Abstract
Immune mediators and leukocyte engagement of brain microvascular endothelial cells (BMVECs) contribute to blood–brain barrier impairment during neuroinflammation. Glycogen synthase kinase 3β (GSK3β) was recently identified as a potent regulator of immune responses in in vitro systems and animal models. However, the role of GSK3β in regulation of immune endothelial functions remains undetermined. Here we evaluated the effect of GSK3β inhibition on the regulation of inflammatory responses in BMVECs. A focused PCR gene array of 84 genes was performed to identify the cytokine and chemokine gene expression profile in tumor necrosis factor (TNF) α-stimulated BMVECs after GSK3β inactivation by specific inhibitors. Fifteen of 39 genes induced by TNFα stimulation were down-regulated after GSK3β inhibition. Genes known to contribute to neuroinflammation that were most negatively affected by GSK3β inactivation included IP-10/CXCL10, MCP-1/CCL2, IL-8/CXCL8, RANTES/CCL5, and Groα/CXCL1. GSK3β suppression resulted in diminished secretion of these proinflammatory mediators by inflamed BMVECs detected by ELISA. GSK3β inhibition in BMVECs reduced adhesion molecule expression as well as monocyte adhesion to and migration across cytokine stimulated BMVEC monolayers. Interactions of monocytes with TNFα-activated BMVECs led to barrier disruption, and GSK3β suppression in the endothelium restored barrier integrity. GSK3β inhibition in vivo substantially decreased leukocyte adhesion to brain endothelium under inflammatory conditions. In summary, inhibition of GSK3β emerges as an important target for stabilization of the blood–brain barrier in neuroinflammation.
The blood–brain barrier (BBB) is composed of endothelial cells with a unique phenotype. Compared with endothelial cells from other vascular beds, brain microvascular endothelial cells (BMVECs) characteristically have very low permeability to solutes, high electrical resistance, complex tight junctions, and an array of transport systems that both supply the brain with nutrients and eliminates byproducts of brain metabolism.1 Low permeability is thought to be important in protecting the brain from toxins circulating in the blood as well as restricting the migration of leukocytes into the neuropil. Neuroinflammation can lead to a loss of barrier function, which is manifested by an increase in permeability. This breach of the barrier results in accumulation of serum neurotoxins and proteins exacerbating brain inflammatory response and neuronal injury.2 The triggers of BBB permeability occurring during the course of neuroinflammation (ie, multiple sclerosis and HIV-1 encephalitis [HIVE]) include proinflammatory mediators and leukocyte engagement of the BMVECs.3 As a consequence of immune and endothelial cell interactions, the BBB could be further compromised because of enhanced and continuous passage of immune cells across the endothelium. It is this combination of immune cells and immune mediators, such as proinflammatory cytokines and chemokines, which contributes to the disruption of neuronal homeostasis.3
Glycogen synthase kinase 3β (GSK3β) is a ubiquitous serine/threonine protein kinase, which is involved in numerous and diverse biological functions including: glycogen metabolism, regulation of cell division, differentiation, and apoptosis.4 Unlike most kinases, GSK3β is constitutively active in cells, and a wide range of extracellular stimuli exerts their effects by inhibiting GSK3β activity.5 GSK3β activity is regulated by signals originating from numerous signaling pathways (for example, the phosphoinositide 3-kinase-AKT pathway, protein kinase A, protein kinase C, and the WNT pathway) which lead to inhibition of the kinase by phosphorylation of the Ser 9 residue in the N-terminal domain of GSK3β (inactive GSK3β).6 However, phosphorylation at the tyrosine 216 residue of GSK3β either by autophosphorylation or by other kinases increases the activity of the kinase (active GSK3β).7
Recently, GSK3β has been implicated as a key regulator of the inflammatory response. The anti-inflammatory effects of GSK3β inhibition have been shown in vitro and in several in vivo models of acute and chronic inflammation.4,8,9 In the endotoxin shock model, GSK3β inhibition attenuated multiorgan injury, improved survival rates, and decreased proinflammatory cytokine production before and after the administration of lethal doses of Escherichia coli (E. coli) lipopolysaccharide (LPS).8,10 These anti-inflammatory effects of GSK3β inhibitors are in part attributable to suppression of the inflammatory response in the vascular endothelium. GSK3β inactivation prevented vascular cell adhesion molecule-1 (VCAM-1) upregulation by tumor necrosis factor (TNF) α in aortic endothelial cells11 and E-selectin expression in umbilical vein endothelium.12 GSK3β inhibition has also been shown to promote endothelial barrier properties in pulmonary arterial endothelial cells.13 Anti-inflammatory and neuroprotective effects of GSK3β inhibition have been shown in models of stroke14 and spinal cord injury.15 Although it has been shown that GSK3β inhibition can attenuate inflammation globally and in many cell types, the role of GSK3β in regulating inflammatory responses in the brain endothelium remains largely unexplored.
Using primary human BMVECs we investigated the effects of GSK3β inhibition under inflammatory conditions. Suppression of GSK3β activity in inflamed brain endothelium prevented the enhanced adhesion of leukocytes in vitro and in vivo, down-regulated expression of adhesion molecule, protected barrier function, and diminished migration of monocytes across BMVEC monolayers in response to relevant proinflammatory factors. Furthermore, GSK3β inhibition down-regulated secretion of inflammatory factors in TNFα-stimulated BMVECs. Relevance of these observations was further confirmed by the presence of active GSK3β in brain endothelium in human brain tissue affected by neuroinflammation. These results suggest that GSK3β could be a potential target for the treatment of BBB injury associated with proinflammatory insult and leukocyte infiltration of the central nervous system, thus providing both an anti-inflammatory and neuroprotective effect.
Materials and Methods
Cell Culture and Reagents
Primary cultures of human BMVECs (provided by Drs. Michael Bernas and Marlys Witte, University of Arizona, Tucson, AZ) were isolated from microvessels derived from temporal or hippocampal tissue removed during operative treatment of epilepsy as described.16 Tissue used for BMVEC isolation was outside epileptogenic foci. The procedures were approved by the Temple University Institutional Review Board. BMVEC cultures were expanded and maintained in DMEM/F-12 media containing 10% fetal bovine serum, endothelial cell growth supplement (BD, Franklin Lakes, NJ), heparin (1 mg/ml, Sigma, St. Louis, MO), amphotericin B (2.5 μg/ml), penicillin (100 U/ml), and streptomycin (10 μg/ml). BMVEC cultures were used at low passage (between 2 and 5). Unless otherwise stated, reagents used for cell culture were purchased from Invitrogen (Carlsbad, CA).
