagocytic ability of macrophages, a phagocytosis assay was performed using peritoneal macrophages freshly isolated from vehicle or PGE2-treated mice. As expected, peritoneal macrophages derived from PGE2-treated mice (those with lower levels of CD36) had significantly lower phagocytic ability (Figure 6D). The mean percentage values (n = 10/group) of phagocytic peritoneal macrophages were 17.6 ± 2.0%, 12.3 ± 1.9%, and 10.6 ± 1.8% for the vehicle, low-dose, and high-dose PGE2-treated groups, respectively (Figure 6E), and the corresponding phagocytosis index values were 156.6 ± 6.3, 112.9 ± 3.5, and 115.1 ± 4.8 (Figure 6E). These data demonstrate that PGE2 significantly increases the number of endometriotic lesions probably by inhibiting the phagocytic activity of peritoneal macrophages.
Inhibition of Peritoneal PGE2 Production Suppresses Endometriosis
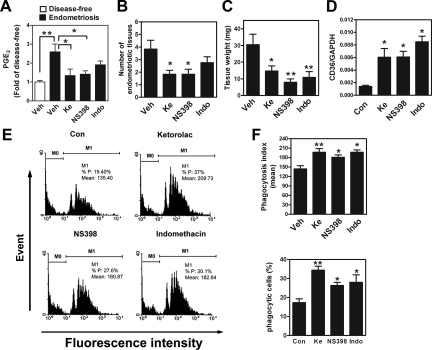
Because PGE2 can suppress the phagocytic activity of macrophages and consequently increase the number of endometriotic lesions, we next evaluated the potential of preventive/therapeutic intervention by abolishing local PGE2 production. Induction of endometriosis in mice caused an increase in the peritoneal PGE2 level, but that elevated level was significantly reduced on treatment with a selective COX-1 inhibitor (ketorolac)35 or a selective COX-2 inhibitor (NS398) (Figure 7A); a COX-1/2 inhibitor (indomethacin) only partially reduced the elevated level peritoneal PGE2 concentration (Figure 7A). Concordantly, a significant decrease in the number of established lesions was observed in the ketorolac and NS398 groups (Figure 7B); treatment with indomethacin apparently reduced the number of lesions by 32%, but this change was not statistically significant owing to large interindividual variations (Figure 7B). Nevertheless, the endometriotic lesions were smaller (Figure 5C), and the total wet-weight of established lesions was reduced in all treatment groups (Figure 7C).
Figure 7.
Inhibition of PGE2 production increases the phagocytic ability of macrophages and reduces endometriosis formation in vivo (n=10 animals per group). A: Concentrations of PGE2 in the peritoneal fluid of mice without (disease-free) or with surgery-induced endometriosis. Mice with endometriosis were further divided into four groups and treated with vehicle (Veh), COX-1 inhibitor (ketorolac, Ke, 10 mg/kg body weight), COX-2 inhibitor (NS398, 10 mg/kg body weight), or COX-1/-2 inhibitor (indomethacin, Indo, 10 mg/kg body weight) for 4 weeks. B: Mean and SD of the number of endometriotic-like lesions in mice treated with COX inhibitors. C: Mean and SD of total wet-weight of endometriotic lesions in mice treated as described above. D: CD36 expression in mouse peritoneal macrophages after surgery-induced endometriosis and drug treatment. E: Representative flow cytometric results of phagocytic ability of freshly isolated peritoneal macrophages after surgery-induced endometriosis and drug treatment. F: Mean and SD of the percent of phagocytic macrophages (lower panel) and mean phagocytosis index (upper panel) in peritoneal macrophages freshly isolated from surgery-induced endometriotic mice. M0: no bead was ingested; M1: with at least one bead ingested *P < 0.05, **P < 0.01, ***P < 0.001, as compared with control.
When peritoneal macrophages were isolated and analyzed, treatment with the various COX inhibitors led to an increase in CD36 mRNA expression (5.3-fold for ketorolac, 2.95-fold for NS398, and 5.23-fold for indomethacin, compared with control) (Figure 7D) without affecting the mRNA levels of other scavenger receptors (Supplemental Figure S2B at http://ajp.amjpathol.org). Most importantly, when peritoneal macrophages isolated from COX inhibitor-treated groups were used in the phagocytosis assay, both the percentage of phagocytic macrophages and the phagocytosis index increased markedly over control values (Figure 7, E and F). Taken together, these data clearly demonstrate that inhibition of PGE2 production is an effective method to reduce the formation of endometriosis.