The following four GSK3β inhibitors were tested: lithium chloride (LiCl, used at 2 mmol/L or 10 mmol/L, purchased from Sigma/Aldrich Co., Ltd.; St. Louis, MO), 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8, used at 0.5 μmol/L or 1 μmol/L, purchased from Calbiochem; San Diego, CA), 5-iodo-indirubin-3′-monoxime (I3′M, used at 1 μmol/L or 3 μmol/L, purchased from Calbiochem) and N-(4-methoxybenzyl)-N′-(5-nitro-1,3-thiazol-2-yl)urea (AR-A014418, used at 5 μmol/L or 10 μmol/L purchased from Sigma/Aldrich). Concentrations of inhibitors were based on previously published work11,17 and our initial dose-dependent experiments with different exposure times. The selected doses did not cause toxicity in the BMVECs when cells were treated at short (4 hours) or for longer (24 hours) periods of time (Supplemental Figure S1, see http://ajp.amjpathol.org).
Human peripheral blood monocytes were obtained from the Human Immunology Core at the University of Pennsylvania (Philadelphia, PA). The core facility isolates monocytes by countercurrent elutriation, generating greater than 90% purely enriched monocytes. Monocytes were used within 24 hours of elutriation and were maintained in DMEM media containing heat-inactivated 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 U/ml), and l-glutamine (2 mmol/L).
PCR Array (Inflammatory Response and Autoimmunity)
A PCR-based microarray for evaluating the expression of genes commonly involved in inflammatory response was performed using the RT2-Profiler PCR Array (SABioscience Corporation, Frederick, MD). The array (PAHS-077C) is configured in a 96-well plate consisting of a focused panel of 84 gene-specific primer sets, along with primers for five housekeeping genes and assay controls. Briefly, total RNA was isolated using the RT2 qPCR-Grade RNA Isolation Kit (SABioscience) from BMVEC monolayers exposed to various experimental conditions, as indicated in the figure. The RNA was then converted to first-strand cDNA using the RT2 First Strand Kit (SABioscience). Next, the cDNA was added to a RT2 SYBR® Green qPCR Master Mix (SABioscience) and then introduced to the array plates. The real-time PCR and data collection was then performed using a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). The amplification data were analyzed using the ΔΔCt method, using the web-based PCR array data analysis software available from SABioscience. After normalization to internal controls, the gene fold expression was calculated from the difference between the gene expression of the experimental condition and the untreated control.
Cytokine and Chemokine ELISA
Genes identified from the PCR array to be affected by GSK3β inhibitors during inflammatory insult were evaluated for protein expression using ELISA. After BMVECs were treated as indicated in the figure, the conditioned medium was collected and the following secreted products were measured: IFNγ-inducible protein of 10 kDa (IP-10) or CXCL10, monocyte chemoattractant protein 1 (MCP-1) or CCL2, interleukin (IL-8) or CXCL8, IL-6, regulated on activation normal T cell expressed and secreted (RANTES) or CCL5, and growth-related oncogene α (Gro α) or CXCL1. Secreted products IP-10/CXCL10, MCP-1/CCL2, IL-8 and RANTES/CCL5 were detected using conventional double sandwich ELISA from RayBiotech Inc. (Norcross, GA). Production of CXCL1/Groα and IL-6 was detected with ELISAs from R&D Systems (Minneapolis, MN). Assays were performed according to the manufacturer’s instructions.
Flow Cytometry
Analysis of the surface expression of adhesion molecules in BMVECs was performed by flow cytometry. Briefly, cells were detached with 2 mmol/L EDTA at 4°C and washed with PBS, then incubated with staining solution (2% BSA in PBS with 0.5% sodium azide). Fluorochrome conjugated antibodies intracellular adhesion molecule-1 (ICAM-1)–allophycocyanin, and VCAM-1–FITC (BD Biosciences) were then added to the cells at the concentration suggested by the manufacturer, and incubated for 30 minutes on ice. Cells were then washed with staining buffer and with PBS, then fixed with 2% methanol-free formaldehyde (Thermo Scientific, Rockford, IL) in PBS. The labeled cells were then acquired using a FACSCalibur flow cytometer (BD Biosciences). Acquisition parameters and gating was performed with CellQuest software (BD Biosciences) and analysis of the data were performed with FlowJo software (Tree Star Inc, Ashland, OR). At least three independent experiments using cells from different donors were performed. Representative histograms are shown indicating the median fluorescence intensity of at least 10,000 events, expressed as the percentage of the maximum median fluorescence intensity.
Transendothelial Electrical Resistance
To determine the effect of GSK3β inhibitors on the compromised barrier integrity of stimulated brain endothelial monolayers exposed to monocytes, transendothelial electrical resistance (TEER) measurements were performed using the 1600R ECIS System (Applied Biophysics, Troy, NY). Using the free ions in the culture media, the instrument generates an AC current flow between an electrode and counterelectrode located in specialized tissue culture arrays and measures the change in impedance. The instrument measures real-time complex impedance, providing readouts for impedance, resistance, and capacitance. Briefly, endothelial cells were plated on collagen type I–coated electrode arrays (8W10E, Applied Biophysics) and monitored until confluence and monolayer formation occurred. After achieving basal TEER readings attributable to monolayer and tight junction formation, the endothelial cells were exposed to the following experimental conditions: untreated (control), TNFα (20 ng/ml), AR-A014418, and I3′M (GSK3β inhibitors). Untreated or stimulated endothelial cells in the presence of GSK3β inhibitors were also exposed to monocytes added at 5 × 104 cells per well. Measurements were taken every 10 minutes at 400 hz. Average baseline TEER readings varied between 1500 and 2400 Ω/cm2. The results are represented as the average percent change of baseline TEER (expressed as average ± SEM) from at least three independent experiments consisting of at least two replicates.
Adhesion and Migration Assays
Monocyte cell adhesion to BMVECs was measured using a fluorescence-based assay as previously described.16 Briefly, BMVECs were plated at a density of 2.5 × 104 cells per well on 96-well plates. After formation of monolayers, the BMVECs were activated with TNFα (20 ng/ml) or simultaneously treated with the indicated test compounds for 24 hours. Thereafter, monocytes were labeled with the fluorescent probe, calcein-am (Invitrogen). The adhesion assay was started after the introduction of fluorescently labeled monocytes (2.5 × 105 cells per well) to the endothelial monolayers. The cells were incubated for 15 minutes to allow adhesion and then rinsed 3 times with PBS to eliminate nonadherent monocytes. Fluorescence measurements from attached monocytes were acquired with a Spectramax fluorescence plate reader (Molecular Devices, Sunnyvale CA). Calculations for determining the number of adherent cells were derived from the fluorescence measurements of external standards. The results are represented as the mean ± SEM fold adhesion (number of adherent monocytes in experimental condition divided by the basal adhesion of the untreated control).
The transendothelial migration assay was performed using a previously described method.16 FluoroBlok (BD Falcon) cell culture inserts, designed to block the transmission of fluorescent light between 490 and 700 nm, were used in the assay to allow for continuous detection of fluorescently labeled monocytes migrating across endothelial monolayers. Briefly, BMVECs were plated at a density of 2.5 × 104 cells per insert on type I collagen–coated FluoroBlok inserts. After one week in culture, the endothelial cells were evaluated for typical barrier formation by measurement of TEER using a voltmeter (EVOM, World Precision Instruments, Sarasota, FL). Then the BMVECs were stimulated for 24 hours with TNFα (20 ng/ml) or IL-6 (10 ng/ml) in the presence or absence of the test compounds, as indicated. After incubation with the test compounds, the BMVECs were rinsed and the medium was replaced. The migration assay was then started by addition of calcein-am fluorescently labeled monocytes. Monocytes were added at a density of 1 × 105 cells per insert to the endothelial monolayers, and CCL2 (50 ng/ml) chemoattractant was added to the lower chamber. After incubation, the fluorescence of migrating monocytes across the BMVECs was measured with a Spectramax fluorescence plate reader (Molecular Devices). The number of migrating monocytes was calculated from external standards of labeled monocytes. The data are shown as the average fold migration ± SEM; the fold migration is derived from the number of migrated monocytes from each experimental condition divided by the number of migrated monocytes in the untreated, no chemoattractant control.
In Vivo Measurement of Leukocyte-Endothelial Interactions
The in vivo leukocyte adhesion studies were performed on 8-week-old male C57BL/6 mice from Taconic Farms (Hudson, NY). All experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee at Temple University. Cranial windows were implanted under anesthesia (i.p. injection of ketamine [100 mg/ml] and xylazine [20 mg/kg] mixture [1:1] at a dose of 1 ml/kg). The head was shaved and positioned in a stereotactic head holder. A 1-cm area of skin on the dorsal surface of the skull over the right cortical hemisphere was excised and the periosteum was removed. A 4-mm-diameter circular craniotomy was performed using a high-speed drill (Champ-Air Dental Drill Benco Dental, Dallas, TX) over the right parietal cortex extending from attachment of the temporal muscle to the midpoint of the sagittal suture in the coronal direction and aligned to the middle of the sagittal suture. A 5-mm coverslip was then placed over the exposed brain, and an airtight seal was produced using Nexaband Quick Gel. A recovery period of four days was allowed between implantation of the cranial window and intravital microscope observation.
On the day of the experiment, animals were anesthetized and immobilized. Intravital microscopy was performed with an epiluminiscence microscope (BX10, Olympus, Japan) equipped with a digital camera Cooke 1600 (Cooke Corporation, Romulus, MI). Leukocytes were stained in vivo by a bolus injection of 50 μl of a 0.01% solution of rhodamine 6G (Sigma/Aldrich, St Louis, MO) into the facial vein as described.18 Leukocytes were visualized by fluorescent light (601 nm excitation). Selective filtering allowed visualization of the fluorescent cells on a dark background. The image from the camera was then displayed on the computer monitor, captured, and recorded by Camfire software at a video frame rate of 25 frames per second. Effects of GSK3β inhibition on leukocyte adhesion elicited by LPS (E. coli 0127:B8, 6 mg/kg, Sigma/Aldrich) were studied at 0, 4, and 24 hours. GSK3β inhibitors (AR-A014418 at 5 mg/kg and TDZD-8 at 2 mg/kg) were injected i.p. simultaneously with LPS. LPS used at this dose has been shown to cause multiorgan failure that was prevented by GSK3β inhibition.8 Adhering leukocytes were defined as the total number of the leukocytes firmly attached to the microvascular endothelium that did not change their location during the entire 30 seconds of observation period. Adhering leukocytes were scored as the number of cells per mm2 of the vascular surface area, calculated from the diameter and standardized length (100 μm) of the vessel segment under investigation.
Human Brain Tissue
Frontal cortex specimens derived from five HIVE cases of different severity (three severe and two moderate according to previously described criteria)19 and four seronegative age-matched controls were provided by the National NeuroAIDS Consortium (Washington, DC). Approval was obtained from the Institutional Review Board for these studies. Serial frozen sections (5 μm thick) were cut and double immunostained (indirect immunofluorescence) for the endothelial cell marker, rhodamine labeled Ulex europeus agglutinin 1 (2 μg/ml, Vector Laboratories, Burlingame, CA), total GSK3β (dilution 1:50, Abcam, Cambridge, MA), GSK3β active form phosphorylated on tyrosine 216 (Tyr216 from Abcam, dilution 1:50), and active GSK3α/β at Tyr216/279 (Abcam, dilution 1:50).
Statistical Analysis
The values shown in all figures and those mentioned in the text represent the average ± SEM. For the RT-PCR array, the ΔΔCt method was used and the data are expressed as fold differences between cytokine-stimulated (with or without) inhibitor and untreated. Multiple group comparisons were performed by one-way analysis of variance with Dunnett’s posthoc tests (adhesion assay, migration assay, FACS, and RT-PCR array). Unpaired two-tailed Student t test was used to compare the values between two treated groups (indicated by brackets) from the resulting leukocyte adhesion acquired under cranial window. Statistical analyses were performed using Prism v5 software (GraphPad Software Inc., La Jolla, CA) and the web based RT2 Profiler PCR Array Data Analysis suite (SA Biosciences). Differences were considered significant at P values <0.05.