Discussion
Immune dysfunction has been proposed to play important roles in endometriosis development,36,37,38 but the mechanism by which the phagocytic capability of macrophages is suppressed is poorly understood, largely owing to the difficulty of collecting peritoneal macrophages from patients. In this study, we provide in vitro and in vivo evidence to demonstrate that PGE2, one of the pro-inflammatory factors produced by endometriotic tissue and peritoneal macrophages,27,39,40,41 inhibits the expression of CD36 by macrophages, thereby suppressing their phagocytic ability. This suppression contributed to the development of endometriosis, and blockade of PGE2 production by nonsteroidal anti-inflammatory drugs significantly enhanced the phagocytic capability of macrophages and effectively reduced the number and size of endometriotic lesions. These data provide both a molecular basis for macrophage loss of function during the development of endometriosis and a functional link between COX-2 overexpression and endometriosis progression.27,39,40,41
CD36, in addition to its well-established function of uptake of oxidized low-density lipoprotein,8 was one of the first macrophage receptors to be implicated in the recognition of aged or apoptotic cells.42,43 Owing to its broad ligand-binding specificity, CD36 has been reported to contribute to several pathological processes such as atherosclerosis and Alzheimer’s disease.8,19,20,21 Recently, we reported that decreased CD36 expression in macrophages is associated with the pathogenesis of endometriosis.15 CD36 levels are markedly reduced in peritoneal macrophages derived from endometriosis patients, and those macrophages are deficient in phagocytic ability.15 In our present study, we demonstrated that PGE2 is a potent locally acting factor that inhibits the expression of CD36 in peritoneal macrophages. Administration of PGE2-inhibited CD36 expression in both primary-culture peritoneal macrophages and TPA-induced monocytic U937cells, indicating that this effect is likely to be a common phenomenon. More importantly, we demonstrated that ectopic expression of CD36 in peritoneal macrophages derived from women with endometriosis not only increased basal phagocytic activity but also restored PGE2-inhibited phagocytic ability. Taken together, our data and previous findings clearly indicate that CD36 plays an indispensible role in controlling the phagocytic ability of macrophages and the development and severity of endometriosis.
The signaling that occurs downstream of PGE2 is very complex, as actions of PGE2 can be mediated by one or more of the four EP receptors. In this study, we demonstrated that inhibition of CD36 expression by PGE2 was mediated by the EP2 receptor. PGE2, via binding to the EP2 receptor, can activate protein kinase A and the mitogen-activated protein kinase pathway.44 Indeed, administration of the protein kinase A inhibitor, H89, or mitogen-activated protein kinase kinase inhibitor, U0126, significantly reversed PGE2-induced CD36 down-regulation. In contrast, treatment of macrophages with an EP2 receptor agonist was sufficient to inhibit CD36 expression. Suppression of CD36 by PGE2 probably occurs at the transcriptional level because our preliminary data revealed that PGE2 can inhibit CD36 promoter activity (Chuang, unpublished data). We are currently trying to elucidate the molecular mechanism responsible for inhibition of CD36 gene expression by PGE2. Nevertheless, our current study shows for the first time that EP2 receptor-dependent PGE2 signaling is an important factor for impairing macrophage function in endometriosis.
Overexpression of COX-2 in endometriotic tissue and peritoneal macrophages of women with endometriosis has been documented.27,39,40,41 The concentration of PGE2 in peritoneal fluid collected from endometriosis patients is markedly higher compared with that collected from healthy women.27 In this study, we also demonstrated that the PGE2 level in peritoneal fluid of surgery-induced endometriotic mice was greater than that in sham control animals. We previously showed that the level of peritoneal PGE2 in endometriosis patients was sufficient to activate the EP2 receptor-dependent signaling pathway in macrophages to suppress the expression and enzymatic activity of matrix metalloproteinase-9.34 Taken together, these results strongly suggest that peritoneal PGE2 is the endogenous factor that suppresses the expression of CD36 in macrophages. Indeed, peritoneal macrophages from mice subjected to surgery-induced endometriosis (with greater PGE2 concentration) expressed less CD36 and had reduced phagocytic ability. In contrast, inhibition of peritoneal PGE2 production by COX inhibitors significantly enhanced CD36 expression and the phagocytic ability of peritoneal macrophages. These data provide direct evidence that overproduction of PGE2 in peritoneal fluid can inhibit the expression of CD36 by macrophages and thus reduce their phagocytic ability.
The multiple functions of PGE2 in controlling the development of endometriosis suggest it as a promising therapeutic target. Clinically, nonsteroidal anti-inflammatory drugs have been used as pain relievers for endometriosis patients. In this study, we demonstrated that daily injection of nonsteroidal anti-inflammatory drugs could effectively suppress the number and size of endometriotic lesions in mice. The important clinical challenge is how to effectively inhibit the aberrant production of PGE2 in endometriosis patients. The short in vivo half-lives of currently available COX inhibitors hampers the treatment efficiency because the continuous expression of COX-1 and COX-2 by peritoneal macrophages27 and ectopic lesions41 often overwhelms the inhibitory effect of the drugs. In addition, the potentially severe adverse effects of nonsteroidal anti-inflammatory drugs make them unfavorable for long-term use. Therefore, in light of our current results, restoring or enhancing the phagocytic capability of macrophages by targeting the EP2 signaling pathway may represent a new direction in the development of strategies to treat endometriosis.