Results
Active Forms of GSK3β Are Found in Brain Endothelium in Neuroinflammation
First, we established whether activated forms of GSK3β are present within human brain tissues affected by neuroinflammation (like HIVE). We used antibodies detecting the active form of GSK3β (p-GSK3β, phosphorylated at Tyr216) and GSK3α/β (p-GSK3α/β, phosphorylated at Tyr216/Tyr279). Control brains demonstrated minimal to no staining with antibodies to p-GSK3β (Figure 1, A–D) and p-GSK3α/β (data not shown). Antibodies to p-GSK3β highlighted endothelial cells in microvessels in brain tissues affected by HIVE (Figure 1, E–H, I–L, and M–P). In addition, glial cells and mononuclear cells infiltrating perivascular spaces in HIVE cases were also stained with p-GSK3 antibodies while there was no immunostaining in controls (data not shown). There was no difference in staining for total GSK3β in control and HIVE brain tissues (data not shown). These data suggest that GSK3β is indeed activated in brain endothelium during neuroinflammation (such as HIVE).
Figure 1.
Active forms of GSK3β detected in brain endothelium in HIV encephalitis (HIVE). Five-μm-thick frozen sections of control human brains (A–D) or HIVE (E–P) were cut. The sections were triple immunostained with Ulex europeus agglutinin 1 (endothelial marker, red, A, E, I, M) and anti–p-GSK3 antibodies (green, B, F, J, N) or DAPI (blue, C, G, K, O). Arrows depict locations of microvessels in panels A–P. Microvessels in control brain tissue (A) showed minimal staining for p-GSK3β (B, green, arrow). In HIVE, the brain endothelium (E, I, M) featured a high level of p-GSK3β (green, arrows, F, J, N). Original magnification: ×200 (A–P).
GSK3β Inhibitors Suppress Expression of Proinflammatory Genes in TNFα-Stimulated BMVECs
It has been previously shown that during inflammatory conditions, GSK3β inhibitors can down-regulate expression of key inflammatory mediators in various cell types. To evaluate whether the anti-inflammatory effect of GSK3β inhibition applies to brain endothelial cells, we profiled the expression of genes commonly involved in the regulation of inflammatory response and autoimmunity. Using a commercial PCR-based focused array, 84 genes relevant to inflammatory responses (ie, cytokines/chemokines and their respective receptors) were analyzed in untreated BMVECs (control), BMVECs activated with TNFα (20 ng/ml, 24 hours), and TNFα-activated BMVECs in the presence of the GSK3β inhibitors, AR-A014418, or I3′M. The array analyses revealed that 39 gene targets were upregulated more than twofold in the TNFα-treated BMVECs when compared with untreated control cells. The addition of either GSK3β inhibitor resulted in the suppression of 15 genes of the 39 upregulated by TNFα. Figure 2, A and B, shows the CC chemokines, CXC chemokines and other proinflammatory mediators that were most affected by inhibition of GSK3β. When compared with BMVECs that were treated with TNFα only, cytokine-stimulated BMVECs exposed to I3′M showed a 99.9% reduction in IP-10/CXCL10 expression (8.34 × 104 down to 59.8 fold), an 89.3% attenuation in CCL2 expression (323.5 down to 27.3 fold), a 70% decrease in CXCL8 expression (274.7 down to 65.4 fold), a 60% diminution in CCL5 (45.6 down to 15.3), and an 82.3% suppression for CXCL1 expression (17.8 down to 1.3 fold). A similar degree of gene inhibition was observed with a second GSK3β inhibitor, AR-A014418. When compared with the TNFα-activated BMVECs, AR-A014418 exposure resulted in a 99.8% reduction of CXCL10 expression (8.34 × 104 down to 728.7 fold), an 87% decrease in CCL2 expression (323.5 down to 42.1 fold), a 60% attenuation of CXCL8 expression (274.7 fold down to 99.5), a 55% decrease in CCL5 expression (45.6 down to 20.5 fold), and a 74% diminution in CXCL1 expression (17.8 down to 4.8 fold). Of note, resting BMVECs treated with GSK3β inhibitors showed no induction of inflammatory genes or toxicity (data not shown). A list of genes that were upregulated by TNFα but showed no significant suppression by any of the GSK3β inhibitors are provided in Supplemental Table S1, see http://ajp.amjpathol.org. Taken together, the PCR array analyses indicate that inactivation of GSK3β suppresses the induction of key proinflammatory mediators in brain endothelial cells.
Figure 2.
GSK3β inhibitors reduced expression of inflammatory genes identified by RT2 Profiler arrays in activated BMVECs. A real-time PCR array was used to profile inflammatory response from BMVECs treated in the presence or absence of TNFα (20 ng/ml, 24 hours) or coincubated with either of the following GSK-3β inhibitors: I3′M (3 μmol/L) or AR-A014418 (10 μmol/L). Total RNA was extracted, reverse-transcribed, and the corresponding cDNA was evaluated by real-time PCR as described in the Methods. Expression profiles were then analyzed with GEArray Expression Analysis Suite 2.0. A and B show the genes for CC chemokines, CXC chemokines, and other proinflammatory mediators affected by inhibition of GSK3β. The results for the TNFα set are represented as the average fold change ± SEM in gene expression compared with the untreated control. For the other groups, the data are represented as the average fold change ± SEM in TNFα plus inhibitor compared with the untreated control. Statistical group comparisons were performed between TNFα-treated and TNFα plus inhibitor (dashed line indicates onefold). (*P < 0.001).
GSK3β Inhibitors Diminish Secretion of Proinflammatory Molecules in Activated Brain Endothelial Cells
To confirm the results obtained from the real-time PCR microarray, we measured the inflammatory molecules corresponding to genes affected by GSK3β inhibition. The following soluble and secreted cytokines (measured by ELISA) were chosen because of their highest degree of suppression by the two tested GSK3β inhibitors and their well-established contribution to neuroinflammation: CXCL10, CCL2, CXCL8, CCL5, and CXCL1. In addition to the ATP binding pocket GSK3β inhibitors, I3′M and AR, we also tested two commonly used non-ATP competitive GSK3β inhibitors, LiCl and TDZD-8. For each compound the effects of low versus high concentration was compared. Doses of individual inhibitors were similar to those previously used by other investigators; the most efficacious concentrations that did not affect cellular viability (Supplemental Figure S1, see http://ajp.amjpathol.org) were established in preliminary experiments. Figure 3 (A–E) demonstrates that all inhibitors that suppress in the PCR array also prevented chemokine/proinflammatory mediator secretion by TNFα-stimulated BMVECs, thus confirming the PCR-array analysis. Inhibitors applied in high nontoxic concentrations suppressed secretion of CXCL10 (70% to 85%), CCL2 (50%), CXCL8 (65% to 75%), CCL5 (50% to 80%), and CXCL1 (30% to 80%, P < 0.001). However, in certain cases the lower concentration of inhibitor did not result in inhibition of cytokine production (for example, CXCL10 at low concentration of I3′M and CCL2 at low concentration of LiCl). The PCR array was also validated for IL-6 (Figure 3F), an inflammatory mediator whose mRNA level was observed to be upregulated by addition of TNFα but unaffected by GSK3β inhibition. Taken together, these results indicate that GSK3β inhibitors effectively suppress potent inflammatory mediators secreted by inflamed BMVECs.
Figure 3.
GSK3β inhibitors diminished production of inflammatory molecules in TNFα-activated BMVECs. BMVECs were incubated for 1 hour in the absence or presence of two concentrations of the GSK-3β inhibitors, LiCl (2 or 10 mmol/L), TDZD (0.5 or 1 μmol/L), AR (5 or 10 μmol/L), or 13′M (1 or 3 μmol/L). TNFα was then added at 20 ng/ml and the BMVECs were incubated for 24 hours. The chemokines were detected in the cell culture supernatants by ELISA (kits from RayBiotech or R&D Systems). GSK3β inhibition in TNFα activated BMVECs resulted in diminished production of all tested molecules (A–E), except for IL-6 (F), when compared with cells treated with only TNFα. Data are expressed as mean values ± SEM from 3 independent determinations in BMVECs from multiple donors. *P < 0.05 as compared with cells treated with only TNFα.
GSK3β Inhibition Protects BBB Function
An important component of neuroinflammation is the enhanced engagement of immune cells with the cerebral microvasculature. The adhesion and engagement of leukocytes with the BMVECs enhances barrier permeability (early neuroinflammatory events). The barrier is further disrupted during immune cell migration across the BMVECs (late neuroinflammatory events). We evaluated whether the anti-inflammatory effect of GSK3β inhibitors could also protect barrier function in activated endothelium during increased immune–endothelial cell interactions. We assessed barrier integrity by measuring TEER. Monocytes were added to BMVEC monolayers (plated on ECIS electrode chambers) with or without TNFα exposure in the presence of GSK3β inhibitors (Figure 4A). Addition of monocytes to untreated BMVECs induced a transient decrease in TEER, resulting in 7% to 9% decrease in TEER. Monocytes added to TNFα-stimulated BMVECs produced a rapid and steady decrease, reaching a maximum drop in TEER of 30% by 12 hours when compared with the initial basal level. The GSK3β inhibitor, I3′M, protected barrier function by preventing half the drop in resistance (15%) between 0 and 6 hours. Interestingly, the second inhibitor, AR-A014418, prevented the decrease in electrical resistance after 10 hours of exposure. It is important to note that the concentration of TNFα used did not produce a decrease in electrical resistance. Therefore, GSK3β inactivation protects against the loss of barrier integrity during immune cell insult to brain endothelium.
Figure 4.
Inhibition of GSK3β improves barrier function in the presence of cytokine and monocyte insult. A: BMVEC monolayers were formed on ECIS electrode culture slides, and continuous measurements of TEER were acquired. The measurements shown represent recordings acquired at 10-minute intervals at the parameters described in the Methods. Endothelial cell monolayers were treated with the following experimental conditions: untreated (blue line), TNFα at 20 ng/ml (green line), monocytes only (black line), TNFα + monocytes + A014418 at 10 μmol/L (red line), and TNFα + monocytes + I3′M at 3 μmol/L (orange line). Monocytes were added at 1 × 105 cells per well at time = 0 (indicated by the arrow). The data are presented as percent change from baseline, which is the resistance measured posttreatment divided by the resistance acquired before treatment introduction. B: Adhesion assays of untreated and TNFα-activated endothelial cells exposed for 24 hours to various GSK-3β inhibitors (LiCl at 2 mmol/L, I3′M at 3 μmol/L, TDZD-8 at 1 μmol/L, and AR-A014418 at 10 μmol/L). Before the assay, all treatments were removed and calcein-am labeled monocytes were added to the BMVECs and allowed to adhere to the BMVECs for 15 minutes. BMVEC monolayers were then rinsed to remove unattached cells and the fluorescence was measured. The data are represented as mean ± SEM fold difference, which is the adhesion value from treated BMVECs divided by the basal adhesion value from untreated cells. Statistical significance (P < 0.05), * compares cytokine-stimulated versus untreated; ** compares cytokine-stimulated plus inhibitor versus cytokine stimulated. C: Transendothelial migration of monocytes was performed across TNFα (20 ng/ml) or IL-6 (10 ng/ml)-stimulated BMVEC monolayers in the presence of the indicated inhibitors; chemotaxis was toward CCL2 (50 ng/ml). Monocytes were migrated for 2 hours and fluorescence was measured as described in the Methods. The figure shows the effect that GSK3β inhibition has on migration when monocytes are treated with inhibitors. The data are represented as mean + SEM fold difference of migration (left y axis) and percentage of input (right y axis), which is the value from migration of treated cells divided by the basal migration of untreated cells without chemoattractant. All data collected are from at least three independent experiments performed in triplicate. Statistical significance (P < 0.05), * compares cytokine-stimulated and chemoattractant versus untreated; ** compares cytokine-stimulated plus inhibitor and chemoattractant versus cytokine stimulated and chemoattractant; # compares cytokine-stimulated versus untreated.