Acknowledgments
We thank Ms Ya-Hui Wang for excellent technical help on flow cytometry. We also thank Ono Pharmaceutical Company for the generous gift of all of the EP receptor agonists.
Footnotes
Address reprint requests to Shaw-Jenq Tsai, Ph.D., Department of Physiology, National Cheng Kung University Medical College, Tainan 701, Taiwan, Republic of China, E-mail: seantsai@mail.ncku.edu.tw.
Supported by grants from National Science Council of Taiwan, Republic of China (NSC95-2320-B-006-047-MY3 and NSC97-2314-B-006-020-MY3).
Ono Pharmaceuticals Company supplied all EP receptor agonists.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–425. [Google Scholar]
- Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med. 2007;9:1–20. doi: 10.1017/S146239940700021X. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann NY Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. doi: 10.1530/jrf.0.0820707. [DOI] [PubMed] [Google Scholar]
- Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35:696–698. doi: 10.1016/s0015-0282(16)45567-6. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton MF, Fazio S. Class A scavenger receptors, macrophages, and atherosclerosis. Curr Opin Lipidol. 2001;12:489–495. doi: 10.1097/00041433-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PC, Wu MH, Shoji Y, Tsai SJ. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol. 2009;219:232–241. doi: 10.1002/path.2588. [DOI] [PubMed] [Google Scholar]
- Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–1115. [PubMed] [Google Scholar]
- Alessio M, Ghigo D, Garbarino G, Geuna M, Malavasi F. Analysis of the human CD36 leucocyte differentiation antigen by means of the monoclonal antibody NL07. Cell Immunol. 1991;137:487–500. doi: 10.1016/0008-8749(91)90096-t. [DOI] [PubMed] [Google Scholar]
- Guy E, Kuchibhotla S, Silverstein R, Febbraio M. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36o/apoEo mice. Atherosclerosis. 2007;192:123–130. doi: 10.1016/j.atherosclerosis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta M, Rigamonti AE, Scarpini E, Galimberti D, Bonomo SM, Venturelli E, Muller EE, Cella SG. The leukocyte expression of CD36 is low in patients with Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2007;28:515–518. doi: 10.1016/j.neurobiolaging.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Janabi M, Yamashita S, Hirano K, Sakai N, Hiraoka H, Matsumoto K, Zhang Z, Nozaki S, Matsuzawa Y. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 2008;149:1180–1189. doi: 10.1210/en.2007-1168. [DOI] [PubMed] [Google Scholar]
- ASRM The American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis:1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Chuang PC, Sun HS, Chen TM, Tsai SJ. Prostaglandin E2 induces fibroblast growth factor 9 via EP3-dependent protein kinase Cdelta and Elk-1 signaling. Mol Cell Biol. 2006;26:8281–8292. doi: 10.1128/MCB.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol. 2007;170:590–598. doi: 10.2353/ajpath.2007.060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Lu CW, Huang BM, Wu MH, Tsai SJ. Cyclic adenosine 3′,5′-monophosphate response element-binding protein and CCAAT/enhancer-binding protein mediate prostaglandin E2-induced steroidogenic acute regulatory protein expression in endometriotic stromal cells. Am J Pathol. 2008;173:433–441. doi: 10.2353/ajpath.2008.080199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P. Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod. 1999;14:2944–2950. doi: 10.1093/humrep/14.12.2944. [DOI] [PubMed] [Google Scholar]
- Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC, Huang MF, Tsai SJ. Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis. Am J Pathol. 2005;167:1061–1069. doi: 10.1016/S0002-9440(10)61195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA: 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmowski WP, Braun D, Gebel H. Endometriosis: genetic and immunologic aspects. Prog Clin Biol Res. 1990;323:99–122. [PubMed] [Google Scholar]
- Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- Raiter-Tenenbaum A, Baranao RI, Etchepareborda JJ, Meresman GF, Rumi LS. Functional and phenotypic alterations in peritoneal macrophages from patients with early and advanced endometriosis. Arch Gynecol Obstet. 1998;261:147–157. doi: 10.1007/s004040050214. [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16:561–566. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87:3263–3273. doi: 10.1210/jcem.87.7.8594. [DOI] [PubMed] [Google Scholar]
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90:286–295. doi: 10.1210/jc.2004-1612. [DOI] [PubMed] [Google Scholar]
- Navazo MD, Daviet L, Savill J, Ren Y, Leung LL, McGregor JL. Identification of a domain (155-183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J Biol Chem. 1996;271:15381–15385. doi: 10.1074/jbc.271.26.15381. [DOI] [PubMed] [Google Scholar]
- Trial J, Rice L. Erythropoietin withdrawal leads to the destruction of young red cells at the endothelial-macrophage interface. Curr Pharm Des. 2004;10:183–190. doi: 10.2174/1381612043453423. [DOI] [PubMed] [Google Scholar]
- Sun HS, Hsiao KY, Hsu CC, Wu MH, Tsai SJ. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology. 2003;144:3934–3942. doi: 10.1210/en.2003-0289. [DOI] [PubMed] [Google Scholar]