GSK3β Inhibition in Brain Endothelium Attenuates Monocyte Adhesion and Transendothelial Migration
We next determined whether the observed protection of barrier integrity by GSK3β inhibition is the result of diminished monocyte adhesion to endothelial cells. Adhesion assays revealed that exposure to TNFα induced a sixfold increase in the number of monocytes attaching to the BMVEC monolayers (Figure 4B). Similarly, the proinflammatory cytokine, IL-6, produced an approximate fivefold increase in monocyte adhesion over untreated BMVECs. Application of GSK3β inhibitors to BMVECs provided significant (approximately threefold) reduction in adhesion when compared with TNFα-activated BMVECs. Similar level of reduction in adhesion events was observed when GSK3β inhibitors were applied in combination with IL-6 (P < 0.001). The results indicate that GSK3β inhibition effectively attenuates the adhesion of monocytes to the TNFα stimulated BMVECs. These results also suggest that although levels of IL-6 (mRNA or secreted protein; see Supplemental Table S1 at http://ajp.amjpathol.org and Figure 3) were not inhibited by inactivation of GSK3β, it does appear that IL-6 signaling leading to the induction of greater adhesion can be suppressed by inhibiting GSK3β.
A decrease in adhesion could also result in a diminution in the number of cells crossing the BBB. Using migration assays in an in vitro BBB model, we tested whether GSK3β inhibition in endothelial cells could prevent monocyte passage across BMVEC monolayers. We used CCL2 as a relevant cytokine upregulated in the central nervous system under neuroinflammatory conditions. Application of CCL2 to the lower chamber of BBB constructs increased monocyte migration twofold as compared with models without chemokine addition. Stimulation of endothelial monolayers with either TNFα or IL-6 further increased migration to CCL2 × 3.9-fold and 3.2-fold, respectively (P < 0.001, Figure 4C). In the TNFα and CCL2 groups, migration was attenuated by 64% after pretreatment of BMVECs with TDZD-8 and 58% after application of LiCl, I3′M, and AR-A014418. Monocyte passage induced by exposure to IL-6/chemokine was inhibited by 48% by I3′M, whereas LiCl, AR-A014418, and TDZD-8 reduced migration by ≈33%. Of note, there was no difference in monocyte migration across control or inhibitor-treated BMVEC monolayers without inflammatory stimuli (data not shown). These results suggest that inhibition of GSK3β in BMVECs reduces both immune cell adhesion and migration across the activated endothelial monolayers.
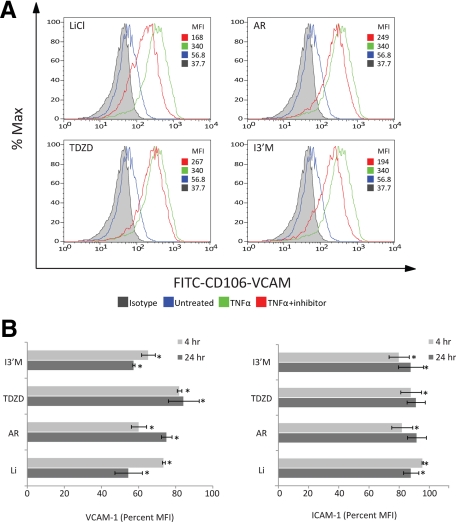
GSK3β Inhibitors Diminish Expression of Vascular Cell Adhesion Molecule in Activated Brain Endothelium
The results gathered from adhesion and migration assays suggest that endothelial-immune cell interaction is blocked by the selective inhibition of GSK3β in the BMVECs. One possible explanation is that active GSK3β is involved in promoting the upregulation of adhesion molecules on the surface of a cytokine-stimulated BMVECs. Adhesion molecules such as VCAM-1 and ICAM-1 are required for the firm engagement of immune cells with the endothelium. Using FACS analysis, we assessed the surface expression level of the adhesion molecules, VCAM-1 and ICAM-1, after cytokine activation of the BMVECs and determined the effect of GSK3β inhibitors on these adhesion molecules. The expression was evaluated for treatments performed at 4 and 24 hours. Figure 5A shows BMVEC surface expression for the 24-hour time point in BMVECs that were untreated (basal level), TNFα-treated, and TNFα-treated with LiCl, TDZD-8, AR-A014418, or I3′M. In activated endothelial cells the induction of VCAM-1 was upregulated between four- and sixfold when compared with the untreated control. Quantitative results comparing four and 24 hours from the FACS analysis demonstrated the differential suppression of VCAM-1 by the different inhibitors (Figure 5B). At 24 hours, suppression of VCAM-1 was observed for all inhibitors; however, I3′M and LiCl provided the best inhibition (55%) and TDZD-8 and AR-A014418 provided 21% inhibition. The four-hour treatment provided a different inhibitor efficacy for VCAM-1 suppression, with AR-A014418 and I3′M resulting in 39% suppression; LiCl and TDZD-8 showed 22% inhibition. Surprisingly, the expression of ICAM-1 was only marginally sensitive to GSK3β inhibition. As expected, TNFα induced ICAM-1 expression between four- and eightfold. However in the presence of inhibitors, a 4% suppression (average of all inhibitors) of ICAM-1 was observed. Furthermore, short as compared with long inhibitor exposure did not appear to differentially affect the minor reduction of ICAM-1 expression. Of note, none of the GSK3β inhibitors affected the basal levels of the adhesion molecules (data not shown). Taken together, these data indicate that GSK3β inhibition can diminish the induction of VCAM-1 in TNFα-activated BMVECs.
Figure 5.
GSK3β inhibitors diminish expression of adhesion molecules in cytokine-activated BMVECs. Adhesion molecule expression was measured in BMVECs stimulated with TNFα (20 ng/ml). Panel A shows representative histograms of FACS analysis for surface expression of vascular cell adhesion molecule-1 (VCAM-1) at 24 hours in untreated, TNFα-treated, and TNFα-treated BMVECs in combination with the indicated GSK3β inhibitors. The data are represented as the mean fluorescence intensity of VCAM-1 as a percentage of the maximum intensity. Panel B shows the accumulative data for surface expression of VCAM-1 (left graph) and ICAM-1 (right graph), comparing 4 hours with 24 hours. The data shown are the average median fluorescence intensity of the experimental (inhibitor plus TNFα) divided by the TNFα only condition; the results are from at least three independent experiments. *P < 0.05.
GSK3β Inhibition Decreases Leukocyte Adhesion to Brain Endothelium in Vivo
Next, we investigated whether GSK3β inhibition would diminish leukocyte adhesion in vivo. Mice were implanted with cranial windows allowing visualization of leukocytes in brain micovessels. Two different selective GSK3β inhibitors were injected simultaneously with LPS (6 mg/kg) and white cell adhesion was monitored at 0, 4, and 24 hours by intravital fluorescence microscopy as described in Methods. LPS injection substantially increased leukocyte adhesion by four hours (≈50-fold, Figure 6, A and B) both inhibitors significantly attenuated leukocyte adhesion after LPS injection (55% by AR and 28% by TDZD, respectively; P < 0.001, Figure 6). By 24 hours, adhesion still was increased by ≈35-fold, and both inhibitors diminished endothelial-leukocyte interactions by 65% (P < 0.001, Figure 5). In summary, intravital microscopy confirmed effects of GSK3β inhibition on monocyte adhesion in vitro.
Figure 6.
In vivo administration of GSK3 inhibitors reduces endogenous leukocyte adhesion after LPS injection. C57BL/6 mice were injected with LPS (6 mg/kg). Two treatment groups were injected i.p. with AR-A014418 (5 mg/kg) or TDZD-8 (2 mg/kg) simultaneously with LPS. The control group received vehicle. A: Leukocyte adhesion in pial microvessels was visualized at 0, 4, and 24 hours by intravital microscopy (cranial window) as previously described.18 Shown in B are the measurements taken of attached leukocytes appearing in A under cranial window. The leukocytes counted included only cells that were under firm adhesion and not rolling during the period of observation. Adhering leukocytes from multiple vessels and from at least three fields of view per animal (3 animals per group) were scored as the number of cells per mm2 of the vascular surface area; calculated from the diameter and standardized length (100 micron) of the vessel segment under investigation. The results indicate that LPS increased adhesion by 50-fold (4 hours) and 35-fold (24 hours), whereas both inhibitors significantly reduced adhesion at both time points. The data are shown as the mean adhesion + SEM (n = 3). *P < 0.001 between the groups compared (bracket).
Discussion
In recent years, the role of chronic BBB impairment has become more apparent in a wide range of diseases contributing to neurodegeneration.20 Pathological conditions such as Alzheimer disease, Parkinson disease, multiple sclerosis, and HIVE have been shown to have various degrees of barrier dysfunction.3 HIVE is characterized by mononuclear cell infiltration (monocytes, T-cells) driven by chemokine production and antiviral responses, and it is accompanied by increased permeability of the BBB.21 Secretory factors from virus-infected activated macrophages are implicated in the neurological decline of HIV-1–infected patients.22 Therefore, approaches leading to a decrease in leukocyte migration and barrier stabilization may prevent central nervous system damage in HIV-1 infection and neuroinflammation. Using primary human BMVECs, we demonstrated that GSK3β inhibition preserved barrier function, diminished monocyte adhesion and migration across endothelial monolayers, and decreased secretion of proinflammatory factors by brain endothelium. Furthermore, we detected active forms of GSK3β in BMVECs in HIVE (featuring BBB injury23) but not control brain tissues. These observations clearly point to GSK3β activation in endothelium under inflammatory conditions.
GSK3β suppression has been suggested as a potential therapeutic approach for a number of diseases, including diabetes, Alzheimer disease and other neurodegenerative disorders, stroke, and traumatic brain injury.14,24,25,26 The GSK3β inhibitor, lithium, was recently recognized as a clinically beneficial drug for treatment of HIV-1–associated cognitive impairment.27 Anti-inflammatory effects of GSK3β inhibition have been documented in several experimental studies in vivo and in vitro.8,9,28 GSK3β inhibition ameliorated arthritis in a mouse model and decreased production of several proinflammatory molecules (such as TNFα, IL-1β, IL-6, iNOS, and COX-2, oxidative stress markers, chemokines).15 GSK3β inhibitors attenuated the systemic inflammation and multiple organ injury caused by endotoxin.8,10 Anti-inflammatory and neuroprotective effects of GSK3β inhibition were shown in animal models of multiple sclerosis,29 stroke,14 cord injury,15 and Alzheimer disease.30 However, the role of GSK3β in BBB injury has not been previously studied.
Inflammation and subsequent tissue injury are controlled by endothelial cells via expression of adhesion molecules facilitating the first step (adhesion) in the passage across endothelial monolayers.31 Adhesion and migration of leukocytes across endothelial cell barriers (like the BBB) lead to disruption and increase in endothelial permeability.19 Our experiments consistently showed up to a sixfold increase in monocyte adhesion after TNFα or IL-6 stimulation and 60% reduction in adhesion after GSK3β inhibition in BMVECs. Of note, GSK3β inhibition did not decrease adhesion of monocytes to unstimulated BMVECs. Furthermore, these observations correlated with a marked suppression in VCAM-1 expression after upregulation by TNFα. Surprisingly, GSK3β inactivation had only a minimal effect on ICAM-1, which is constitutively present on brain endothelium.32 Our results differ from observations by Vines et al17 showing that TNFα and IL-1β treatment of primary cultures of human microvascular endothelial cells reduced endogenously active GSK3β protein levels, and inhibition of GSK3β activity enhanced inflammatory mediators (IL-6, CCL2, VCAM-1) after cytokine stimulation. On the other hand, our data are in agreement with the findings of Eto and colleagues that demonstrated that GSK3β inhibition suppressed the TNFα-stimulated expression of VCAM-1 on aortic endothelial cells via inhibition of NF-κB DNA binding.11 In another study, GSK3β suppression diminished the expression of the adhesion molecule, E-selectin, in vivo and decreased leukocyte accumulation in inflamed renal tissue.12 Relevance of our in vitro results was confirmed in vivo by intravital microscopy. LPS injection increased leukocyte adhesion to brain endothelium 35- to 50-fold in mice, and GSK3β inhibitors reduced it by 28% to 65%.
Our results demonstrate that the diminished adhesion of leukocytes to the endothelium during GSK3β inhibition paralleled the decrease in monocyte migration across BMVEC monolayers. Indeed, migration assays showed a three- to fourfold increase in monocyte passage across cytokine-activated BMVEC monolayers in response to the neuroinflammatory relevant chemokine, CCL2. Pretreatment of BMVECs with various GSK3β inhibitors significantly reduced the migration of monocytes (by 30% to 64%). Although our studies focused on the inhibition of GSK3β in endothelial cells, migration experiments complement those of Tickenbrok and colleagues indicating that GSK3β inhibition in monocytes prevented their migration.33
Because GSK3β inhibition in the brain endothelium produced strong anti-inflammatory effects, we investigated whether it could also protect barrier function. Previously, GSK3β phosphorylation (inactivation) by AKT in lung endothelium enabled the tightening of adherent junctions and enhanced TEER.13 GSK3β activation was implicated in causing a “leakier” BBB in mice deficient of platelet endothelial cell adhesion molecule, which develops a more severe experimental autoimmune encephalomyelitis.34 Our experiments showed that interactions of monocytes with TNFα-stimulated BMVECs resulted in diminished barrier function (TEER decrease); GSK3β inactivation significantly protected the barrier from injury. We have previously shown that engagement of BMVECs by monocytes results in activation of Rho GTPases (like RhoA, Rac)16 inducing an increase in barrier permeability and phosphorylation of tight junction proteins (occludin, claudin-5); consequently, inhibition of RhoA/Rho kinase prevented these effects.19 Whether GSK3β inhibition prevents tight junction modifications or interferes with GTPase activity that lead to barrier dysfunction remains to be determined.
Although endothelial cells of various vascular beds are the targets of pro-inflammatory molecules, the endothelium can produce a multitude of cytokines, chemokines,35 or other molecules36 that can affect barrier function in a paracrine fashion and promote inflammation.37 Given the significant anti-inflammatory activity of GSK3β inhibition in macrophages and other cells, we analyzed such effects in BMVECs after TNFα stimulation. TNFα stimulation led to a 50,000- to 80,000-fold increase in mRNA for a number of proinflammatory factors, and GSK3β dramatically decreased this transcriptional activity. Of the numerous targets analyzed, only certain inflammatory mediators were suppressed after GSK3β inhibition, thus indicating differential regulation by GSK3β. The most prominent effect was achieved at the 24-hour time point. In vivo data suggested that, even after single administration, GSK3β inhibitors were efficient 24 hours later, with more pronounced effect when compared with 4 hours. Multiple targets of GSK3β inhibition, some requiring transcription and new protein synthesis, may explain the delayed effects and warrants further studies.
In summary, GSK3β inhibition emerges as a potent therapeutic approach to decrease various aspects of inflammation-associated BMVEC dysfunction including the generation of proinflammatory molecules, barrier destabilization, increased monocyte adhesion, and migration. The latter feature is of special interest since up to now glucocorticoids are the only class of drugs that are used for tightening the BBB. Our future studies will attempt to uncover the underlying mechanism of the effects of GSK3β on BBB function.
Acknowledgments
We thank the NIH National NeuroAIDS Consortium and Dr. Benjamin Gelman at the Texas NeuroAIDS Research Center for providing brain tissue specimens.
Footnotes
Address reprint requests to Yuri Persidsky, M.D., Ph.D., Temple University School of Medicine, Department of Pathology and Lab Medicine, 3401 N. Broad St., Philadelphia, PA 19140. E-mail: yuri.persidsky@tuhs.temple.edu.
Supported in part by research grants from the National Institutes of Health: NS043985, AA015913, and MH65151 (to Y.P.).
Brain tissue specimens were provided by the Texas NeuroAIDS Research Center (Dr. Benjamin Gelman; NIH grants U01-MH083507 and R24-NS45491).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Stolp HB, Dziegielewska KM. Review: role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Ohashi PS. GSK3: an in-Toll-erant protein kinase? Nat Immunol. 2005;6:751–752. doi: 10.1038/ni0805-751. [DOI] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugo L, Collin M, Allen DA, Patel NS, Bauer I, Mervaala EM, Louhelainen M, Foster SJ, Yaqoob MM, Thiemermann C. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- Takada Y, Singh S, Aggarwal BB. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M, Kouroedov A, Cosentino F, Luscher TF. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-alpha. Circulation. 2005;112:1316–1322. doi: 10.1161/CIRCULATIONAHA.105.564112. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Dworkin LD. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney Int. 2006;69:1166–1174. doi: 10.1038/sj.ki.5000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model, Proc Natl Acad Sci USA. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Collin M, Esposito E, Bramanti P, Thiemermann C. Glycogen synthase kinase-3 beta inhibition reduces secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2006;318:79–89. doi: 10.1124/jpet.106.102863. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines A, Cahoon S, Goldberg I, Saxena U, Pillarisetti S. Novel anti-inflammatory role for glycogen synthase kinase-3beta in the inhibition of tumor necrosis factor-alpha- and interleukin-1beta-induced inflammatory gene expression. J Biol Chem. 2006;281:16985–16990. doi: 10.1074/jbc.M602446200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 Suppl 1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, Durelle J, Grant I, Everall I. Lithium improves HIV-associated neurocognitive impairment. Aids. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, 3rd, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific. NF-kappaB-dependent transcription, Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, Yang F, Chen P, Ubeda OJ, Kim PC, Davies P, Ma Q, Cole GM, Frautschy SA. GSK3 inhibitors show benefits in an Alzheimer’s disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol Dis. 2009;33:193–206. doi: 10.1016/j.nbd.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. Molecular mechanisms that control leukocyte extravasation through endothelial cell contacts. Ernst Schering Found Symp Proc. 2007:151–167. doi: 10.1007/2789_2007_063. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- Tickenbrock L, Schwable J, Strey A, Sargin B, Hehn S, Baas M, Choudhary C, Gerke V, Berdel WE, Muller-Tidow C, Serve H. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol. 2006;79:1306–1313. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subileau EA, Rezaie P, Davies HA, Colyer FM, Greenwood J, Male DK, Romero IA. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:227–240. doi: 10.1097/NEN.0b013e318197eca7. [DOI] [PubMed] [Google Scholar]
- Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134:431–441. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]